Abstract
Urine collection can be challenging in studies involving small rodents like mice, as the actual methods of collection are anxiogenic and constrain animal welfare while having high variability in the volume of urine collected. To improve the current methods and eventually reduce the impact on the well-being of mice, we developed an innovative 3D-printed urine collection device (UCD). This two-compartment UCD is shaped to fit in classical husbandry cages and allows urine collection by spontaneous urination from two mice housed in their own cage without cross-contamination while enabling potential social interactions. We used our UCD to study the evolution of urinary parameters related to renal functions in a model of antibody-mediated chronic kidney disease. Overall, we report here a time-saving and affordable method for urine collection providing a large amount of uncontaminated urine and which we believe may improve animal welfare in comparison with other methods.
Keywords: Ethics and welfare, experimental design, in vivo, organisms and models, refinement, rodents, techniques, urine collection
Introduction
Studying mouse models of kidney diseases requires urine sample collection to accurately measure the evolution of the disease. However, ensuring the collection of a sufficient volume of uncontaminated urine with ease and at low cost, while maintaining the animal welfare, remains tedious.
Metabolic cages allow the recording of multiple parameters, and ensure the collection of uncontaminated urine from mice. 1 However, metabolic cages require the isolation of mice for long periods on a small floor area, which does not meet European regulations. 2 Housing in metabolic cages leads to high and prolonged stress levels, 3 which can be reduced by grouped housing. 4 In addition, metabolic cages are expensive and require a suitable rack in the animal facility.
Recently, more affordable methods were described, such as the use of hydrophobic sands 5 or stimulation of natural micturition. These methods require physical restraint or complete isolation of an animal and volumes of urine that are collected vary greatly. All these methods have variable efficiencies regarding the quality and quantity of urine collected and affect the well-being of the animal. To respect the principle of the 3Rs and particularly to refine urine collection, we developed an affordable, easy-to-use and innovative urine collection device (UCD) using 3D printing, also adaptable to aluminium.
Results
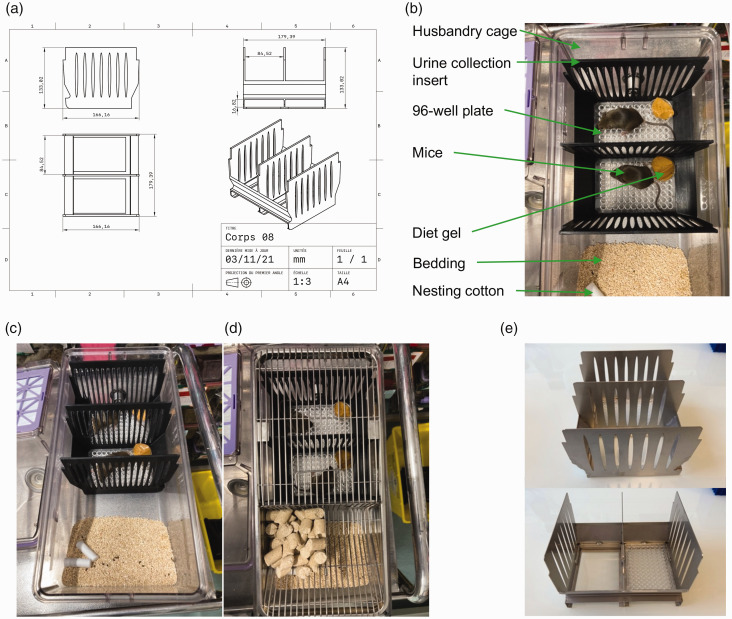
Our 3D-printed UCD contains two compartments, fits to the husbandry cages from Allentown (NexGen Mouse 500) or Techniplast (Green line GM500) and maintains the airflow in the cage through its perforated walls, when using ventilated racks (Figure 1(a)). The UCD permits urine collection from two separated mice without any cross-contamination (Figure 1(b) to (d); files for 3D printing available in the Supplementary material online). Each compartment can receive a disposable, sterile, 96-well plate (polystyrene) fitting at the bottom of the UCD constituting the floor and spanning across one-third of the cage (140 cm2/mouse; Figure 1(a) to (d)).
Figure 1.
Development of a new urine collection device. Representative images of the (a) 3D design with precise dimensions for Allentown cages, (b) top view, (c) side view and (d) top view (with the cage’s grid) of the urine collection insert and (e) Side views of the aluminium urine collection device.
