Abstract
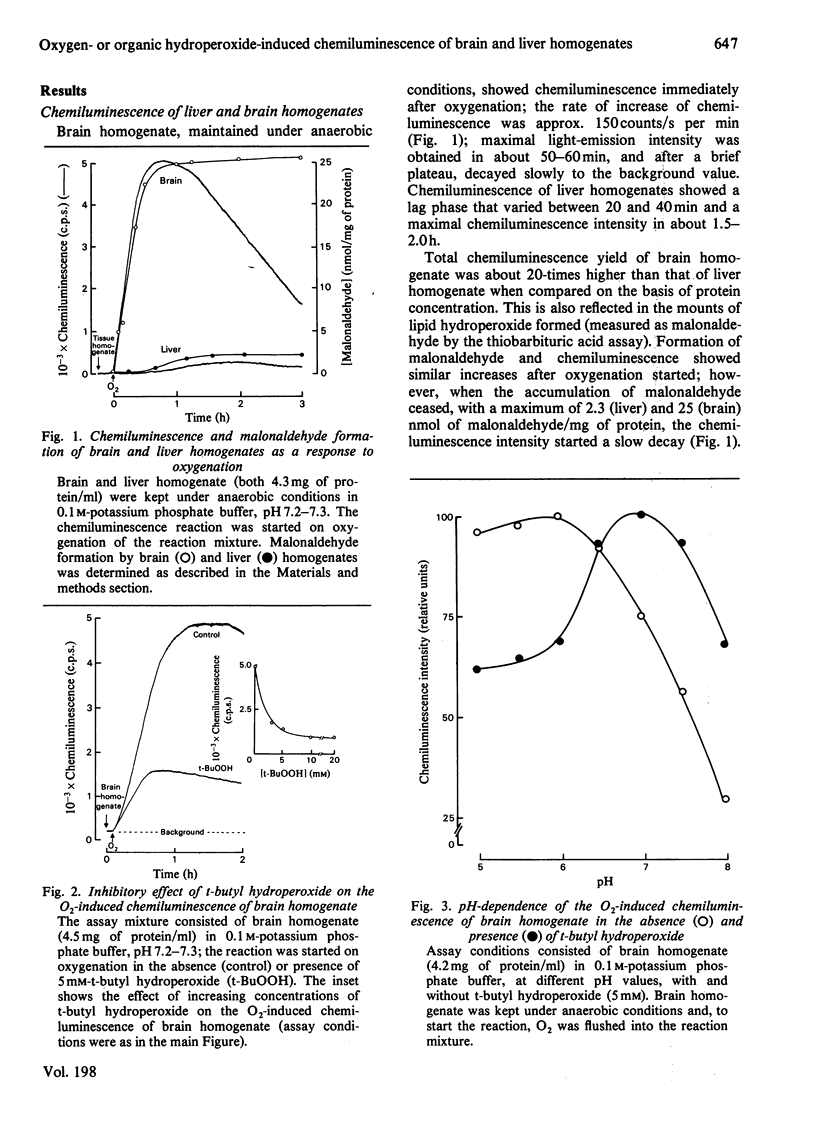
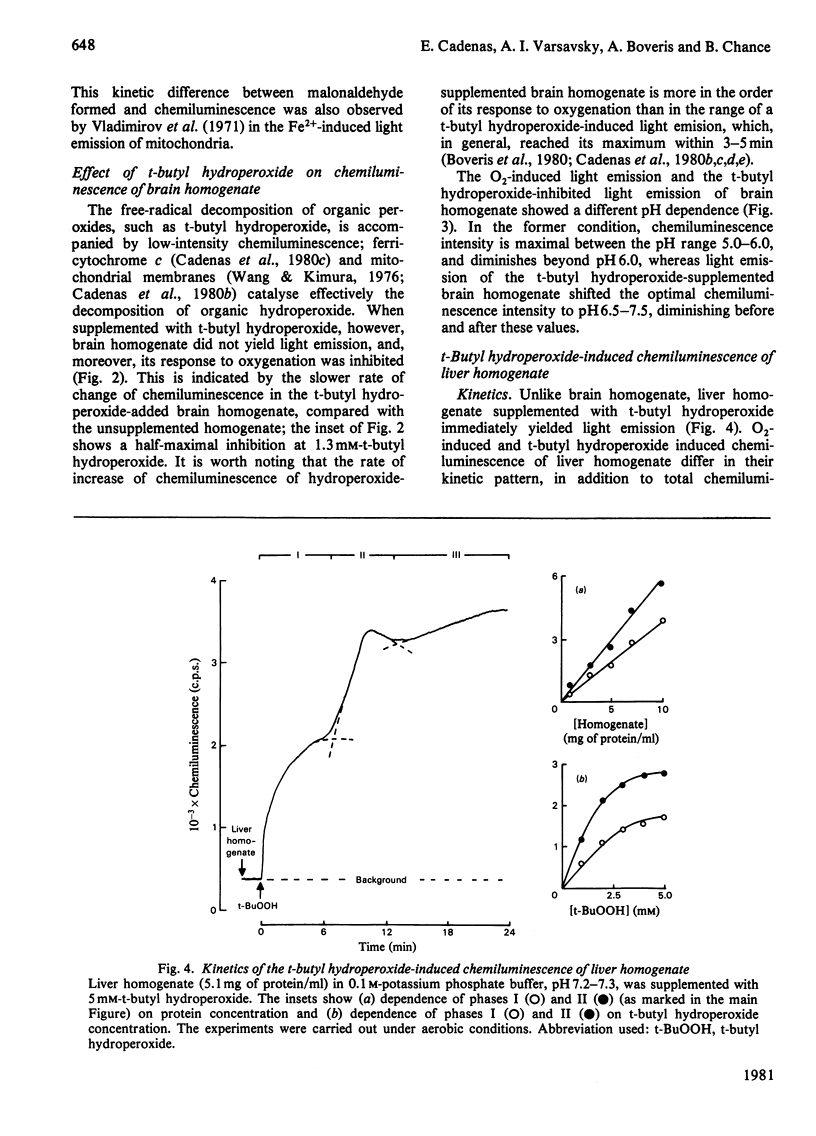
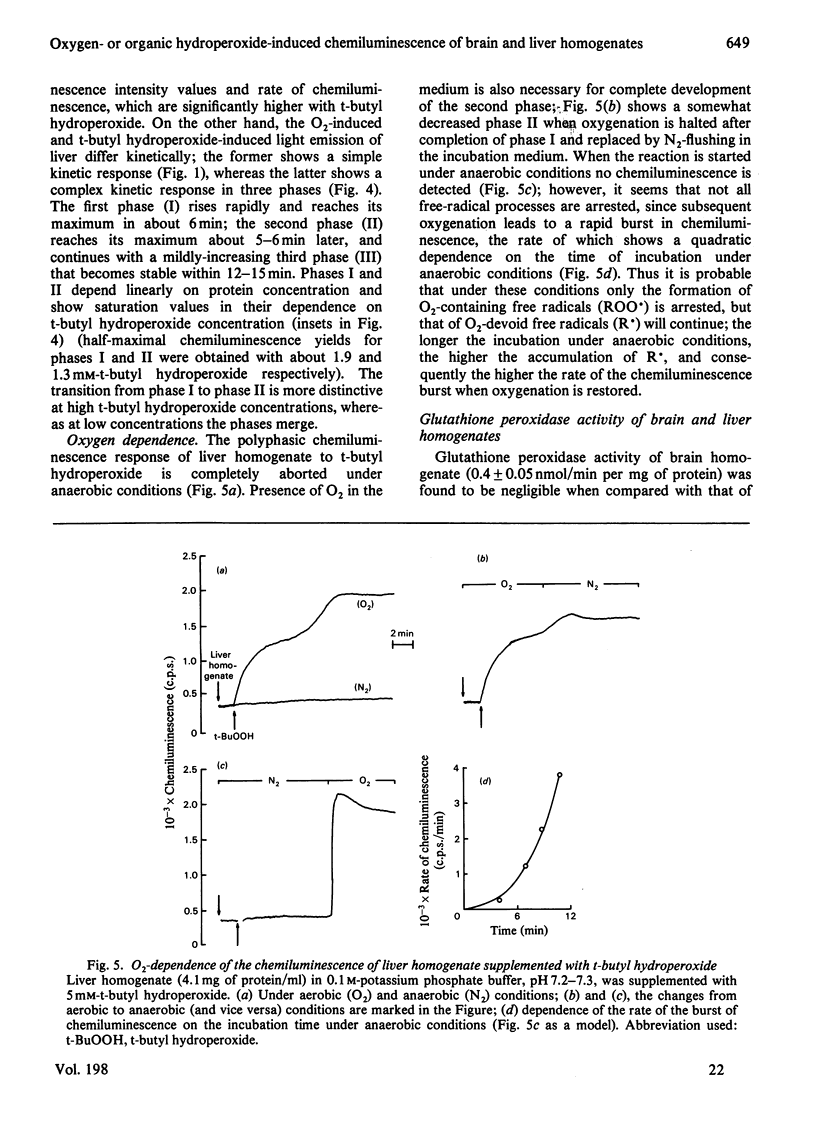
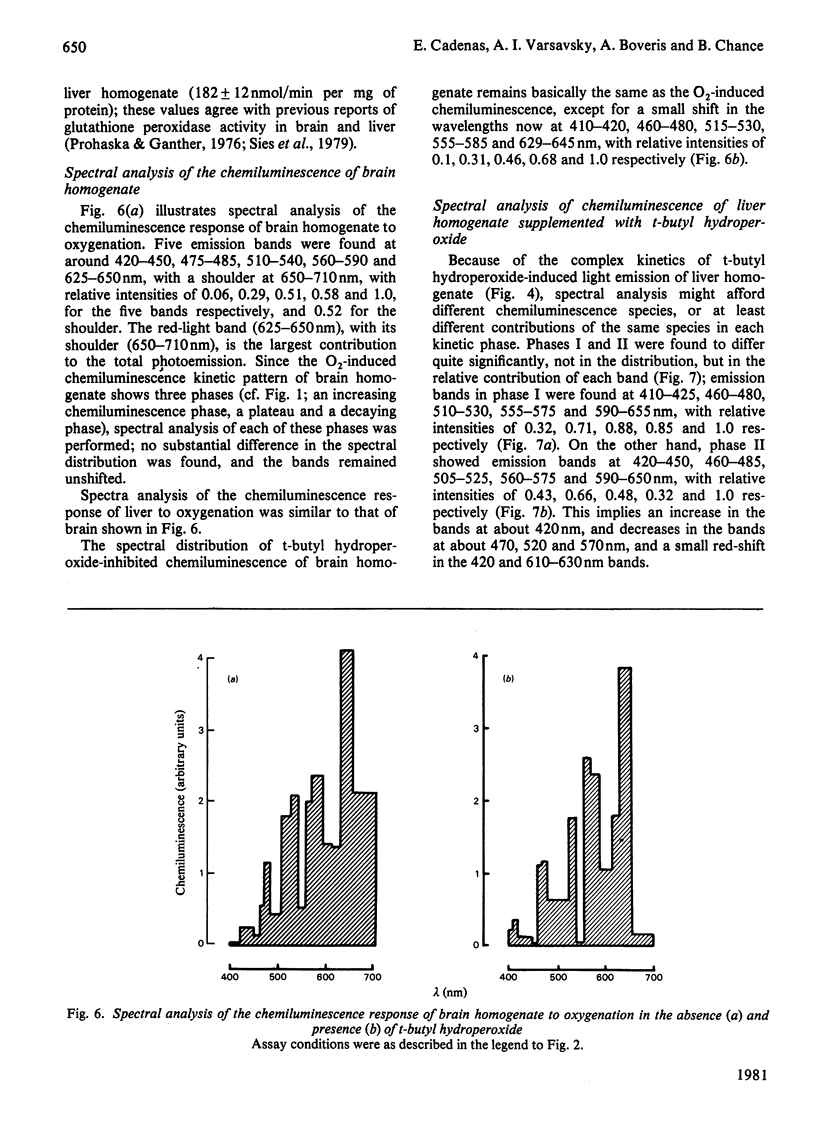
Oxygenation of anaerobically isolated brain and liver homogenates is associated with chemiluminescence and formation of lipid hydroperoxides, the latter determined by the thiobarbituric acid assay. Light emission and formation of malonaldehyde are 20-fold higher in the brain than in liver; chemiluminescence of both decays when accumulation of malonaldehyde ceases. Exogenous organic peroxides, such as t-butyl hydroperoxide, inhibit the light-emission response to oxygenation by brain homogenate, whereas they enhance that of liver homogenate. t-Butyl hydroperoxide-induced photoemission of liver homogenate shows a polyphasic kinetic pattern that is O2-dependent. The spectral analysis of chemiluminescence arising from brain and liver homogenates on oxygenation shows a spectrum with five emission bands at 420-450, 475-485, 510-540, 560-580 and 625-640 nm. These bands are subjected to intensity changes or shifts of the wavelength whenever t-butyl hydroperoxide is present, either inhibiting or stimulating light emission. The blue-band chemiluminescence, around 435 nm, is possibly due to the weak light emission arising from excited carbonyl compounds [Lloyd (1965) J. Chem. Soc. Faraday Trans. 