Abstract
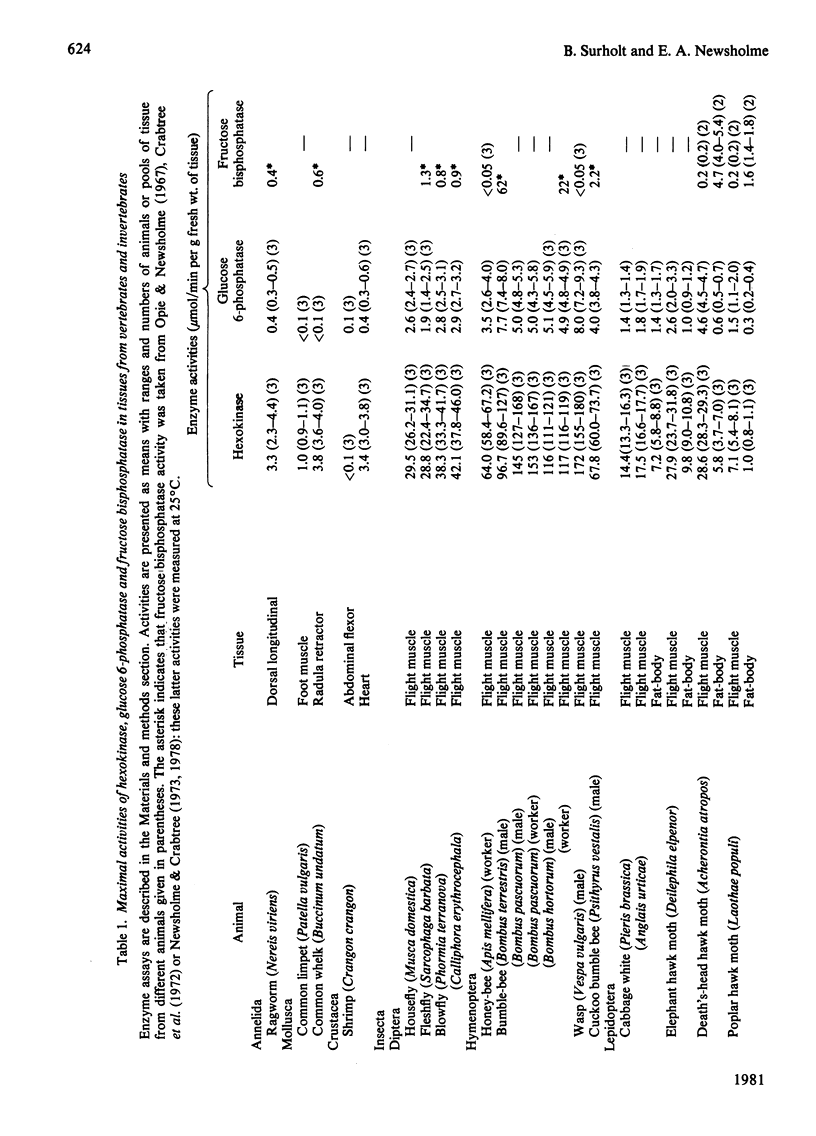
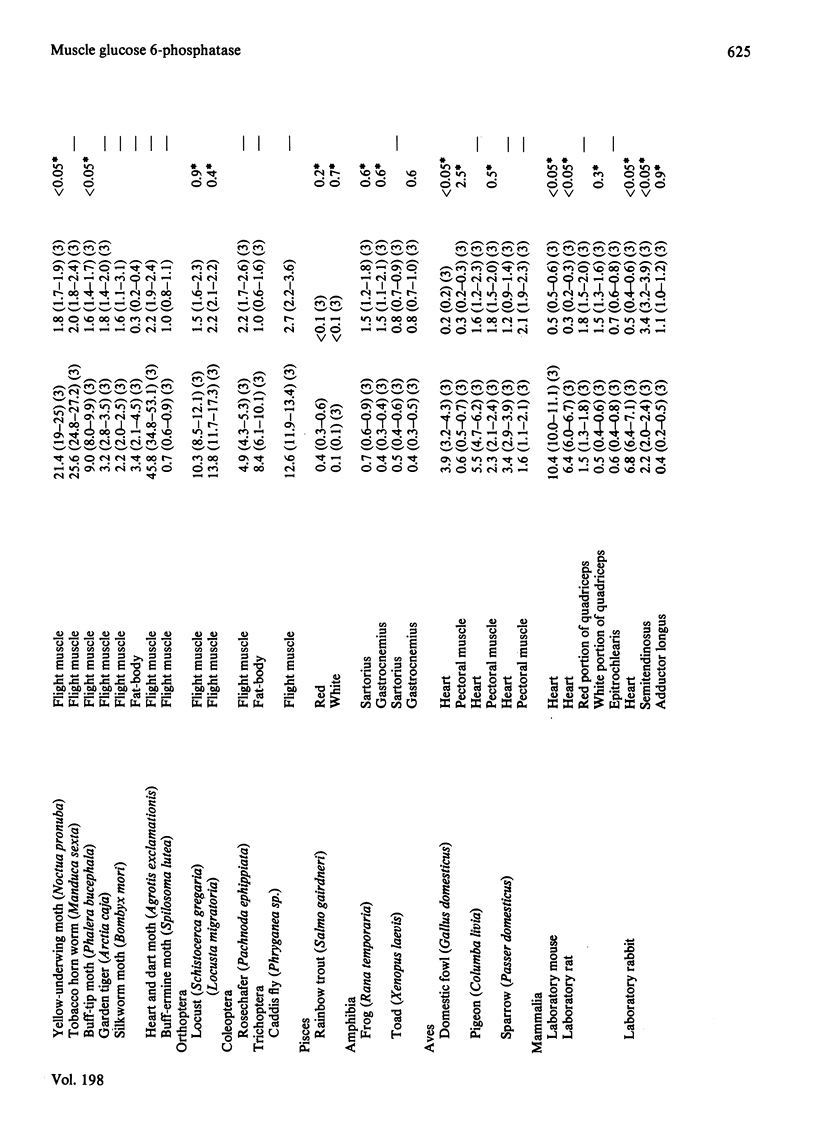
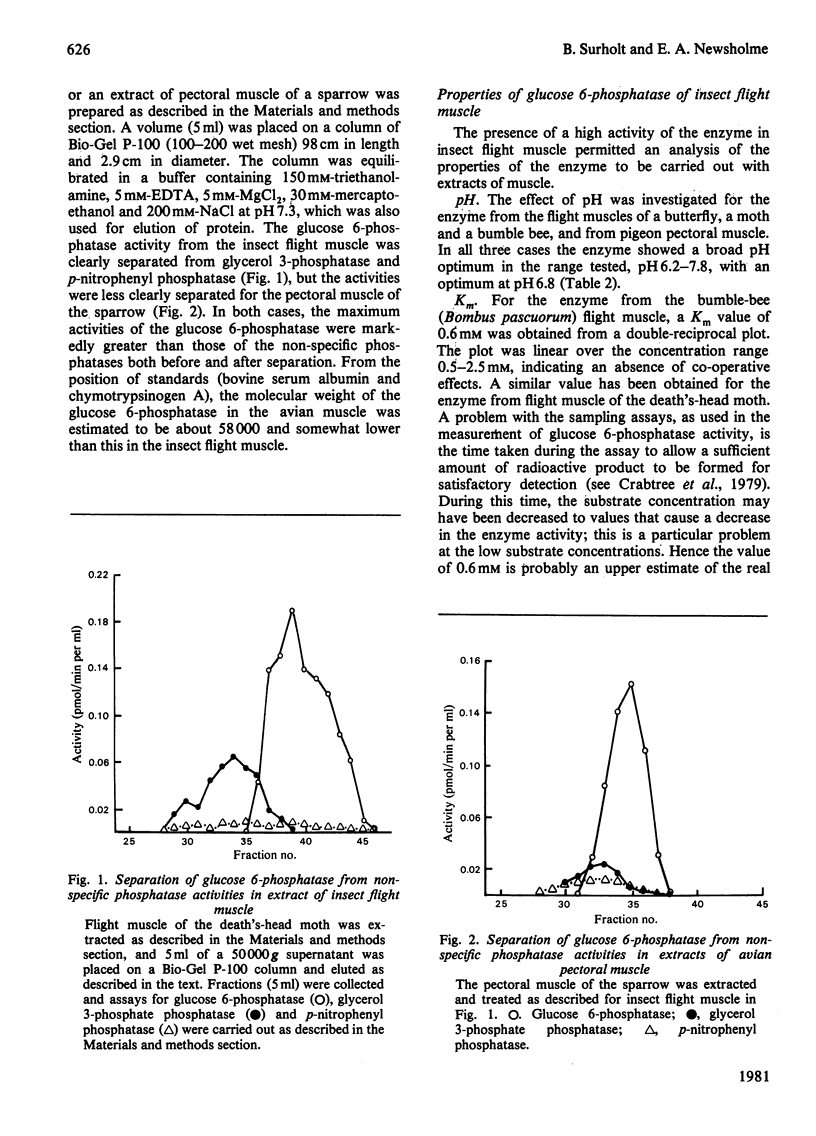
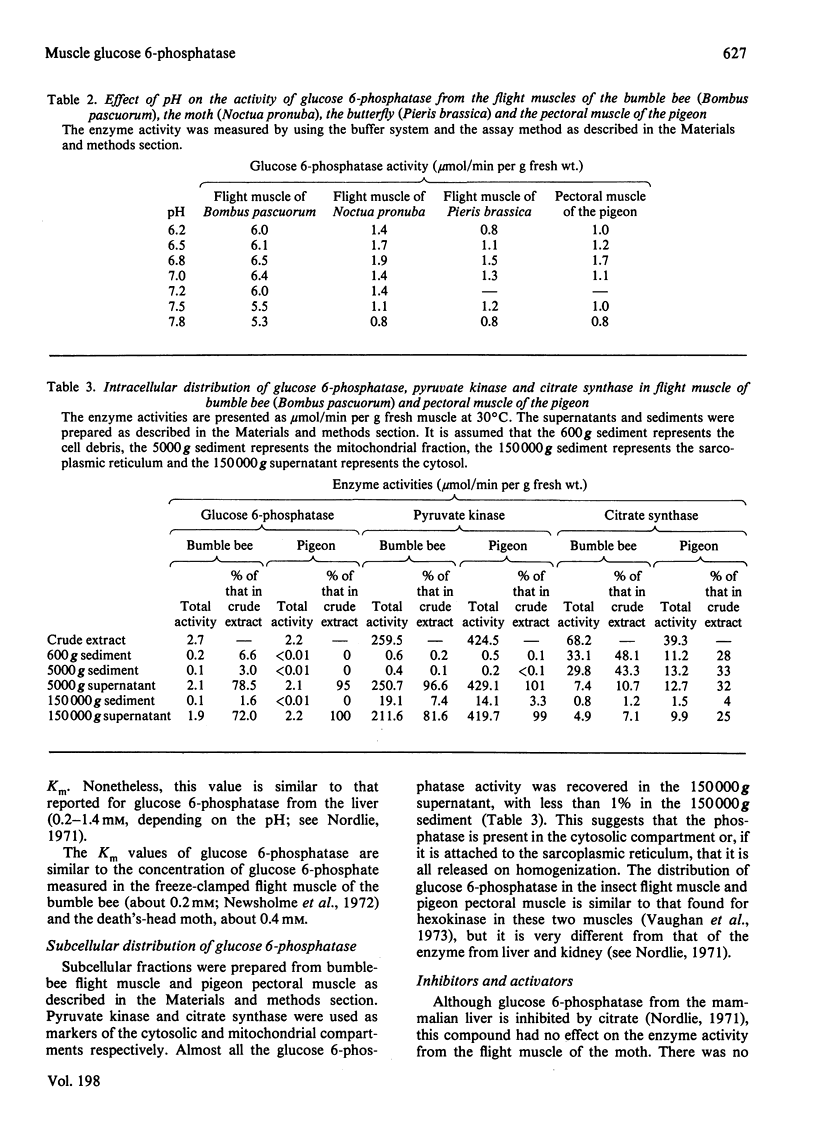
1. The maximum catalytic activities of glucose 6-phosphatase were measured in a large number of muscles from vertebrates and invertebrates. The activities range from less than 0.1 to 8.0 mumol/min per g fresh wt. at 30 degrees C: the highest activity, observed in the flight muscle of the wasp (Vespa vulgaris), is similar to that in rat liver. The hydrolytic activity was shown to be specific towards glucose 6-phosphate. 2. The pH optimum was 6.8 and the Km was approx. 0.6 mM (flight muscle of a moth). 3. Almost all of the glucose 6-phosphatase activity from extracts of the flight muscle of a moth and the pectoral muscle of a pigeon were recovered in the cytosolic fraction (i.e. 150,000 g supernatant). 4. During development of the locust (Schistocerca gregaria), the activity of the phosphatase in the flight muscle increased during the first 3 days after the final moult. 5. The activity of glucose 6-phosphatase from insect and avian muscle was separated from that of non-specific phosphatase on a Bio-Gel P-100 column. 