All animal housing and procedures followed the guidelines of the Directive 2010/63/EU of the European Parliament, and were reviewed and approved by our local ethical committee (Comité d’Ethique Paris Nord 121; APAFIS no. 17819). To assess the efficacy of our device, we used adult 129S6/SvEvTac mice (10–11 weeks old) from both sexes, bred in-house. Mice are housed in standard cages in ventilated racks with bedding (litter and cottons), enrichments (cardboard roll) and free access to food and water. For urine collection, mice are placed in the UCD installed in their cage, overnight (<12 h). The time of the day and duration for collection have to be adjusted to the species, strain and sex of the animals. In the UCD, animals have free access to food/water as a hydrated gel in a cup (5 g/collection period; DietGel® 76A Clear H2O). At the end of the procedure, urine is easily collected, only from uncontaminated wells of the 96-well plates using a pipette, from each compartment. Next, the plates are discarded, the UCD is removed from the cage and completely disinfected with 70% ethanol, and mice are placed back in the cage with free access to food, water, bedding and nesting.
Initially, our 3D-printed UCD (Figure 1(b) and (c)) was made out of a temperature- and pressure-sensitive polylactic acid and could not be autoclaved. Based on the blueprints of the 3D-printed UCD, we manufactured an easy to sterilize (autoclave) and affordable (€75/device and long-lasting lifespan) aluminium-based UCD (Figure 1(e)). Both 3D-printed and aluminium-based UCDs are loaded with 96-well plates and showed similar efficiency for urine collection. The next experiments were conducted with aluminium-based UCDs.
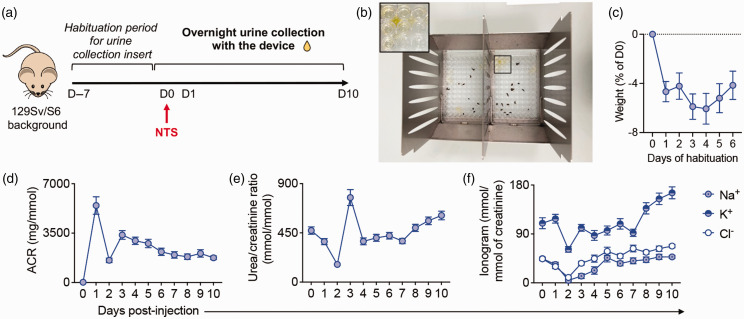
To reduce anxiety, mice were placed in the UCD overnight, every day for a week, to get accustomed to the device and to the food (Figure 2(a)). During this ‘habituation period’, uncontaminated urine samples were collected to measure baseline parameters (Figure 2(b)). Mice lost about 4% of their weight after the first night (p < 0.05, one-way analysis of variance, Prism software) in the UCD, most likely due to the change in the diet and to a slight environment-induced anxiety. However, weights remained steady on the following days (Figure 2(c)). The habituation period is essential, and we believe that the prolonged use of this device does not overstress mice. Using our UCD, we collected 50 to 700 µl of uncontaminated urine, per mouse, per collection period.
Figure 2.
Urine collection for renal function analysis in a model of antibody-mediated chronic kidney disease. (a) Experimental workflow (n=13 mice, from two independent experiments). (b) Representative image showing uncontaminated urine in the 96-well plate of the device ready to be collected. Kinetic analysis of (c) the percentage of weight change compared with day 0 (D0) during the habituation period and ((d) to (f)) urinary parameters: (d) albumin, (e) urea and (f) the ionogram (Na+, K+, Cl–) normalized on urinary creatinine after nephrotoxic serum (NTS) injection. The results are displayed as the mean ± SEM.
D: day; ACR: Albumin/creatinine ratio (ACR)
To validate this method of urine collection, we used the UCD to analyse renal functions in a model of antibody-mediated chronic kidney disease (AMCKD) induced by the injection of nephrotoxic serum (NTS) (Probetex, reference PTX-001S-Ms). 6 Mice were monitored daily for 10 days following the intravenous injection of NTS (50 µl, day 0) and euthanized at the end of the experiment (Figure 2(a)). Mice were not subjected to other interventions. The collected urine samples allowed the efficient measurement of the urinary albumin/creatinine ratio, of the excretion of urea and of the urinary ionogram during AMCKD (Figure 2(d) to (f)) using an automated biochemical analyser, Respons®920 (Diasys) 6 and its reagents (references: 102429910921; 117599910920; 131019910920; 136009910921; 960430). Results from all of the animals are presented (no exclusion).