61, 2182-2193; Vassil'ev (1965) Opt. Spectrosc. (USSR) 18, 131-135], whereas the presence of other bands suggests generation of singlet molecular oxygen either in the process triggered on oxygenation (lipid oxygenation) or after supplementation with organic hydroperoxides. We offer several explanations for the spectral analysis presented here.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen B. R., Lint T. F., Brendzel A. M. Chemically shifted singlet oxygen spectrum. Biochim Biophys Acta. 1978 Sep 6;542(3):527–536. doi: 10.1016/0304-4165(78)90382-3. [DOI] [PubMed] [Google Scholar]
- Boveris A., Cadenas E., Reiter R., Filipkowski M., Nakase Y., Chance B. Organ chemiluminescence: noninvasive assay for oxidative radical reactions. Proc Natl Acad Sci U S A. 1980 Jan;77(1):347–351. doi: 10.1073/pnas.77.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas E., Arad I. D., Boveris A., Fisher A. B., Chance B. Partial spectral analysis of the hydroperoxide-induced chemiluminescence of the perfused lung. FEBS Lett. 1980 Mar 10;111(2):413–418. doi: 10.1016/0014-5793(80)80839-8. [DOI] [PubMed] [Google Scholar]
- Cadenas E., Boveris A., Chance B. Low-level chemiluminescence of bovine heart submitochondrial particles. Biochem J. 1980 Mar 15;186(3):659–667. doi: 10.1042/bj1860659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas E., Boveris A., Chance B. Low-level chemiluminescence of hydroperoxide-supplemented cytochrome c. Biochem J. 1980 Apr 1;187(1):131–140. doi: 10.1042/bj1870131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Di Luzio N. R., Stege T. E. Enhanced hepatic chemiluminescence following carbon tetrachloride or hydrazine administration. Life Sci. 1977 Nov 15;21(10):1457–1464. doi: 10.1016/0024-3205(77)90200-4. [DOI] [PubMed] [Google Scholar]
- Evans C. D., List G. R., Dolev A., McConnell D. G., Hoffmann R. L. Pentane from thermal decomposition of lipoxidase-derived products. Lipids. 1967 Sep;2(5):432–434. doi: 10.1007/BF02531860. [DOI] [PubMed] [Google Scholar]
- Günzler W. A., Kremers H., Flohé L. An improved coupled test procedure for glutathione peroxidase (EC 1-11-1-9-) in blood. Z Klin Chem Klin Biochem. 1974 Oct;12(10):444–448. doi: 10.1515/cclm.1974.12.10.444. [DOI] [PubMed] [Google Scholar]
- Hastings J. W., Wilson T. Bioluminescence and chemiluminescence. Photochem Photobiol. 1976 Jun;23(6):461–473. doi: 10.1111/j.1751-1097.1976.tb07282.x. [DOI] [PubMed] [Google Scholar]
