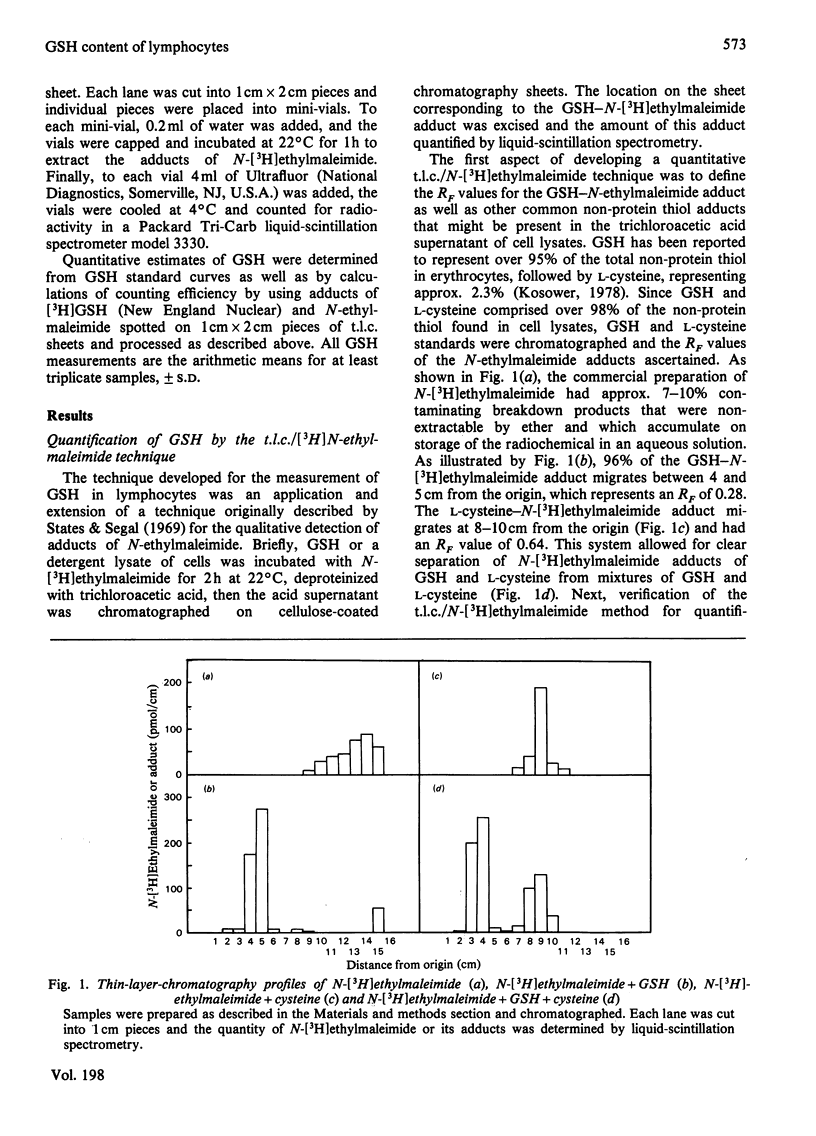
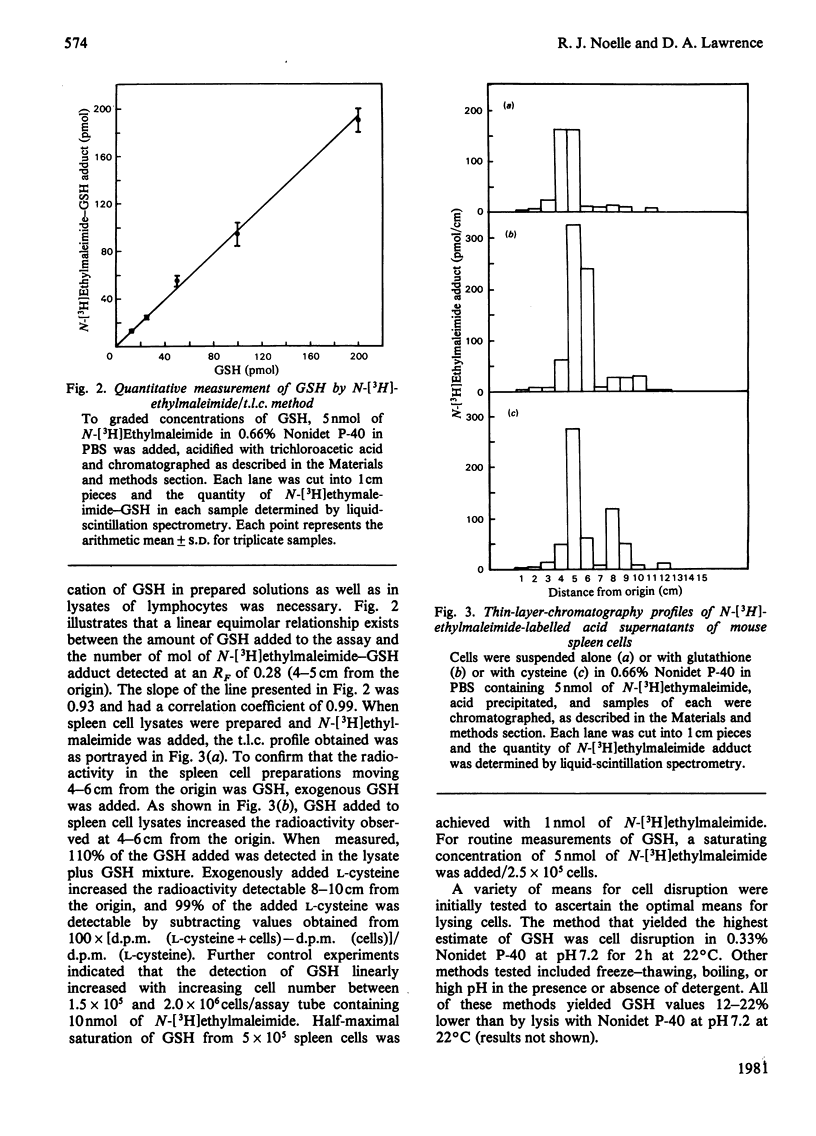
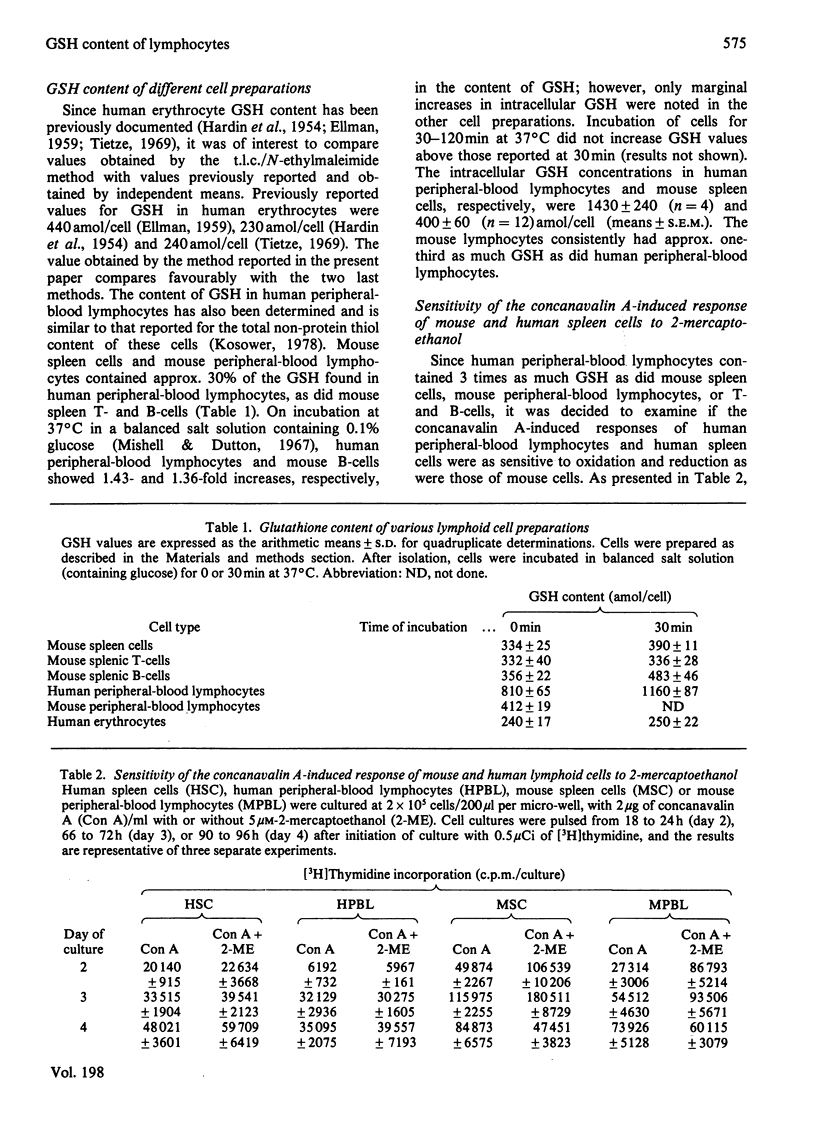
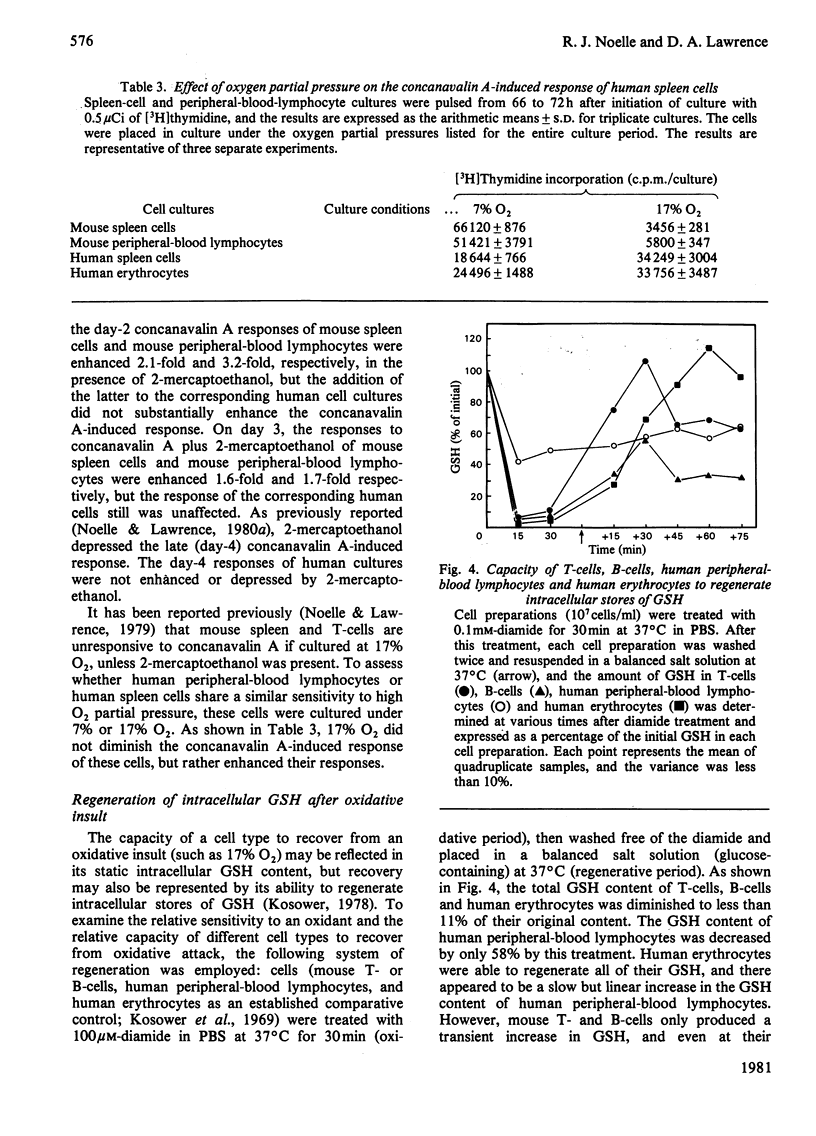
Abstract
The glutathione (GSH) content of mouse T- and B-cells was determined and compared with the GSH content of human peripheral blood lymphocytes and human erythrocytes. Owing to the difficulty of obtaining large numbers of purified lymphocytes, a technique was developed to measure picomolar quantities of GSH. By this technique, mouse T- and B-cells, as well as mouse peripheral-blood lymphocytes, were found to contain approx. 30% of the GSH found in human peripheral-blood lymphocytes. The concanavalin A response of human peripheral-blood lymphocytes and human spleen cells was insensitive to 2-mercaptoethanol as well as to culture in 17% O2, whereas mouse lymphocyte responses were altered by 2-mercaptoethanol and inhibited by 17% O2. The capacity of human peripheral-blood lymphocytes, human erythrocytes, mouse T-cells and mouse B-cells to regenerate GSH stores after chemical oxidation by diamide was tested, and it was found that mouse cells were less capable of regenerating GSH than human erythrocytes or human peripheral-blood lymphocytes. In addition, the latter lymphocytes were less sensitive to oxidation of GSH and to inhibition of proliferation by diamide.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. E., Warner N. L. Ionizing radiation and the immune response. Adv Immunol. 1976;24:215–335. doi: 10.1016/s0065-2776(08)60331-4. [DOI] [PubMed] [Google Scholar]