6. For the activities from 63 muscles, there was a strong positive correlation between those of glucose 6-phosphatase and hexokinase, but no correlation between the activities of glucose 6-phosphatase and fructose bisphosphatase. It is suggested that the role of glucose 6-phosphate in muscle is either to produce glucose from glucose 6-phosphate derived from glycogen or to provide the enzymic basis for a substrate ("futile") cycle between glucose and glucose 6-phosphatase in muscle to improve the sensitivity of the mechanism that regulates the rate of glucose phosphorylation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alp P. R., Newsholme E. A., Zammit V. A. Activities of citrate synthase and NAD+-linked and NADP+-linked isocitrate dehydrogenase in muscle from vertebrates and invertebrates. Biochem J. 1976 Mar 15;154(3):689–700. doi: 10.1042/bj1540689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anchors J. M., Karnovsky M. L. Purification of cerebral glucose-6-phosphatase. An enzyme involved in sleep. J Biol Chem. 1975 Aug 25;250(16):6408–6416. [PubMed] [Google Scholar]
- Beis I., Newsholme E. A. The contents of adenine nucleotides, phosphagens and some glycolytic intermediates in resting muscles from vertebrates and invertebrates. Biochem J. 1975 Oct;152(1):23–32. doi: 10.1042/bj1520023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree B., Higgins S. J., Newsholme E. A. The activities of pyruvate carboxylase, phosphoenolpyruvate carboxylase and fructose diphosphatase in muscles from vertebrates and invertebrates. Biochem J. 1972 Nov;130(2):391–396. doi: 10.1042/bj1300391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARLAND P. B., RANDLE P. J. A rapid enzymatic assay for glycerol. Nature. 1962 Dec 8;196:987–988. doi: 10.1038/196987a0. [DOI] [PubMed] [Google Scholar]