Conclusion
To conclude, our UCD (3D-printed and aluminium-based) is very efficient and easily allows urine collection without invasive intervention on mice. The UCD keeps mice in their own visual and olfactive environment, and possibly maintains social interactions. The physical isolation in each compartment guarantees the quality of the samples without cross-contamination. These advantages and the adaptability of this device to any lab, cage (shape, material) and protocols are what sets this device apart from the other methods of urine collection.
However, the UCD also has some limitations encountered with other methods of urine collection. To avoid urine contamination by food, mice face a new diet (jellified) that requires a period of habituation. Additionally, this diet can impact urinary parameters, so using the same diet for all of the experimental groups is essential. Moreover, the UCD is not a metabolic cage and it does not: 1) allow refrigerated urine collection; 2) determine precisely the volume of urine produced; 3) monitor the precise food/water intake over a period of collection. Finally, Van Loo et al. 7 suggested that ‘living apart together’ can also affect the well-being of mice, and this aspect of the UCD will require further studies.
As it is challenging to maintain the balance between animal welfare and collection of urine, this innovative UCD represents an affordable and good alternative to efficiently collect a large amount of uncontaminated urine suitable for the biochemical analysis of renal functions, while potentially creating a less stressful and a refined environment for the animals.
Supplemental Material
Supplemental material, sj-zip-1-lan-10.1177_00236772231219828 for Refining urine collection in mice: Development of an innovative urine collection device by Mélodie Douté, Céline Monzali, Antonino Nicoletti, Giuseppina Caligiuri and Marc Clement in Laboratory Animals
Supplemental material, sj-pdf-2-lan-10.1177_00236772231219828 for Refining urine collection in mice: Development of an innovative urine collection device by Mélodie Douté, Céline Monzali, Antonino Nicoletti, Giuseppina Caligiuri and Marc Clement in Laboratory Animals
Supplemental material, sj-zip-3-lan-10.1177_00236772231219828 for Refining urine collection in mice: Development of an innovative urine collection device by Mélodie Douté, Céline Monzali, Antonino Nicoletti, Giuseppina Caligiuri and Marc Clement in Laboratory Animals
Supplemental material, sj-pdf-4-lan-10.1177_00236772231219828 for Refining urine collection in mice: Development of an innovative urine collection device by Mélodie Douté, Céline Monzali, Antonino Nicoletti, Giuseppina Caligiuri and Marc Clement in Laboratory Animals
Supplemental material, sj-zip-5-lan-10.1177_00236772231219828 for Refining urine collection in mice: Development of an innovative urine collection device by Mélodie Douté, Céline Monzali, Antonino Nicoletti, Giuseppina Caligiuri and Marc Clement in Laboratory Animals
Supplemental material, sj-pdf-6-lan-10.1177_00236772231219828 for Refining urine collection in mice: Development of an innovative urine collection device by Mélodie Douté, Céline Monzali, Antonino Nicoletti, Giuseppina Caligiuri and Marc Clement in Laboratory Animals
Supplemental material, sj-zip-7-lan-10.1177_00236772231219828 for Refining urine collection in mice: Development of an innovative urine collection device by Mélodie Douté, Céline Monzali, Antonino Nicoletti, Giuseppina Caligiuri and Marc Clement in Laboratory Animals
Supplemental material, sj-zip-8-lan-10.1177_00236772231219828 for Refining urine collection in mice: Development of an innovative urine collection device by Mélodie Douté, Céline Monzali, Antonino Nicoletti, Giuseppina Caligiuri and Marc Clement in Laboratory Animals
Supplemental material, sj-zip-9-lan-10.1177_00236772231219828 for Refining urine collection in mice: Development of an innovative urine collection device by Mélodie Douté, Céline Monzali, Antonino Nicoletti, Giuseppina Caligiuri and Marc Clement in Laboratory Animals
Acknowledgement
We thank B Clement from the SLTS metal solution company for his help in the creation of the aluminium based UCD.
