ABSTRACT
Respiratory syncytial virus (RSV) is a common cause of acute respiratory illness in individuals of all ages, with adults aged ≥60 years and adults with certain chronic conditions at increased risk of severe RSV-related outcomes. This study evaluates the cost-effectiveness of the adjuvanted RSVPreF3 vaccine versus no vaccine in adults aged ≥60 years in the United States (US). A multi-cohort Markov model was developed with a 5-year time horizon and 1-month cycle length to compare outcomes for no vaccination and one-time adjuvanted RSVPreF3 vaccination (assuming the same vaccination as for influenza vaccines). Clinical parameters (e.g., vaccine efficacy) were based on phase 3 clinical trial data over 3 seasons, with all other inputs obtained from public US sources and scientific literature. Outcomes included total and incremental quality-adjusted life year (QALY) losses and costs, as well as incremental cost-effectiveness ratios (ICERs). Sensitivity analyses were conducted to evaluate the sensitivity of results to inputs. In the base case, the model estimated that vaccinating 52.7 million adults aged ≥60 years with the adjuvanted RSVPreF3 vaccine once would result in 244,424 fewer QALY losses and an incremental societal cost of $4.5 billion over 5 years, with vaccination costs partially offset by reduced disease-related costs. From the societal perspective, adjuvanted RSVPreF3 vaccination resulted in an ICER of $18,430 per QALY gained. Results were relatively robust across sensitivity analyses and indicate that adjuvanted RSVPreF3 vaccination is a cost-effective option for the prevention of RSV in US adults aged ≥ 60 years, reducing the substantial burden within this population.
KEYWORDS: Respiratory syncytial virus, adjuvanted RSVPreF3 vaccine, cost-effectiveness, older adults, vaccination, United States
Introduction
Respiratory syncytial virus (RSV) is a common cause of acute respiratory illness (ARI) in individuals of all ages in the United States (US).1,2 In adults, RSV-ARI cases are often classified as upper respiratory tract disease (URTD), with mild to moderate symptoms (e.g., nasal congestion, sore throat).2 RSV-ARI cases that are classified as lower respiratory tract disease (LRTD) have airway involvement and, as a result, are accompanied by more severe symptoms (e.g., cough, dyspnea, wheezing). Adults aged ≥60 years and adults with certain chronic conditions are at increased risk of severe RSV-related outcomes (e.g., hospitalization, pneumonia complications, and mortality).3–7 Older adults experience age-related decline in their immune system function, which makes them more susceptible to viral infections such as RSV.8 Additionally, many risk factors for severe RSV-related outcomes (e.g., chronic obstructive pulmonary disease [COPD], congestive heart failure [CHF], coronary artery disease [CAD], diabetes mellitus) are more prevalent in adults aged ≥60 years.9–12 A study conducted in New York between 2017–2020 reported that RSV-related hospitalization rates were multiple fold higher among adults aged ≥65 years with COPD, CHF, CAD, and diabetes versus individuals without each of these respective conditions.13
In the US, a previous landmark study conducted during four winter seasons (1999–2003) in New York found that each year 3–7% of healthy adults aged ≥65 years and 4–10% of adults with chronic conditions that place them at increased risk of severe RSV (aged ≥21 years with CHF or a chronic pulmonary condition) experienced an RSV infection.14 RSV-related mortality is also substantial in adults aged ≥60 years, especially those with comorbidities.6,7 In comparison to influenza, recent studies have found that RSV may be associated with greater morbidity and mortality among hospitalized adults aged ≥60 years.6,15
Even so, the role of RSV as an important respiratory illness in adults aged ≥60 years remains relatively underrecognized, in part due to testing limitations.3 Available rapid antigen tests and immunofluorescence assays have lower sensitivity in adults,16 and while molecular reverse transcriptase polymerase chain reaction (RT-PCR) tests are considered more appropriate for adults, such routine molecular testing is often not performed and still have limitations related to case ascertainment.16–18 As such, epidemiologic estimates for incidence, morbidity, and mortality are likely to underestimate the true burden of RSV.16,19
RSV vaccination offers considerable benefits in reducing RSV disease in adults aged ≥60 years. In May 2023, the US Food and Drug Administration (FDA) approved two RSV vaccines (AREXVY, GSK; ABRYSVO, Pfizer) for the prevention of LRTD caused by RSV in individuals aged ≥60 years.20,21 Both vaccines target the RSV viral F glycoprotein in its functional prefusion conformation, which facilitates RSV cell entry within respiratory epithelium.20,21 In June 2023, the Centers for Disease Control and Prevention’s (CDC’s) Advisory Committee on Immunization Practices (ACIP), recommended that adults aged ≥60 years may receive one dose of an RSV vaccine, using shared clinical decision-making.22 Such recommendations were underpinned by key clinical data demonstrating high vaccine efficacy against RSV-LRTD.23,24 For the adjuvanted RSVPreF3 vaccine, the pivotal Adult Respiratory Syncytial Virus (AReSVi-006) phase 3 placebo-controlled clinical study evaluated the efficacy of one dose given to over 12,000 adults aged ≥60 years. Vaccine efficacy during the first season (median 6.7-month follow-up period) was 82.6% against RSV-related LRTD and 71.7% against RSV-ARI.23 Subsequently reported follow-up data showed cumulative vaccine efficacy over two full seasons (median 17.8-month follow-up period) of 67.2% against RSV-LRTD and 52.7% against RSV-ARI,25 and cumulative vaccine efficacy over three seasons (median 30.6-month follow-up period) of 62.9% against RSV-LRTD and 51.1% against RSV-ARI.26 In May 2024, a third RSV vaccine for use in adults aged ≥60 years was approved by the US FDA (mRESVIA, Moderna).27 In June 2024, the CDC’s ACIP voted to revise their RSV vaccination recommendation to specify that all adults aged ≥75 years and adults aged 60–74 years who are at increased risk for severe RSV disease should receive a single dose of RSV vaccine.28
As with any new vaccine introduction, estimating the clinical and economic impact of RSV vaccination of adults aged ≥60 years can inform decision makers as to the potential benefits of implementing such vaccination within immunization programs. The public health impact of adjuvanted RSVPreF3 vaccination in US adults aged ≥60 years over a 3-year time horizon has previously been reported based on clinical trial data through two seasons (median 17.8-month follow-up period).29 In this previous analysis, we developed a multi-cohort Markov model and used US epidemiologic inputs and data from the AReSVi-006 study to estimate the substantial numbers of RSV cases, hospitalizations, and RSV-related deaths that could potentially be avoided with adjuvanted RSVPreF3 vaccination over 3 years. The objective of the present study was to evaluate the cost-effectiveness of the adjuvanted RSVPreF3 vaccine in US adults aged ≥60 years compared with no vaccination over 5 years, utilizing an updated version of the Markov model with clinical trial data through three seasons (median 30.6-month follow-up period).
Materials and methods
Model overview
A static, multi-cohort Markov model was developed to capture RSV-related disease states as previously reported (Figure 1).29 We chose a static Markov modeling approach with a 1-month cycle length to capture key clinical outcomes and disease transition events associated with RSV infection, accounting for seasonality in RSV disease epidemiology, waning of vaccine efficacy over time, and the ability of individuals to be reinfected with RSV disease over the modeled time horizon. The model structure, data inputs, and underlying assumptions were informed by a targeted literature review and validated throughout model development by a number of RSV expert clinicians, epidemiologists, and health economists. The model was created in Microsoft Excel (Microsoft Corporation; Redmond, Washington) and compares RSV-related clinical, healthcare resource use, quality-adjusted life year (QALY) loss, and cost outcomes that are estimated for simulations with and without adjuvanted RSVPreF3 vaccination among US adults aged ≥60 years. The model has been updated to include the most recent data available on vaccine efficacy and waning. Other model inputs have also been updated to reflect the latest data available (where possible). The revised model includes a time horizon of 5 years in the base-case analysis. This time horizon was selected to capture the expected residual protection of the adjuvanted RSVPreF3 vaccine beyond the median 30.6-month follow-up period that is now available from seasons 1–3 of the AReSVi-006 study.25,26
Figure 1.
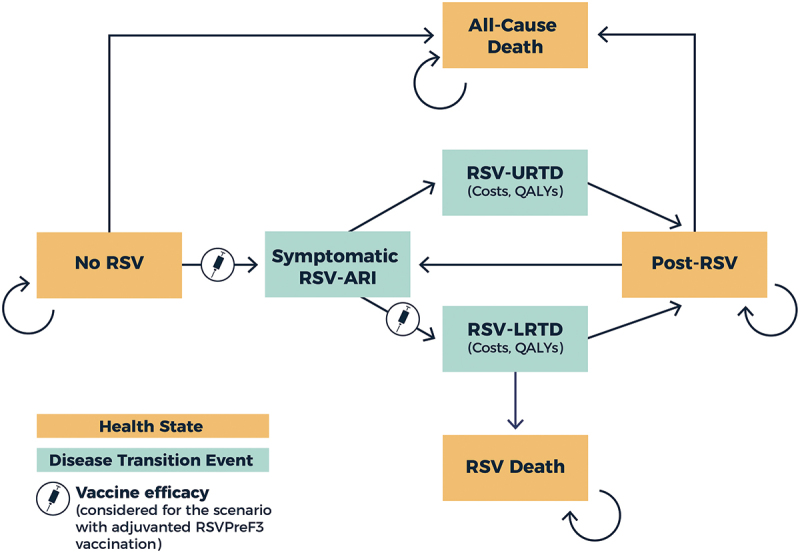
Markov model structure.