- Hue L., Hers H. G. Utile and futile cycles in the liver. Biochem Biophys Res Commun. 1974 Jun 4;58(3):540–548. doi: 10.1016/s0006-291x(74)80454-7. [DOI] [PubMed] [Google Scholar]
- Kitcher S. A., Siddle K., Luzio J. P. A method for the determination of glucose-6-phosphatase activity in rat liver with [U-14C]glucose 6-phosphate as substrate. Anal Biochem. 1978 Jul 15;88(1):29–36. doi: 10.1016/0003-2697(78)90395-0. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B., Higgins S. J., Thornton S. D., Start C. The activities of fructose diphosphatase in flight muscles from the bumble-bee and the role of this enzyme in heat generation. Biochem J. 1972 Jun;128(1):89–97. doi: 10.1042/bj1280089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B. Metabolic aspects of enzyme activity regulation. Symp Soc Exp Biol. 1973;27:429–460. [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B. Substrate cycles in metabolic regulation and in heat generation. Biochem Soc Symp. 1976;(41):61–109. [PubMed] [Google Scholar]
- Newsholme E. A., Taylor K. Glycerol kinase activities in muscles from vertebrates and invertebrates. Biochem J. 1969 May;112(4):465–474. doi: 10.1042/bj1120465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie L. H., Newsholme E. A. The activities of fructose 1,6-diphosphatase, phosphofructokinase and phosphoenolpyruvate carboxykinase in white muscle and red muscle. Biochem J. 1967 May;103(2):391–399. doi: 10.1042/bj1030391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Vaughan H., Thornton S. D., Newsholme E. A. The effects of calcium ions on the activities of trehalase, hexokinase, phosphofructokinase, fructose diphosphatase and pyruvate kinase from various muscles. Biochem J. 1973 Mar;132(3):527–535. doi: 10.1042/bj1320527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A., Beis I., Newsholme E. A. Maximum activities and effects of fructose bisphosphate on pyruvate kinase from muscles of vertebrates and invertebrates in relation to the control of glycolysis. Biochem J. 1978 Sep 15;174(3):989–998. doi: 10.1042/bj1740989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A., Newsholme E. A. The maximum activities of hexokinase, phosphorylase, phosphofructokinase, glycerol phosphate dehydrogenases, lactate dehydrogenase, octopine dehydrogenase, phosphoenolpyruvate carboxykinase, nucleoside diphosphatekinase, glutamate-oxaloacetate transaminase and arginine kinase in relation to carbohydrate utilization in muscles from marine invertebrates. Biochem J. 1976 Dec 15;160(3):447–462. doi: 10.1042/bj1600447. [DOI] [PMC free article] [PubMed] [Google Scholar]