- Hochstein P., Nordenbrand K., Ernster L. Evidence for the involvement of iron in the ADP-activated peroxidation of lipids in microsomes and mitochondria. Biochem Biophys Res Commun. 1964;14:323–328. doi: 10.1016/s0006-291x(64)80004-8. [DOI] [PubMed] [Google Scholar]
- Nakano M., Noguchi T., Sugioka K., Fukuyama H., Sato M. Spectroscopic evidence for the generation of singlet oxygen in the reduced nicotinamide adenine dinucleotide phosphate-dependent microsomal lipid peroxidation system. J Biol Chem. 1975 Mar 25;250(6):2404–2406. [PubMed] [Google Scholar]
- Olson J. A. Some aspects of vitamin A metabolism. Vitam Horm. 1968;26:1–63. doi: 10.1016/s0083-6729(08)60751-7. [DOI] [PubMed] [Google Scholar]
- Pinto R. E., Bartley W. The effect of age and sex on glutathione reductase and glutathione peroxidase activities and on aerobic glutathione oxidation in rat liver homogenates. Biochem J. 1969 Mar;112(1):109–115. doi: 10.1042/bj1120109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohaska J. R., Ganther H. E. Selenium and glutathione peroxidase in developing rat brain. J Neurochem. 1976 Dec;27(6):1379–1387. doi: 10.1111/j.1471-4159.1976.tb02619.x. [DOI] [PubMed] [Google Scholar]
- Pryor W. A. The formation of free radicals and the consequences of their reactions in vivo. Photochem Photobiol. 1978 Oct-Nov;28(4-5):787–801. doi: 10.1111/j.1751-1097.1978.tb07020.x. [DOI] [PubMed] [Google Scholar]