ARI, acute respiratory illness; LRTD, lower respiratory tract disease; QALY, quality-adjusted life year; RSV, respiratory syncytial virus; URTD, upper respiratory tract disease.
Individuals begin in the “No RSV” health state and transition between different health states and disease transition events over the 5-year time horizon. Symptomatic RSV-ARI cases are classified as either URTD or LRTD, using LRTD case definitions from the adjuvanted RSVPreF3 vaccine’s phase 3 clinical trial.23,25 Specifically, RSV-LRTD cases were required to have ≥2 lower respiratory symptoms or signs (including ≥1 sign) or ≥3 lower respiratory symptoms for ≥24 hours.23 RSV-URTD and LRTD cases then transition to the “Post-RSV” recovery health state (although some LRTD-associated deaths can occur). RSV reinfection is common throughout the lifespan, in part due to short-term immune responses. The model allows for reinfection, following the same underlying model structure as for the initial RSV infection.
Symptomatic RSV-ARI cases (URTD and LRTD) each experience QALY losses and incur direct costs associated with healthcare resource use and medications, and indirect costs due to productivity losses. RSV-LRTD cases are more likely to have more severe outcomes and incur greater costs and QALY losses (see further details below). For the vaccination strategy, individuals are able to receive the adjuvanted RSVPreF3 vaccine as a single dose, which provides efficacy against RSV-ARI and RSV-LRTD. Vaccinated individuals incur direct and indirect costs of vaccination, including vaccine acquisition and administration costs. To better capture the full costs and potential disutility of vaccination, the model assumes that some vaccinated individuals also experience Grade 3 local or systemic adverse events (AEs), incurring associated direct and indirect costs as well as QALY losses. The model accounts for all-cause mortality and also RSV-related deaths (assumed to occur only among hospitalized RSV-LRTD cases). The model only includes mortality within 30 days of RSV-LRTD hospitalization and does not account for longer-term mortality due to RSV. All costs were adjusted for inflation as necessary and presented in 2024 US dollars (USD).30,31 All costs and QALYs were discounted at an annual rate of 3%.32 Model reporting was conducted in accordance with Consolidated Health Economic Evaluation Reporting Standards (CHEERS).33 This study did not involve human participants and as a result did not require ethical approval for development of the model.
Model inputs
Key population, epidemiology, vaccine, cost, and utility inputs used in the model are presented in Table 1, with additional details provided in the Supplemental Material. All inputs were based on a targeted review of the literature, using public US sources and scientific literature, as well as clinical trial data for the adjuvanted RSVPreF3 vaccine through three RSV seasons (median 30.6-month follow-up period).
Table 1.
Key model inputs used in base-case analysis.
| Category/input | Base-case value | Source |
|---|---|---|
| Population and epidemiologic inputs | ||
| Population size | 82,737,846 | 34 |
| Annual incidence of symptomatic RSV-ARI per person year at risk | 0.0465 | 14,36 |
| Percentage of symptomatic RSV-ARI cases that are RSV-LRTD | 50.2% | AReSVi-006 phase 3 clinical trial placebo arm.26 |
| Probability of death given RSV-LRTD | 60–64y: 0.002050 65–74y: 0.008208 ≥75y: 0.010839 |
5,7,57 |
| Vaccination coverage and efficacy inputs | ||
| Vaccination coverage | 60–64y: 46.2% ≥65y: 69.7% |
40 |
| VE against RSV-ARI at time t | Time-varying values ranging from 75.5% in month 2 to 19.6% in month 60 | AReSVi-006 phase 3 clinical trial.26 |
| VE against RSV-LRTD at time t | Time-varying values ranging from 86.8% in month 2 to 28.2% in month 60 | |
| Cost inputs | ||
| Vaccine acquisition cost | $294 | 45 |
| Vaccine administration direct cost | $23 | 41,46 |
| Direct cost per symptomatic RSV-ARI case (varies by age) | 4–5,41–45,57 | |
| URTD | $61–$84 | |
| LRTD | $1,133–$1,955 | |
| Indirect cost per symptomatic RSV-ARI case (varies by age). | 4,30,50,51,53 | |
| URTD | $185–$272 | |
| LRTD | $334–$493 | |
| Indirect cost per RSV-related death (varies by age) | $10,700–$744,619 | 30,51 |
| Utility inputs | ||
| QALY loss due to RSV-URTD case | 0.0133 | 55 |
| QALY loss due to RSV-LRTD case | 0.0178 | |
ARI, acute respiratory illness; LRTD, lower respiratory tract disease; QALY, quality-adjusted life year; RSV, respiratory syncytial virus; URTD, upper respiratory tract disease; VE, vaccine efficacy; y, year.
Additional details on model inputs are provided in the Supplemental Material.
The model includes the full population of US adults aged ≥60 years (n = 82,737,846), based on 2024 US Census Bureau population projections.34 To account for differences in outcomes by age, the population is modeled using seven age cohorts: 60–64, 65–69, 70–74, 75–79, 80–84, 85–89, and ≥90 years (Table S1). Monthly probabilities of all-cause mortality by age were derived from 2021 US life tables (the most current data available at the time of model development) and were applied to the full modeled population.35
Many of the RSV epidemiological inputs are as previously reported in a related public health impact analysis.29 Each year, the model includes an incidence of symptomatic RSV-ARI of 0.0465 cases per person-year, based on mean rates reported by Falsey et al. (2005),14 then weighted assuming that 33.7% of individuals have chronic conditions that place them at increased risk of severe RSV and the remaining 66.3% are non-high-risk.36 For sensitivity analyses, the lower and upper bounds (0.0272–0.0627) were based on the minimum and maximum incidence across the four seasons reported by Falsey et al.14 Data from the National Respiratory and Enteric Virus Surveillance System (NREVSS) from 2023–2024 were used to derive seasonality factors to adjust monthly symptomatic RSV-ARI incidence estimates (Table S2).37,38 This approach was used to account for the typical seasonal trends in RSV incidence (i.e., with more RSV-ARI cases observed between the months of October and April). The model also accounted for reinfection. Although limited data are available on risk of RSV reinfection among adults, data for US infants across eight seasons suggest similar risk of reinfection as for an initial episode.39 The present model assumed the same incidence rates for symptomatic RSV-ARI reinfection as for initial infection. The proportion of symptomatic RSV-ARI cases classified as RSV-LRTD was derived from seasons 1–3 data from the placebo arm of the AReSVi-006 phase 3 clinical trial, where 215/428 RSV-ARI cases (50.2%) were classified as RSV-LRTD.26 The remaining 49.8% were considered as RSV-URTD cases. RSV-related mortality rates in RSV-LRTD cases were estimated based on 30-day mortality post-hospitalization from Tseng et al. (2020),7 (i.e., 7.1% for hospitalized cases aged 60–74 years and 9.4% for hospitalized cases aged ≥75 years). These rates were derived based on the relative differences between in-hospital mortality rates by age versus for the overall population aged ≥60 years, as reported by Tseng et al. (2020).7
For the vaccination strategy, the model assumed vaccination at the start of October, using the same coverage rates as those reported for seasonal influenza vaccines during the 2023–2024 season (46.2% for adults aged 60–64 years and 69.7% for adults aged ≥65 years [Table S3]).40 Vaccine efficacy inputs were based on results from the AReSVi-006 phase 3 clinical trial over three seasons (i.e., including a median follow-up time of 30.6 months).26 In this approach, continuous Cox models with time-varying effects were fitted to the trial data to estimate the monthly vaccine efficacy against RSV-ARI and RSV-LRTD during and beyond the clinical trial follow-up period. Specifically, vaccine efficacy by day after vaccination (based on AReSVi-006 data) was analyzed separately for RSV-ARI and RSV-LRTD. Although different types of continuous time-varying functions were considered (e.g., logarithmic continuous, square root, Cox model), Cox models using logarithmic functions of time were selected based on Akaike Information Criteria. In the base-case analysis, vaccine efficacy (VE) at time (t) since vaccination (in days [converted from months by a factor of 365/12], with t = 0 representing 15 days post-vaccination to allow for an immunity buildup period) is estimated using the functions listed below.