Footnotes
The authors have no conflicts of interest to declare.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by INSERM, Fondation pour la Recherche Médicale (FRM, grant no. ARF201809007140), Laboratory of Excellence INFLAMEX (grant no. ANR-11-IDEX-0005-02).
ORCID iDs: Mélodie Douté https://orcid.org/0000-0002-1926-9718
Marc Clement https://orcid.org/0000-0002-6479-8360
References
- 1.Bazare J, Leamons ML, Young JF. Sampling methods for pharmacokinetic studies in the mouse. J Pharmacol Methods 1981; 5: 99–120. [DOI] [PubMed] [Google Scholar]
- 2.Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes. Annex III Section B.1, https://eur-lex.europa.eu/eli/dir/2010/63/oj. (2010, accessed 15 October 2023).
- 3.Kalliokoski O, Jacobsen KR, Darusman HS, et al. Mice do not habituate to metabolism cage housing–a three week study of male BALB/c mice. Reddy H, editor. PLoS One 2013; 8: e58460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muta O, Odaka M, Fujii Y, et al. Difference in endocrine and behavior between short-term single- and paired-housing mice in metabolic cage. Neurosci Lett 2023; 806: 137246. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman JF, Fan AX, Neuendorf EH, et al. Hydrophobic sand versus metabolic cages: A comparison of urine collection methods for rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 2018; 57: 51–57. [PMC free article] [PubMed] [Google Scholar]
- 6.Douté M, Sannier A, Even G, et al. Thrombopoietin-dependent myelo-megakaryopoiesis fuels thromboinflammation and worsens antibody-mediated chronic renal microvascular injury. J Am Soc Nephrol 2023; 34: 1207–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Loo PLP, Kuin N, Sommer R, et al. Impact of ‘living apart together’ on postoperative recovery of mice compared with social and individual housing. Lab Anim 2007; 41: 441–455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-zip-1-lan-10.1177_00236772231219828 for Refining urine collection in mice: Development of an innovative urine collection device by Mélodie Douté, Céline Monzali, Antonino Nicoletti, Giuseppina Caligiuri and Marc Clement in Laboratory Animals
Supplemental material, sj-pdf-2-lan-10.1177_00236772231219828 for Refining urine collection in mice: Development of an innovative urine collection device by Mélodie Douté, Céline Monzali, Antonino Nicoletti, Giuseppina Caligiuri and Marc Clement in Laboratory Animals
Supplemental material, sj-zip-3-lan-10.1177_00236772231219828 for Refining urine collection in mice: Development of an innovative urine collection device by Mélodie Douté, Céline Monzali, Antonino Nicoletti, Giuseppina Caligiuri and Marc Clement in Laboratory Animals
Supplemental material, sj-pdf-4-lan-10.1177_00236772231219828 for Refining urine collection in mice: Development of an innovative urine collection device by Mélodie Douté, Céline Monzali, Antonino Nicoletti, Giuseppina Caligiuri and Marc Clement in Laboratory Animals
Supplemental material, sj-zip-5-lan-10.1177_00236772231219828 for Refining urine collection in mice: Development of an innovative urine collection device by Mélodie Douté, Céline Monzali, Antonino Nicoletti, Giuseppina Caligiuri and Marc Clement in Laboratory Animals
Supplemental material, sj-pdf-6-lan-10.1177_00236772231219828 for Refining urine collection in mice: Development of an innovative urine collection device by Mélodie Douté, Céline Monzali, Antonino Nicoletti, Giuseppina Caligiuri and Marc Clement in Laboratory Animals
Supplemental material, sj-zip-7-lan-10.1177_00236772231219828 for Refining urine collection in mice: Development of an innovative urine collection device by Mélodie Douté, Céline Monzali, Antonino Nicoletti, Giuseppina Caligiuri and Marc Clement in Laboratory Animals
Supplemental material, sj-zip-8-lan-10.1177_00236772231219828 for Refining urine collection in mice: Development of an innovative urine collection device by Mélodie Douté, Céline Monzali, Antonino Nicoletti, Giuseppina Caligiuri and Marc Clement in Laboratory Animals
Supplemental material, sj-zip-9-lan-10.1177_00236772231219828 for Refining urine collection in mice: Development of an innovative urine collection device by Mélodie Douté, Céline Monzali, Antonino Nicoletti, Giuseppina Caligiuri and Marc Clement in Laboratory Animals