- Anundi I., Högberg J., Stead A. H. Glutathione depletion in isolated hepatocytes: its relation to lipid peroxidation and cell damage. Acta Pharmacol Toxicol (Copenh) 1979 Jul;45(1):45–51. doi: 10.1111/j.1600-0773.1979.tb02359.x. [DOI] [PubMed] [Google Scholar]
- Davidson W. F., Parish C. R. A procedure for removing red cells and dead cells from lymphoid cell suspensions. J Immunol Methods. 1975 Jun;7(2-3):291–300. doi: 10.1016/0022-1759(75)90026-5. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Field L., Gallo A. A., Beck F. W., Whitehouse M. W. Lymphocyte surface poisons: disulfides and thiolsulfonates. Chem Biol Interact. 1978 Nov;23(2):215–225. doi: 10.1016/0009-2797(78)90007-8. [DOI] [PubMed] [Google Scholar]
- Goldberg N. D., Graff G., Haddox M. K., Stephenson J. H., Glass D. B., Moser M. E. Redox modulation of splenic cell soluble guanylate cyclase activity: activation by hydrophilic and hydrophobic oxidants represented by ascorbic and dehydroascorbic acids, fatty acid hydroperoxides, and prostaglandin endoperoxides. Adv Cyclic Nucleotide Res. 1978;9:101–130. [PubMed] [Google Scholar]
- HARDIN B., VALENTINE W. N., FOLLETTE J. H., LAWRENCE J. S. Studies on the sulfhydryl content of hyman leukocytes and erythrocytes. Am J Med Sci. 1954 Jul;228(1):73–82. doi: 10.1097/00000441-195407000-00009. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Hydrogen donor system for Escherichia coli ribonucleoside-diphosphate reductase dependent upon glutathione. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2275–2279. doi: 10.1073/pnas.73.7.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M. The glutathione status of cells. Int Rev Cytol. 1978;54:109–160. doi: 10.1016/s0074-7696(08)60166-7. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M., Wertheim B., Correa W. S. Diamide, a new reagent for the intracellular oxidation of glutathione to the disulfide. Biochem Biophys Res Commun. 1969 Nov 6;37(4):593–596. doi: 10.1016/0006-291x(69)90850-x. [DOI] [PubMed] [Google Scholar]
- Kwock L., Wallach D. F., Hefter K. Involvement of sulfhydryl groups in the action of the insulin and radiation on thymocyte Na+-dependent amino acid transport. Biochim Biophys Acta. 1976 Jan 8;419(1):93–103. doi: 10.1016/0005-2736(76)90374-6. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse M. L., Dahl R. H. Cellular glutathione is a key to the oxygen effect in radiation damage. Nature. 1978 Feb 16;271(5646):660–662. doi: 10.1038/271660a0. [DOI] [PubMed] [Google Scholar]
- Noelle R. J., Lawrence D. A. Modulation of T-cell functions. I. Effect of 2-mercaptoethanol and macrophages on T-cell proliferation. Cell Immunol. 1980 Mar 15;50(2):416–431. doi: 10.1016/0008-8749(80)90295-6. [DOI] [PubMed] [Google Scholar]
- Ohara H., Terasima T. Variations of cellular sulfhydryl content during cell cycle of HeLa cells and its correlation to cyclic change of x-ray sensitivity. Exp Cell Res. 1969 Nov;58(1):182–185. doi: 10.1016/0014-4827(69)90133-5. [DOI] [PubMed] [Google Scholar]