RSV-ARI: VE(t) = 1 – e(−2.5614 + 0.3125 * ln(t + 10))
RSV-LRTD: VE(t) = 1 – e(−3.6786 + 0.4465 * ln(t + 10))
Monthly vaccine efficacy inputs were used in the model to match the 1-month cycle length. In month 1, vaccine efficacy was assumed to be 50% of its peak, allowing for an immune buildup period. As a result, the model estimates the highest vaccine efficacy against RSV-ARI and RSV-LRTD during month 2 (75.5% and 86.8%, respectively) (Figure 2). These vaccine efficacy values are projected to wane to 19.6% against RSV-ARI and 28.2% against RSV-LRTD by month 60. 95% confidence intervals (CIs) by month were estimated for use in sensitivity analyses (Figures S1 and S2).
Figure 2.
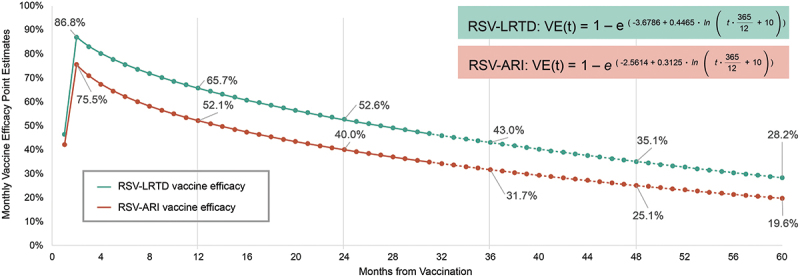
Adjuvanted RSVPreF3 vaccine efficacy against RSV-ARI and RSV-LRTD over modeled 5-year time horizon.
ARI, acute respiratory illness; LRTD, lower respiratory tract disease; RSV, respiratory syncytial virus; VE, vaccine efficacy.
In the first cycle of the model (i.e., the month of first vaccination), the analysis includes 50% of peak VE. Data from the AReSVi-006 phase 3 clinical trial were used to estimate VE waning functions using Cox models for RSV-ARI and RSV-LRTD. In the figure, data from the AReSVi-006 phase 3 clinical trial are represented by solid lines and extrapolations beyond the median trial follow-up period are represented by dashed lines. The figure is shown for the base-case modeled time horizon of 5 years. The time horizon of 5 years is used to capture residual protection of adjuvanted RSVPreF3 vaccine beyond the trial follow-up period. Revaccination schedule is still to be determined.
The model considered both direct and indirect costs of RSV disease from the societal perspective. Direct costs of symptomatic RSV-ARI cases captured only healthcare resource use and medication costs associated with the acute RSV case and did not include longer-term medical costs. A unit costing approach was used in which unit costs from publicly available sources for resources used to treat RSV-URTD and RSV-LRTD cases were applied to the proportion of patients using each resource (see Supplemental Material Tables S4, S5, and S6 for additional details). The model was calibrated so that the modeled outcomes for rates of RSV-related outpatient cases, emergency department (ED) visits, and hospitalizations matched the incidence rates of medically-attended RSV reported in a recent systematic literature review and meta-analysis, after adjusting for under detection due to RSV testing limitations.5 All medically-attended RSV cases were assumed to have one outpatient visit each, with some cases also experiencing ED visits and/or hospitalizations (Table S5). Percentages of RSV-URTD and RSV-LRTD cases resulting in x-rays and medication use were based on data from Belongia et al. (2018).4
Healthcare resource use costs were based on Centers for Medicare and Medicaid Services (CMS) payments for physician services41 and hospital outpatient and inpatient care.42,43 These costs were used for all patients aged ≥65 years. For patients aged 60–64 years, CMS costs were adjusted by a factor of 2 to reflect observations reported by Lopez et al.,44 that commercial insurance rates are approximately double CMS rates. Costs of medication (e.g., antibiotics, bronchodilators, corticosteroids) were based on wholesale acquisition costs from RedBook or assumption.45
Specific unit costs by resource use type, and medication costs are shown in Table S6. All cost inputs (weighted to accommodate the proportion of patients utilizing each resource) were aggregated to generate average costs per case. The mean total direct costs per symptomatic RSV-ARI case ranged from $61–$84 for RSV-URTD cases and $1,133–$1,955 for RSV-LRTD cases (Table 1). The higher mean costs for RSV-LRTD cases reflect the higher costs of ED visits and hospitalizations being incurred only in RSV-LRTD cases.
Direct vaccination costs included vaccine acquisition costs, administration costs, and costs per Grade 3 vaccine-related AE. The adjuvanted RSVPreF3 vaccine acquisition cost ($294) was based on its wholesale acquisition cost.45 The model assumed a $23 vaccine administration cost based on costs reported by Tsai et al. (2019) and CMS payments.41,46 Direct costs of vaccine-related AEs were $376–$752 per Grade 3 AE. These costs were estimated based on healthcare resource use data from the AReSVi-006 phase 3 trial, where 2.3% of vaccinated individuals experienced a Grade 3 vaccine-related AE, of whom 2.5% required hospitalization and 3.5% required outpatient care.47 Most Grade 3 AEs were reactogenic in nature (pain/erythema).23 Unit costs for hospitalization due to AEs were based on commercial costs for adults aged 60–64 years and Medicare costs for adults aged ≥65 years,43,44 with outpatient costs derived from the 2021 US Medical Expenditure Panel Survey Household Component dataset.48
We considered indirect costs within the model to evaluate cost-effectiveness from the societal perspective. Indirect costs associated with productivity loss due to RSV disease, premature death, as well as vaccine administration and AEs were all captured in the model. As recommended by the Second Panel on Cost-Effectiveness in Health and Medicine, all productivity losses included both market and nonmarket productivity losses to fully capture the value of individuals’ time.32 This approach considered not only market wages for those adults aged ≥60 years in the workforce but also nonmarket productive time (e.g., unpaid time spent caregiving, volunteering, performing household activities). Time spent on market and nonmarket productive activities were obtained from the American Time Use Survey.49
Productivity losses were assumed to be 0.5–1 day per nonmedically-attended RSV-ARI case and 3.3 days per RSV-URTD or RSV-LRTD outpatient visit or RSV-LRTD ED visit (based on estimates reported for influenza),50 as well as 7 days per hospitalized RSV-LRTD case (based on estimates reported for RSV).4 For premature RSV-related mortality, we assumed productivity losses throughout the remaining lifetime, derived from Grosse et al.,51 and assumed an annual productivity growth of 1% and a discounting rate of 3%. For the vaccination strategy, we assumed 0.79–1.14 hours of productivity loss per vaccine administration (based on estimates reported for influenza),50 and 1.3 days per Grade 3 AE (based on data reported for adults aged ≥50 years receiving recombinant zoster vaccine).52 Market productivity unit costs per hour were estimated from weekly median wages obtained from US Bureau of Labor Statistics for 2024,53 adjusted as reported by Grosse et al.51 Nonmarket productivity losses were also estimated using the methods reported by Grosse et al.51 The underlying specific indirect cost inputs for market and nonmarket productivity losses stratified by age are presented in the Supplemental Material (Table S4). As for direct costs, indirect costs were similarly aggregated to generate average indirect costs per case. Mean total indirect costs ranged from $185–$272 for RSV-URTD cases and $334–$493 for RSV-LRTD cases. Mean total indirect costs for RSV-related mortality ranged from $10,700–$744,619 (Table 1).
QALY losses were included in the model to account for the impact of RSV-outcomes and vaccine-related AEs on quality of life (Table S7). Baseline age-specific EQ-5D utilities were taken from the published literature for the general US population.54 For RSV-URTD and RSV-LRTD cases, QALY loss inputs were obtained from a US time trade-off study that estimated QALY losses associated with RSV-LRTD and RSV-URTD of 0.0178 and 0.0133 respectively.55 For vaccine-related Grade 3 AEs a QALY loss of 0.000677 was assumed, based on data reported for adults aged ≥50 years receiving recombinant zoster vaccine.52
Modeled outcomes and analyses
The base-case cost-effectiveness analysis was conducted from the societal perspective, including direct and indirect costs associated with symptomatic RSV-ARI cases and RSV vaccination. Cost outcomes are reported overall and stratified by type of cost. Direct costs are reported separately for costs associated with RSV-URTD cases, RSV-LRTD cases, and adjuvanted RSVPreF3 vaccination. Indirect costs are reported separately for costs associated with RSV-URTD cases, RSV-LRTD cases, RSV-LRTD-related deaths, and adjuvanted RSVPreF3 vaccination.
Modeled outcomes with and without vaccination were compared to estimate the incremental impact of adjuvanted RSVPreF3 vaccination on cost and QALY outcomes. The incremental cost-effectiveness ratio (ICER) was calculated as the incremental cost per QALY gained and was compared to a range of commonly used willingness-to-pay thresholds for the US ($50,000, $100,000, and $150,000 per QALY gained).56 Given the updated 2024 ACIP recommendations for RSV vaccination,28 results are also reported separately for the general population of adults aged 60–74 and ≥75 years. Several additional measures were also considered. Estimated costs of adjuvanted RSVPreF3 vaccination per avoided outcome (RSV-ARI case, RSV-LRTD-related hospitalization, and RSV-related death) were calculated by dividing the total incremental costs by the number of avoided outcomes over the 5-year time horizon. Estimates for the number of avoided outcomes per 1 million vaccinations were calculated by dividing the total number of avoided outcomes over the 5-year time horizon by the number of individuals who were vaccinated and then multiplying by 1 million. Numbers needed to vaccinate (NNVs) to avoid each outcome were further calculated by dividing the total number of individuals vaccinated by the number of avoided outcomes over the 5-year time horizon.