- SELIGER H. H. A photoelectric method for the measurement of spectra of light sources of rapidly varying intensities. Anal Biochem. 1960 Jun;1:60–65. doi: 10.1016/0003-2697(60)90019-1. [DOI] [PubMed] [Google Scholar]
- Sies H., Koch O. R., Martino E., Boveris A. Increased biliary glutathione disulfide release in chronically ethanol-treated rats. FEBS Lett. 1979 Jul 15;103(2):287–290. doi: 10.1016/0014-5793(79)81346-0. [DOI] [PubMed] [Google Scholar]
- Sugioka K., Nakano M. A possible mechanism of the generation of singlet molecular oxygen in nadph-dependent microsomal lipid peroxidation. Biochim Biophys Acta. 1976 Feb 16;423(2):203–216. doi: 10.1016/0005-2728(76)90179-1. [DOI] [PubMed] [Google Scholar]
- Vidigal-Martinelli C., Zinner K., Kachar B., Duŕan N., Cilento G. Emission from singlet oxygen during the peroxidase-catalyzed oxidation of malonaldehyde. FEBS Lett. 1979 Dec 1;108(1):266–268. doi: 10.1016/0014-5793(79)81225-9. [DOI] [PubMed] [Google Scholar]
- Wang H. P., Kimura T. Ferrous ion-mediated cytochrome P-450 degradation and lipid peroxidation in adrenal cortex mitochondria. Biochim Biophys Acta. 1976 Mar 12;423(3):374–381. doi: 10.1016/0005-2728(76)90194-8. [DOI] [PubMed] [Google Scholar]