- Oliver J. M., Albertini D. F., Berlin R. D. Effects of glutathione-oxidizing agents on microtubule assembly and microtubule-dependent surface properties of human neutrophils. J Cell Biol. 1976 Dec;71(3):921–932. doi: 10.1083/jcb.71.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redelman D., Hudig D. The mechanism of cell-mediated cytotoxicity. I. Killing by murine cytotoxic T lymphocytes requires cell surface thiols and activated proteases. J Immunol. 1980 Feb;124(2):870–878. [PubMed] [Google Scholar]
- Sagone A. L., Jr, Kamps S., Campbell R. The effect of oxidant injury on the lymphoblastic transformation of human lymphocytes. Photochem Photobiol. 1978 Oct-Nov;28(4-5):909–915. doi: 10.1111/j.1751-1097.1978.tb07039.x. [DOI] [PubMed] [Google Scholar]
- States B., Segal S. Thin-layer chromatographic separation of cystine and the N-ethylmaleimide adducts of cysteine and glutathionen. Anal Biochem. 1969 Feb;27(2):323–329. doi: 10.1016/0003-2697(69)90041-4. [DOI] [PubMed] [Google Scholar]
- Strom T. B., Lundin A. P., Carpenter C. B. The role of cyclic nucleotides in lymphocyte activation and function. Prog Clin Immunol. 1977;3:115–153. [PubMed] [Google Scholar]
- Sutherland R. M., Pihl A. Repair of radiation damage to erythrocyte membranes. The reduction of radiation-induced disulfide groups. Radiat Res. 1968 May;34(2):300–314. [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]