Deterministic and probabilistic sensitivity analyses were conducted to evaluate the sensitivity of ICER results to the model’s input values. For the univariate deterministic sensitivity analysis, key groups of inputs were varied individually, holding all other input parameters constant. Lower and upper bounds of parameter variations were based either on 95% CIs, upper and lower values of source inputs, or applied as ±20% of the mean value. For additional detail, see tables within the Supplemental Material. The probabilistic sensitivity analysis varied key inputs simultaneously and assessed the impact on cost-effectiveness across 5,000 Monte Carlo simulations, computing the percentage of simulations falling within willingness-to-pay thresholds of $50,000, $100,000, and $150,000 per QALY gained. In addition, three scenario analyses were conducted: one scenario from the healthcare sector perspective (excluding any indirect costs associated with symptomatic RSV-ARI cases or vaccination) over the 5-year study horizon, and two additional scenarios evaluating cost-effectiveness from the societal perspective over a shorter 3-year and longer 6-year time horizon.
Results
Base-case and scenario analysis results
Without RSV vaccination, the burden of RSV disease in adults aged ≥60 years in the US over 5 years was estimated to be approximately 17.9 million symptomatic RSV-ARI cases (8.9 million RSV-URTD and 9.0 million RSV-LRTD cases), including 8.2 million outpatient visits, 826,790 hospitalizations, and 65,853 RSV-LRTD-related deaths (Figure S3). From the societal perspective, RSV disease was estimated to incur total costs of more than $41.2 billion over 5 years; including nearly $15.3 billion in direct costs (with $14.7 billion due to RSV-LRTD) and $25.9 billion in indirect costs due to productivity loss (Table 2). Most indirect costs were from productivity losses due to premature RSV-related mortality (approximately $20.2 billion), while productivity losses associated with symptomatic RSV-ARI in the acute phase were approximately $5.7 billion. The highest cost burden was incurred in adults aged 65–69 years (with overall total societal costs of nearly $14.2 billion over 5 years), followed by adults aged 70–74 years ($9.1 billion) and 60–64 years ($7.8 billion). Without vaccination, RSV disease was also estimated to result in 750,861 QALY losses. Results related to the annual burden of RSV disease in the absence of vaccination (i.e., results over the first year of the modeled time horizon) are provided in the Supplemental Material (Table S8 and Table S9).
Table 2.
Base-case cost-effectiveness results for adjuvanted RSVPreF3 vaccine versus no vaccine for US adults aged ≥60 years over a 5-year period.
| Adjuvanted RSVPreF3 | No vaccine | Incremental (adjuvanted RSVPreF3 vs. no vaccine) | |
|---|---|---|---|
| Total direct costs | $27,347,207,309 | $15,280,977,272 | $12,066,230,037 |
| RSV-URTD case | $460,312,937 | $564,130,625 | -$103,817,688 |
| RSV-LRTD case | $9,669,724,469 | $14,716,846,647 | -$5,047,122,177 |
| Adjuvanted RSVPreF3 vaccination | $17,217,169,902 | $0 | $17,217,169,902 |
| Total indirect costs | $18,376,753,964 | $25,938,249,005 | -$7,561,495,041 |
| RSV-URTD case | $1,663,687,471 | $2,045,752,669 | -$382,065,198 |
| RSV-LRTD case | $2,479,950,488 | $3,697,373,917 | -$1,217,423,429 |
| RSV-related death | $13,340,141,814 | $20,195,122,419 | -$6,854,980,605 |
| Adjuvanted RSVPreF3 vaccination | $892,974,191 | $0 | $892,974,191 |
| Total societal costs | $45,723,961,273 | $41,219,226,277 | $4,504,734,996 |
| Total QALY losses | 506,437 | 750,861 | -244,424 |
| RSV-URTD case | 90,815 | 111,983 | -21,168 |
| RSV-LRTD case | 100,736 | 150,636 | -49,900 |
| RSV- related death | 314,077 | 488,242 | -174,165 |
| Adjuvanted RSVPreF3 vaccination AE | 810 | – | 810 |
| ICER (cost per QALY gained) | N/A | N/A | $18,430 |
AE, adverse event; ICER, incremental cost-effectiveness ratio; LRTD, lower respiratory tract disease; N/A, not applicable; QALY, quality-adjusted life year; RSV, respiratory syncytial virus; URTD, upper respiratory tract disease; US, United States.
All costs discounted at 3% per year.
For the vaccination strategy, one-time adjuvanted RSVPreF3 vaccination of nearly 52.7 million individuals was estimated to result in nearly 4.6 million fewer symptomatic RSV-ARI cases, 2.4 million fewer RSV-related outpatient visits, 288,933 fewer hospitalizations, and 23,120 fewer deaths over the 5-year period compared with no vaccination (Figure S3). Adjuvanted RSVPreF3 vaccination also was associated with 244,424 fewer QALY losses, primarily due to fewer QALYs lost due to mortality and RSV-LRTD cases (Table 2).
These reductions in RSV-related outcomes with vaccination were accompanied by reductions in disease-related direct and indirect costs. Direct costs due to RSV disease over 5 years decreased by more than $5.1 billion and RSV-related indirect costs decreased by more than $8.4 billion with adjuvanted RSVPreF3 vaccination (Table 2). Reductions in RSV-LRTD cases and associated severe outcomes (hospitalizations and deaths) accounted for the majority of disease-related cost savings over the 5-year time horizon. Compared with no vaccination, RSV-LRTD direct costs decreased from approximately $14.7 billion to $9.7 billion, with indirect costs due to productivity loss decreasing from approximately $23.9 billion to $15.8 billion. In contrast, disease-related cost savings associated with RSV-URTD cases were relatively small. Compared with no vaccination, direct costs decreased from approximately $0.6 billion to $0.5 billion with vaccination and indirect costs decreased from approximately $2.0 billion to $1.7 billion.
These cost savings of $13.6 billion in RSV-related costs over the 5-year period partially offset the costs of adjuvanted RSVPreF3 vaccination, estimated at approximately $18.1 billion. Total vaccination costs included approximately $17.2 billion in direct costs ($15.5 billion for vaccine acquisition, $1.2 billion for vaccine administration, and $0.5 billion for vaccine-related AEs) and $0.9 billion in indirect costs ($0.6 billion for vaccine administration and $0.3 billion for vaccine-related AEs).
In the base-case analysis, the total incremental cost of the adjuvanted RSVPreF3 vaccination strategy was estimated to be $4.5 billion over the 5-year time horizon. As previously noted, vaccination resulted in 244,424 fewer QALY losses over 5 years, resulting in an ICER of $18,430 per QALY gained from the societal perspective (Table 2). ICERs were estimated at $11,915 per QALY gained among adults aged 60–74 years and $31,893 per QALY gained among adults aged ≥75 years (Table S10 and Table S11).
Among all adults aged ≥60 years, adjuvanted RSVPreF3 vaccination costs per avoided outcome were approximately $985 to avoid a symptomatic RSV-ARI case, $15,591 to avoid an RSV-related hospitalization, and $194,844 to avoid an RSV-related death. The 5-year public health impact of adjuvanted RSVPreF3 vaccination translates to an estimated 86,795 avoided symptomatic RSV-ARI cases, 5,483 avoided RSV-related hospitalizations, and 439 avoided RSV-related deaths per 1 million vaccinations. Over the 5-year time horizon, the NNVs to prevent 1 symptomatic RSV-ARI case and 1 RSV-LRTD case were 12 and 18, respectively. A total of 22 individuals would need to be vaccinated to avoid 1 RSV-related outpatient visit, 228 to avoid 1 ED visit, 182 to avoid 1 hospitalization, and 2,279 to avoid 1 RSV-related death.
For the scenario evaluating cost-effectiveness from the healthcare sector perspective, adjuvanted RSVPreF3 vaccination was associated with incremental direct costs of nearly $12.1 billion, resulting in an ICER of $49,366 per QALY gained (Table S12). For the scenario with a 3-year time horizon, one-time adjuvanted RSVPreF3 vaccination at the beginning of year 1 was associated with total incremental societal costs of $7.7 billion over 3 years; $13.3 billion in total direct costs, offset by a $5.5 billion reduction in indirect costs (Table S13). The resulting ICER over the 3-year period was $40,212 per QALY gained. Using a 6-year time horizon, one-time adjuvanted RSVPreF3 vaccination was associated with total incremental societal costs of $3.5 billion, resulting in an ICER of $13,421 per QALY gained (Table S14).
Sensitivity analysis results
Results of the deterministic and probabilistic sensitivity analyses are presented in Figures 3 and 4, respectively. The deterministic sensitivity analysis found that results were most sensitive to key RSV epidemiological and vaccine efficacy inputs, including the annual incidence of symptomatic RSV-ARI, vaccine efficacy against RSV-LRTD, percentage of RSV-LRTD cases resulting in death, and percentage of symptomatic RSV-ARI cases that are LRTD. Results for the top 15 inputs are shown in Figure 3. Across all deterministic sensitivity analysis runs, the ICER ranged from a low of $1,960 to a high of $64,001 per QALY gained (generated when applying the upper and lower bounds for annual incidence of first symptomatic RSV-ARI, respectively). Using similar lower rates for RSV reinfection of 2.7% had limited impact on the resultant ICER, with only a marginal increase in the ICER to $21,488 per QALY gained (Figure 3). Indeed, most of the input variations (84.2% of all input variations) resulted in changes to the ICER that were less than $5,000 per QALY gained in either direction.
Figure 3.
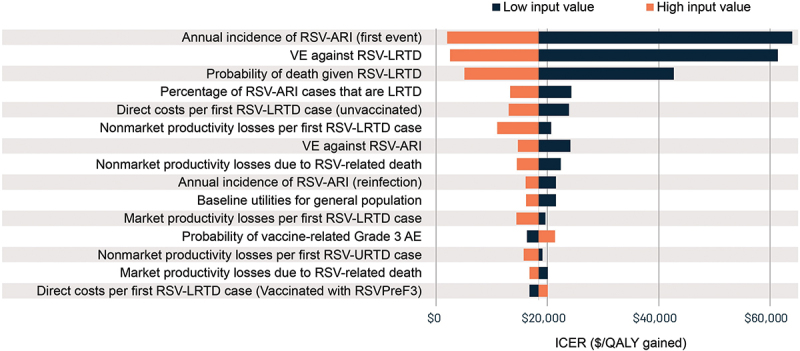
Deterministic sensitivity analysis results for the cost-effectiveness of adjuvanted RSVPreF3 vaccine versus no vaccine for US adults aged ≥60 years over a 5-year period.
Base-case ICER is $18,430 per QALY gained. Results are shown for the top 15 inputs.
AE, adverse event; ARI, acute respiratory illness; ICER, incremental cost-effectiveness ratio; LRTD, lower respiratory tract disease; QALY, quality-adjusted life year; RSV, respiratory syncytial virus; URTD, upper respiratory tract disease; US, United States; VE, vaccine efficacy.
Figure 4.
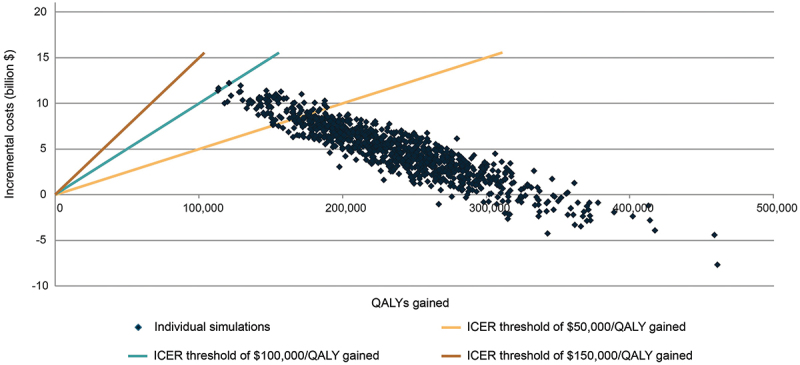
Probabilistic sensitivity analysis results for the cost-effectiveness of adjuvanted RSVPreF3 vaccine versus no vaccine for US adults aged ≥60 years over a 5-year period.
Incremental costs versus incremental QALYs are displayed from 5,000 probabilistic sensitivity analysis simulations.
ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year; US, United States.
The probabilistic sensitivity analysis showed that the adjuvanted RSVPreF3 vaccine was cost-effective in 100.0% of simulations using a willingness-to-pay threshold of $150,000 per QALY gained, in 99.8% of simulations using a willingness-to-pay threshold of $100,000 per QALY gained, and in 91.2% of simulations using a willingness-to-pay threshold of $50,000 per QALY gained (Figure 4). A total of 6.0% of simulations resulted in adjuvanted RSVPreF3 being cost saving (i.e., resulting in improved health outcomes and reduced societal costs).
Discussion
Findings from the current study using clinical data through three RSV seasons indicate that one-time adjuvanted RSVPreF3 vaccination is estimated to have a substantial impact in reducing RSV disease burden over 5 years and is cost-effective for the prevention of RSV disease in US adults aged ≥60 years. ICER results from both the societal and healthcare sector perspectives ($18,430 and $49,366 per QALY gained, respectively) were both below commonly used US willingness-to-pay thresholds of $100,000 and $150,000 per QALY gained.56 These results were relatively robust to a wide range of input values used in sensitivity analyses. Results were most sensitive to assumptions related to key vaccine efficacy and RSV epidemiology inputs, including vaccine efficacy against RSV-LRTD. However, the maximum ICER from the deterministic sensitivity analysis was $64,001 per QALY gained and nearly all probabilistic sensitivity analysis simulations (99.8%) resulted in ICERs that were below $100,000 per QALY gained.
Given the relatively recent introduction of RSV vaccines for use among adults aged ≥60 years in the US, there are relatively few published economic/cost-effectiveness evaluations.57–59 While direct comparison of our findings with those previously reported is hindered by differences in model inputs and perspectives, some general observations can be made. Herring et al. evaluated a hypothetical RSV vaccine (assuming 50% efficacy against RSV overall and 65% efficacy against moderate-to-severe RSV-LRTD) and estimated reductions in RSV outcomes and associated costs over one year, although only direct costs from the healthcare sector perspective were included in the analysis.57 The study evaluated two scenarios using different RSV incidence inputs, including one based upon Falsey et al.,14 as used in the present study. In that scenario, as may be expected, RSV disease burden in the absence of vaccination was generally similar to what we report over the first year. However, their analysis estimated a larger number of RSV-related deaths because they included 12-month mortality post-hospitalization.57 Two other studies evaluated the cost-effectiveness of adjuvanted RSVPreF3 vaccination in preventing medically-attended RSV cases, with analyses conducted from the societal perspective using 2-year time horizons.58,59 First, the discrete-event simulation model by Moghadas et al. estimated reductions in RSV outcomes with adjuvanted RSVPreF3 vaccination over 2 years.58 Assuming vaccine costs of $210 per dose and sigmoidal vaccine efficacy, adjuvanted RSVPreF3 vaccination was associated with total incremental societal costs of $10.6 billion over 2 years, with a resultant ICER of $94,432 per QALY gained.58 The other cost-effectiveness model developed by Hutton et al. assumed lower incidence rates for RSV-related hospitalizations, based on epidemiologic data from the CDC’s RSV Hospitalization Surveillance Network (RSV-NET).59 The model estimated a higher ICER of $196,842 per QALY gained over 2 years, using a vaccine price of $280. In a scenario analysis, the model reported a lower ICER of $127,049 per QALY gained over a 3-year time horizon.59 However, the models reported by Moghadas et al. and Hutton et al. do not capture the additional benefits of RSV vaccination that are projected beyond these 2–3 year time periods following vaccination.
Differences between results from the current analysis and other publicly available analyses can be attributed to differences in the underlying model structures, input values, and assumptions. In particular, comparisons of results should be interpreted taken into account the modeled populations, time horizons, and assumptions related to RSV epidemiological data. Each of the analyses demonstrated the value of RSV vaccination in substantially reducing disease burden among adults aged ≥60 years, although specific economic outcomes varied across the studies.
The present study has some caveats. Estimating the incidence of RSV-ARI without vaccination based on the most robust data available was crucial to our analysis. Here, RSV-ARI incidence was based on estimates from Falsey et al., conducted within New York between 1999 through 2003.14 Given limited alternative data sources that have captured symptomatic RSV-ARI using multiple testing modalities, the study by Falsey et al. remains an important benchmark for RSV burden within US adults and continues to be used as an input source in other recent analyses on RSV disease burden in the US.57,60 In our sensitivity analysis, we found that varying RSV-ARI incidence had one of the greatest impacts on cost-effectiveness. Applying the lower bound for RSV-ARI incidence increased the ICER to $64,001 per QALY gained over 5 years. There remains a need for more robust data on RSV-ARI burden in US adults. Such data can be used in subsequent updates to the present model as further epidemiological data become available. Our model also accounted for RSV-ARI reinfection, assuming similar rates as for the incidence of initial RSV-ARI, in part due to limited data for reinfection in adults, and where pediatric data suggest no overall reduction in susceptibility to further infection following previous recent infection.39 While it remains possible that reinfection rates could be lower, this was examined in our sensitivity analysis, where applying the lower bound on the RSV-ARI reinfection rate had a limited impact on the ICER (increasing to $21,488 per QALY gained). Furthermore, it should be noted that across the 5-year period, reinfections accounted for a relatively small proportion of all RSV-ARI cases (approximately 10%). Nevertheless, a better understanding of RSV-ARI reinfection patterns can help to inform future analyses. Another point relates to our model structure. We used a static model, and so did not account for any dynamic transmission aspect, and any broader indirect impact of vaccination on RSV transmission (including any potential herd immunity), which is an inherent limitation of such static models.
Our model includes some conservative assumptions. Some relate to adjuvanted RSVPreF3 vaccine efficacy. Specifically, the model did not consider any disease attenuation for breakthrough cases (i.e., symptomatic RSV-ARI cases in vaccinated individuals), even though in the AReSVi-006 phase 3 clinical trial, less severe symptoms and less healthcare resource use were observed among vaccinated cases as compared with placebo.61 Furthermore, while the model used efficacy against symptomatic RSV-ARI and RSV-LRTD, it conservatively did not consider vaccine efficacy against severe RSV-LRTD. In the AReSVi-006 study, efficacy against severe RSV-LRTD of 94.1% was reported during one RSV season, with 78.8% efficacy across 2 full seasons and 67.4% across 3 seasons.23,25,26 Beyond these vaccine efficacy assumptions, the model also conservatively did not consider any additional, spill-over caregiver-related productivity and QALY losses. In addition, while coadministration studies have found that the adjuvanted RSVPreF3 vaccine can be administered with all commonly used influenza and shingles vaccines,20,62–65 the possibility of vaccine coadministration was not accounted for in the present model. Lastly, the model assumes that RSV-related deaths only occur among hospitalized RSV-LRTD cases, potentially underestimating the total number of RSV-related deaths that may occur across all care settings. Our model also only accounted for short-term mortality (within 30 days of admission), and did not consider any additional longer-term mortality (i.e., within 12-months), which may be considerable.6,7 As such, the mortality reduction associated with vaccination may greatly underestimate the overall benefits in reducing RSV-related deaths.
Accepting these caveats, our study has some additional limitations. First, the efficacy data we used for adjuvanted RSVPreF3 vaccination were based on data from the AReSVi-006 phase 3 clinical study and may not reflect vaccine effectiveness seen in real-world settings (where effectiveness and duration of protection may be lower). In addition, the input data we used for efficacy waning were derived from predictive modeling rather than directly from reported clinical data to be able to account for vaccine efficacy beyond the clinical trial median follow-up period of 30.6 months. Second, in the present study we evaluated a single vaccine strategy, comparing adjuvanted RSVPreF3 vaccination versus no vaccination. We did not conduct a direct comparison with any available alternative RSV vaccine, in part due to the lack of direct or indirect evidence evaluating the relative treatment effect of different vaccines.
A further limitation relates to our study population. Our analyses were conducted across the general population of US adults aged ≥60 years and reflect outcomes that could be expected if individuals in this age range were vaccinated uniformly, without restriction to certain groups of individuals who are at increased risk of severe RSV-related outcomes due to medical comorbidities. Given ACIP’s June 2024 recommendation specifying that one dose of RSV vaccine should be administered to all adults aged ≥75 years and adults aged 60–74 years who are at increased risk of severe RSV disease,28 it is possible that adults aged ≥60 years who are at highest risk of severe RSV outcomes will be prioritized for vaccination. Although not examined in the present study, it may be expected that adjuvanted RSVPreF3 vaccination would have an even greater impact in preventing severe outcomes, leading to a more favorable cost-effectiveness profile in such high-risk individuals than reported here for the general population of adults aged ≥60 years. However, the policy implications of our results suggest that even routine vaccination of all adults aged ≥60 years with the adjuvanted RSVPreF3 vaccine would be an efficient use of healthcare resources.
It is also important to recognize that implementation of RSV vaccines within existing adult vaccination programs may carry additional costs to the healthcare sector, in terms of training, communication, and public awareness activities. As an additional limitation, these broader program implementation and policy considerations were not included in the current analysis, where the focus was on evaluating the cost-effectiveness of adjuvanted RSVPreF3 vaccination assuming universal access for all adults aged ≥60 years. During the 2023–2024 season, more than 10.5 million US adults aged ≥60 years received an RSV vaccination.66 With RSV vaccine implementation moving into the second season of vaccine availability, additional efforts will be needed to ensure that individuals at highest-risk of severe RSV disease are able to get vaccinated.
In conclusion, the results from this study highlight the value of the adjuvanted RSVPreF3 vaccine for the prevention of RSV disease among adults aged ≥60 years in the US, where vaccination is a cost-effective strategy, resulting in substantial reductions in RSV-related morbidity and mortality. Additional analyses are needed to better understand the public health impact and cost-effectiveness of the adjuvanted RSVPreF3 vaccine among adults with chronic conditions that place them at increased risk of severe RSV-related outcomes.
Supplementary Material
Acknowledgments
The authors would like to thank Zinan Yi, Will Herring, Laure-Anne Van Bellinghen, and Ilse Van Vlaenderen for their study support. The authors would also like to thank Business & Decision Life Sciences Medical Communication Service Center for editorial assistance and manuscript coordination, on behalf of GSK. Ashish Agrawal and Iain O’Neill (freelance, on behalf of GSK) provided writing support.
Biography
Elizabeth La, PhD, is a Director in the US Health Economics and Outcomes Research team for Vaccines at GSK. Her current research focuses on the burden of respiratory syncytial virus (RSV) disease in adults aged ≥60 years and adults who are at increased risk of severe RSV disease, as well as the potential impact of RSV vaccination in reducing disease burden. Prior to joining GSK, Dr. La was an Associate Director of Health Economics at RTI Health solutions, conducting research across a number of therapeutic areas, including infectious and vaccine-preventable diseases. Dr. La has more than 15 years of experience conducting and managing health economics and outcomes research studies. Before working at RTI Health Solutions, Elizabeth obtained her PhD in Health Policy and Management at the University of North Carolina at Chapel Hill. Her research has been published in several peer-reviewed journals, including Vaccine, Pharmacoeconomics, and Human Vaccines & Immunotherapeutics, and presented at various professional conferences.
Funding Statement
GSK funded this study [GSK study identifier: VEO-000319] and was involved in all stages of study conduct, including analysis of the data. GSK also took in charge all costs associated with the development and publication of this manuscript.
Disclosure statement
Elizabeth La, David Singer, Sara Poston, Desmond Curran, Jessica Pickett, and Frederik Verelst are employed by and hold financial equities in GSK. Daniel Molnar was employed by GSK at the time of the study. Jonathan Graham is employed by RTI Health Solutions, which received funding from GSK for the conduct of this study. All authors declare no other financial or non-financial relationships and activities.
Author contributions
All authors participated in the design, implementation, and/or analysis, as well as the interpretation of study results and the development of this manuscript. All authors had full access to the data and gave final approval before submission. All authors agree to be accountable for all aspects of the work.
Data availability statement
The model used in this study is proprietary property of GSK and is not able to be shared.
Trademark
AREXVY is a trademark owned by or licensed to GSK.
ABRYSVO is a trademark of Pfizer.
mRESVIA is a trademark of Moderna.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2432745
References
- 1.Walsh EE. Respiratory syncytial virus infection: an illness for all ages. Clin Chest Med. 2017;38(1):29–12. doi: 10.1016/j.ccm.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coultas JA, Smyth R, Openshaw PJ. Respiratory syncytial virus (RSV): a scourge from infancy to old age. Thorax. 2019;74(10):986–993. doi: 10.1136/thoraxjnl-2018-212212. [DOI] [PubMed] [Google Scholar]
- 3.Branche AR, Falsey AR. Respiratory syncytial virus infection in older adults: an under-recognized problem. Drugs Aging. 2015;32(4):261–269. doi: 10.1007/s40266-015-0258-9. [DOI] [PubMed] [Google Scholar]
- 4.Belongia EA, King JP, Kieke BA, Pluta J, Al-Hilli A, Meece JK, Shinde V. Clinical features, severity, and incidence of RSV illness during 12 consecutive seasons in a community cohort of adults ≥60 years old. Open Forum Infect Dis. 2018;5(12):ofy316. doi: 10.1093/ofid/ofy316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLaughlin JM, Khan F, Begier E, Swerdlow DL, Jodar L, Falsey AR. Rates of medically attended RSV among US adults: a systematic review and meta-analysis. Open Forum Infect Dis. 2022;9(7):ofac300. doi: 10.1093/ofid/ofac300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ackerson B, Tseng HF, Sy LS, Solano Z, Slezak J, Luo Y, Fischetti CA, Shinde V. Severe morbidity and mortality associated with respiratory syncytial virus versus influenza infection in hospitalized older adults. Clin Infect Dis. 2019;69(2):197–203. doi: 10.1093/cid/ciy991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tseng HF, Sy LS, Ackerson B, Solano Z, Slezak J, Luo Y, Fischetti CA, Shinde V. Severe morbidity and Short- and mid- to long-term mortality in older adults hospitalized with respiratory syncytial virus infection. J Infect Dis. 2020;222(8):1298–1310. doi: 10.1093/infdis/jiaa361. [DOI] [PubMed] [Google Scholar]
- 8.Stephens LM, Varga SM. Considerations for a respiratory syncytial virus vaccine targeting an elderly population. Vaccines (Basel). 2021;9(6):624. doi: 10.3390/vaccines9060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pleasants RA, Heidari K, Ohar J, Donohue JF, Lugogo NL, Kanotra SM, Kraft M, Mannino DM, Strange CB. Respiratory symptoms among US adults: a cross-sectional health survey study. Pulm Ther. 2022;8(3):255–268. doi: 10.1007/s41030-022-00194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan J, Pravosud V, Mannino DM, Siegel K, Choate R, Sullivan T. National and state estimates of COPD morbidity and mortality – United States, 2014–2015. Chronic Obstr Pulm Dis. 2018;5(4):324–333. doi: 10.15326/jcopdf.5.4.2018.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Beaton AZ, Boehme AK, Buxton AE, et al. Heart disease and stroke statistics-2023 update: a report from the American heart association. Circulation. 2023;147(8):e93–e621. doi: 10.1161/CIR.0000000000001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Li X, Wang Z, Bancks MP, Carnethon MR, Greenland P, Feng YQ, Wang H, Zhong VW. Trends in prevalence of diabetes and control of risk factors in diabetes among US adults, 1999-2018. JAMA. 2021;326(8):1–13. doi: 10.1001/jama.2021.9883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Branche AR, Saiman L, Walsh EE, Falsey AR, Sieling WD, Greendyke W, Peterson DR, Vargas CY, Phillips M, Finelli L. Incidence of respiratory syncytial virus infection among hospitalized adults, 2017–2020. Clin Infect Dis. 2022;74(6):1004–1011. doi: 10.1093/cid/ciab595. [DOI] [PubMed] [Google Scholar]
- 14.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 15.Begley KM, Monto AS, Lamerato LE, Malani AN, Lauring AS, Talbot HK, Gaglani M, McNeal T, Silveira FP, Zimmerman RK, et al. Prevalence and clinical outcomes of respiratory syncytial virus vs influenza in adults hospitalized with acute respiratory illness from a prospective multicenter study. Clin Infect Dis. 2023;76(11):1980–1988. doi: 10.1093/cid/ciad031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onwuchekwa C, Moreo LM, Menon S, Machado B, Curcio D, Kalina W, Atwell JE, Gessner BD, Siapka M, Agarwal N, et al. Underascertainment of respiratory syncytial virus infection in adults due to diagnostic testing limitations: a systematic literature review and meta-analysis. J Infect Dis. 2023;228(2):173–184. doi: 10.1093/infdis/jiad012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rozenbaum MH, Judy J, Tran D, Yacisin K, Kurosky SK, Begier E. Low levels of RSV testing among adults hospitalized for lower respiratory tract infection in the United States. Infect Dis Ther. 2023;12(2):677–685. doi: 10.1007/s40121-023-00758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rozenbaum MH, Begier E, Kurosky SK, Whelan J, Bem D, Pouwels KB, Postma M, Bont L. Incidence of respiratory syncytial virus infection in older adults: limitations of current data. Infect Dis Ther. 2023;12(6):1487–1504. doi: 10.1007/s40121-023-00802-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Kulkarni D, Begier E, Wahi-Singh P, Wahi-Singh B, Gessner B, Nair H. Adjusting for case under-ascertainment in estimating RSV hospitalisation burden of older adults in high-income countries: a systematic review and modelling study. Infect Dis Ther. 2023;12(4):1137–1149. doi: 10.1007/s40121-023-00792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.AREXVY [Package Insert] . GlaxoSmithkline biologicals. 2024. [Accessed 2024 Oct 7]. https://www.fda.gov/media/167805/download.
- 21.ABRYSVO [Package Insert]. Pfizer Inc . 2024. [Accessed 2024 Oct 7]. https://www.fda.gov/media/168889/download?attachment.
- 22.Melgar M, Britton A, Roper LE, Talbot HK, Long SS, Kotton CN, Havers FP. Use of respiratory syncytial virus vaccines in older adults: recommendations of the advisory committee on immunization practices — United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(29):793–801. doi: 10.15585/mmwr.mm7229a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papi A, Ison MG, Langley JM, Lee DG, Leroux-Roels I, Martinon-Torres F, Schwarz TF, van Zyl-Smit RN, Campora L, Dezutter N, et al. Respiratory syncytial virus prefusion F protein vaccine in older adults. N Engl J Med. 2023;388(7):595–608. doi: 10.1056/NEJMoa2209604. [DOI] [PubMed] [Google Scholar]
- 24.Walsh EE, Perez Marc G, Zareba AM, Falsey AR, Jiang Q, Patton M, Polack FP, Llapur C, Doreski PA, Ilangovan K, et al. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N Engl J Med. 2023;388(16):1465–1477. doi: 10.1056/NEJMoa2213836. [DOI] [PubMed] [Google Scholar]
- 25.Ison MG, Papi A, Athan E, Feldman RG, Langley JM, Lee DG, Leroux-Roels I, Martinon-Torres F, Schwarz TF, van Zyl-Smit RN, et al. Efficacy and safety of respiratory syncytial virus prefusion F protein vaccine (RSVPreF3 OA) in older adults over 2 RSV seasons. Clin Infect Dis. 2024;78(6):1732–1744. doi: 10.1093/cid/ciae010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ison MG, Papi A, Athan E, Feldman RG, Langley JM, Lee D-G, Leroux-Roels I, Martinon-Torres F, Schwarz TF, van Zyl-Smit RN, et al. The efficacy of a single dose of the respiratory syncytial virus prefusion F protein vaccine in adults ≥60 years of age over 3 RSV seasons. In: Presented at CHEST. Boston, United States. 2024. [Accessed 2024 Oct 6–9]. [Google Scholar]
- 27.MRESVIA [Package Insert] . Moderna. 2024. [Accessed 2024 Oct 7]. https://www.fda.gov/media/179005/download.
- 28.Britton A, Roper LE, Kotton CN, Hutton DW, Fleming-Dutra KE, Godfrey M, Ortega-Sanchez IR, Broder KR, Talbot HK, Long SS, et al. Use of respiratory syncytial virus vaccines in adults aged ≥60 years: updated recommendations of the advisory committee on immunization practices – United States, 2024. MMWR Morb Mortal Wkly Rep. 2024;73(32):696–702. doi: 10.15585/mmwr.mm7332e1. [DOI] [PubMed] [Google Scholar]
- 29.Molnar D, La EM, Verelst F, Poston S, Graham J, Van Bellinghen LA, Curran D. Public health impact of the adjuvanted RSVPreF3 vaccine for respiratory syncytial virus prevention among older adults in the United States. Infect Dis Ther. 2024;13(4):827–844. doi: 10.1007/s40121-024-00939-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US Bureau of Economic Analysis . Gross Domestic Product: Implicit Price Deflator [GDPDEF], retrieved from FRED, federal reserve bank of St. Louis. 2024. [Accessed 2024 Oct 7]. https://fred.stlouisfed.org/series/GDPDEF.
- 31.US Bureau of Labor Statistics . Consumer price index for all urban consumers: medical care in US city average, not seasonally adjusted (half 1 2024). 2024. [Accessed 2024 Oct 7]. https://www.bls.gov/cpi/data.htm.
- 32.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, Kuntz KM, Meltzer DO, Owens DK, Prosser LA, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 33.Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, Caulley L, Chaiyakunapruk N, Greenberg D, Loder E, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health. 2022;25(1):3–9. doi: 10.1016/j.jval.2021.11.1351. [DOI] [PubMed] [Google Scholar]
- 34.US Census Bureau . Projected population by single year of age, sex, race, and Hispanic origin for the United States: 2023 to 2100. [Accessed 2024 May 22]. https://www.census.gov/data/datasets/2023/demo/popproj/2023-popproj.html.
- 35.Arias E, Xu J, Kochanek K. United States life tables, 2021. Natl Vital Stat Rep. 2023;72(12):1–64. doi: 10.15620/cdc:132418. [DOI] [PubMed] [Google Scholar]
- 36.DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, Pollak R, Christoff J, Earl J, Landolfi V, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371(7):635–645. doi: 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- 37.Hamid S, Winn A, Parikh R, Jones JM, McMorrow M, Prill MM, Silk BJ, Scobie HM, Hall AJ. Seasonality of respiratory syncytial virus –United States, 2017–2023. MMWR Morb Mortal Wkly Rep. 2023;72(14):355–361. doi: 10.15585/mmwr.mm7214a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention (CDC) . Percent positivity of RSV nucleic acid amplification tests by HHS region, national respiratory and enteric virus surveillance system (NREVSS). 2024. [Accessed 2024 Oct 7]. https://data.cdc.gov/Laboratory-Surveillance/Percent-Positivity-of-Respiratory-Syncytial-Virus-/3cxc-4k8q/about_data.
- 39.Nduaguba SO, Tran PT, Choi Y, Winterstein AG, Abidi SH. Respiratory syncytial virus reinfections among infants and young children in the United States, 2011–2019. PLOS ONE. 2023;18(2):e0281555. doi: 10.1371/journal.pone.0281555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention (CDC) . Flu vaccination coverage, United States, 2023–24 influenza season. 2024. [Accessed 2024 Oct 7]. https://www.cdc.gov/fluvaxview/coverage-by-season/2023-2024.html.
- 41.Centers for Medicare and Medicaid Services (CMS) . Physician fee schedule lookup tool. 2024. [Accessed 2024 Oct 7]. https://www.cms.gov/medicare/physician-fee-schedule/search/overview.
- 42.Centers for Medicare and Medicaid Services (CMS) . Hospital outpatient prospective payment system. NFRM OPPS Cost Statistics Files; [Accessed 2024 Oct 7]. https://www.cms.gov/medicare/payment/prospective-payment-systems/hospital-outpatient/regulations-notices/cms-1786-fc. [Google Scholar]
- 43.Centers for Medicare and Medicaid Services (CMS) . Medicare inpatient hospitals - by geography and service (2022 dataset). 2024. [Accessed 2024 Oct 7]. https://data.cms.gov/provider-summary-by-type-of-service/medicare-inpatient-hospitals/medicare-inpatient-hospitals-by-geography-and-service.
- 44.Lopez E, Neuman T, Jacobson G, Levitt L. How much more than medicare do private insurers pay? A review of the literature. Kaiser family foundation (KFF). 2020. [Accessed 2024 Oct 7]. https://www.kff.org/medicare/issue-brief/how-much-more-than-medicare-do-private-insurers-pay-a-review-of-the-literature/.
- 45.Merative Micromedex RED BOOK . [Accessed 2024 Oct 7]. https://www.micromedexsolutions.com/home/dispatch.
- 46.Tsai Y, Zhou F, Lindley MC. Insurance reimbursements for routinely recommended adult vaccines in the private sector. Am J Prev Med. 2019;57(2):180–190. doi: 10.1016/j.amepre.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.GSK data on file: AReSVi-006 phase 3 clinical trial.
- 48.Agency for Healthcare Research and Quality (AHRQ) . Mean expenditure per person with care ($) by condition and event type, United States, 2021. Medical expenditure panel survey household component data. [Accessed 2024 May 5]. https://datatools.ahrq.gov/meps-hc.
- 49.US Bureau of Labor Statistics. American Time Use Survey (ATUS) . Table 3. Time spent in primary activities for the civilian population by age, sex, race, Hispanic or Latino ethnicity, marital status, and educational attainment, annual averages. 2024. [Accessed 2024 Oct 7]. https://www.bls.gov/news.release/archives/atus_06272024.htm.
- 50.Prosser LA, O’Brien MA, Molinari NA, Hohman KH, Nichol KL, Messonnier ML, Lieu TA. Non-traditional settings for influenza vaccination of adults: costs and cost effectiveness. Pharmacoeconomics. 2008;26(2):163–178. doi: 10.2165/00019053-200826020-00006. [DOI] [PubMed] [Google Scholar]
- 51.Grosse SD, Krueger KV, Pike J. Estimated annual and lifetime labor productivity in the United States, 2016: implications for economic evaluations. J Med Econ. 2019;22(6):501–508. doi: 10.1080/13696998.2018.1542520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmader KE, Levin MJ, Grupping K, Matthews S, Butuk D, Chen M, Idrissi ME, Fissette LA, Fogarty C, Hartley P, et al. The impact of reactogenicity after the first dose of recombinant zoster vaccine on the physical functioning and quality of life of older adults: an open-label, phase III trial. J Gerontol A Biol Sci Med Sci. 2019;74(8):1217–1224. doi: 10.1093/gerona/gly218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.US Bureau of Labor Statistics . Usual weekly earnings of wage and salary workers, second quarter 2024. [Accessed 2024 Oct 7]. https://www.bls.gov/news.release/pdf/wkyeng.pdf.
- 54.Janssen B, Szende A. Population norms for the EQ-5D. In: Szende A; Janssen BCabases J, editors. Self-reported population health: an international perspective. Dordrecht, The Netherlands: Springer; 2014. p. 19–30. [PubMed] [Google Scholar]
- 55.Rendas-Baum R, Molnar D, Curran D, Bjorner JB. A time trade-off study to estimate respiratory syncytial virus (RSV) related utility values for older adults (OA) in the United States. In: Presented at ISPOR. Boston (MA). 2023. [Accessed 2023 May 7–10]. [Google Scholar]
- 56.Neumann PJ, Kim DD. Cost-effectiveness thresholds used by study authors, 1990–2021. JAMA. 2023;329(15):1312–1314. doi: 10.1001/jama.2023.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herring WL, Zhang Y, Shinde V, Stoddard J, Talbird SE, Rosen B. Clinical and economic outcomes associated with respiratory syncytial virus vaccination in older adults in the United States. Vaccine. 2022;40(3):483–493. doi: 10.1016/j.vaccine.2021.12.002. [DOI] [PubMed] [Google Scholar]
- 58.Moghadas SM, Shoukat A, Bawden CE, Langley JM, Singer BH, Fitzpatrick MC, Galvani AP. Cost-effectiveness of prefusion F protein-based vaccines against respiratory syncytial virus disease for older adults in the United States. Clin Infect Dis. 2023;78(5):1328–1335. doi: 10.1093/cid/ciad658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hutton DW, Prosser LA, Rose AM, Mercon K, Ortega-Sanchez IR, Leidner AJ, Havers FP, Prill MM, Whitaker M, Roper LE, et al. Cost-effectiveness of vaccinating adults aged 60 years and older against respiratory syncytial virus. Vaccine. 2024;42(24):126294. doi: 10.1016/j.vaccine.2024.126294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carrico J, Hicks KA, Wilson E, Panozzo CA, Ghaswalla P. The annual economic burden of respiratory syncytial virus in adults in the United States. J Infect Dis. 2023;230(2):e342–e352. doi: 10.1093/infdis/jiad559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Curran D, Matthews S, Cabrera ES, Perez SN, Breva LP, Ramet M, Helman L, Park DW, Schwarz TF, Melendez IMG, et al. The respiratory syncytial virus prefusion F protein vaccine attenuates the severity of respiratory syncytial virus-associated disease in breakthrough infections in adults ≥60 years of age. Influenza Other Respir Viruses. 2024;18(2):e13236. doi: 10.1111/irv.13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chandler R, Montenegro N, Llorach C, Aguirre LN, Germain S, Kuriyakose SO, Lambert A, Descamps D, Olivier A, Hulstrom V. Immunogenicity, reactogenicity, and safety of AS01E-adjuvanted RSV prefusion F protein-based Candidate vaccine (RSVPreF3 OA) when Co-administered with a seasonal quadrivalent influenza vaccine in older adults: results of a phase 3, open-label, randomized controlled trial. Clin Infect Dis. 2024; ciad786. doi: 10.1093/cid/ciad786. [DOI] [PubMed] [Google Scholar]
- 63.Clark R, Davies S, Labrador J, Loubet P, Natalini Martinez S, Morinigo HM, Nicolas JF, Vera MP, Ramet M, Rebollo-Rodrigo MH, et al. Safety and immunogenicity of respiratory syncytial virus prefusion F protein vaccine when Co-administered with adjuvanted seasonal quadrivalent influenza vaccine in older adults: A phase 3 randomized trial. Clin Infect Dis. 2024;79(4):1088–1098. doi: 10.1093/cid/ciae365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buynak R, Cannon K, DeAtkine D, Kirby J, Usdan L, Bhavsar A, Gerard C, Kuznetsova A, Jayadev A, Amare H, et al. Randomized, open-label phase 3 study evaluating immunogenicity, safety, and reactogenicity of RSVPreF3 OA coadministered with FLU-QIV-HD in adults aged ≥65. Infect Dis Ther. 2024;13(8):1789–1805. doi: 10.1007/s40121-024-00985-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dennis P. Co-administration of the adjuvanted respiratory syncytial virus (RSV) prefusion F protein vaccine (RSVPreF3 OA) with the adjuvanted recombinant zoster vaccine (RZV) in adults ≥50 years of age. In: Presented at European geriatric medicine society (EuGMS). Valencia, Spain. [Accessed 2024 Sep 18–20]. [Google Scholar]
- 66.Centers for Disease Control and Prevention (CDC) . RSVVaxView. Weekly RSV vaccination dashboard. [Accessed 2024 Oct 16]. https://www.cdc.gov/rsvvaxview/data/index.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The model used in this study is proprietary property of GSK and is not able to be shared.


