Abstract
Background:
Maintaining optimal hearing health and preventing ear infections is crucial across all life stages, spanning from before birth and infancy to middle age and beyond. The primary aim of the research was to evaluate and compare the effectiveness of the telemedicine-enabled otoscope (TEO) in diagnosing ear diseases with that of the conventional otoscope.
Materials and Methods:
The databases PubMed, Cochrane, and Embase were thoroughly searched to find original studies on TEO at the community as well as hospital levels along with meta-analysis for comparison with standard diagnostic methods using traditional otoscopes.
Results:
The telemedicine-equipped otoscope displays a sensitivity of 82% (confidence interval 0.78–0.95) and a specificity of 95% (confidence interval 0.93–0.96). When employed by physicians, the combined sensitivity and specificity become 84% (confidence interval 0.79–0.88) and 91% (confidence interval 0.87–0.94), respectively. Community Health Workers (CHWs) using telemedicine-enabled otoscopes exhibit a collective sensitivity of 80% (confidence interval 0.72–0.87) and a collective specificity of 97% (confidence interval 0.95–0.98). Snapping multiple photographs and removal of cerumen where necessary in increasing the quality of the photographs for diagnosis.
Conclusions:
The TEO holds the promise of substantially enhancing the accessibility of audiology services, encompassing evaluation, public outreach, and fundamental care. Its implementation has the potential to fortify primary health care environments, contributing to the prevention of ear diseases and assisting in mitigating the shortage of skilled ear care professionals.
Keywords: Ear diseases, ENT, otoscope, pooled sensitivity, telemedicine
BACKGROUND
A significant proportion of the global population, approximately 10%, experiences mild to severe hearing loss, establishing it as a prominent contributor to disability on a global scale. Adult-onset hearing loss is positioned as the ninth leading factor in terms of the years of healthy life compromised by disability and the thirteenth among the primary causes of diseases worldwide.[1] As life expectancy has risen, there has been a diminished focus on disabilities and impairments such as deafness and hearing-related issues. Global burden of hearing loss, particularly chronic otitis media, is significant. If not addressed promptly, ear conditions can lead to sensorineural hearing loss as a subsequent consequence.[2]
In India, approximately six percent of the entire population, totaling around 6.3 crores individuals, experience various degrees of considerable hearing ability impairment. Nearly five out of every 1000 children in the country grapple with severe to profound hearing challenges.[3] The prevalence of adult-onset deafness in India is approximately 7.6%, while childhood-onset deafness is estimated to be around 2%.[4] The 2014 survey in India on the assessment of children (out of school) unveiled 2,20,425 individuals aged between 6 and 13 in India reported having a hearing impairment. Among them, 42,556 were school-going children, constituting 19.31% of the total with hearing disabilities in that age group.[5] Hearing impairment, a significantly overlooked health issue in India, requires effective intervention. The severity of hearing loss and bothersome otorrhea can detrimentally impact education, employment opportunities, and social integration. The lack of adequate facilities for detecting and treating ear conditions often leads to substantial financial and societal repercussions for individuals, communities, and the nation as a whole. Addressing this issue properly is essential for holistic well-being.
In India, obstacles to the timely identification and intervention of hearing impairment stem from various factors. These include insufficient infrastructure, a shortage of skilled professionals, limited understanding of auditory evaluation initiatives amidst primary care providers, and a deficiency of sophisticated technology in primary healthcare facilities. Furthermore, late-onset hearing impairment often goes unnoticed due to financial constraints hindering access to treatment. Respondents also cited time constraints as a significant barrier to undergoing ear check-ups.[6] Consequently, routine or regular hearing assessments were severely overlooked, prompting the need for door-to-door services employing digital health technology.
In the modern era of technological advancements and digitization, telemedicine has emerged as a groundbreaking solution to bridge the gap between healthcare services and patients, particularly in remote or underserved areas.[7] One vital aspect of telemedicine is the development and integration of specialized medical devices that enable remote examination and diagnosis. Telemedicine has seen devices for preventing and treating ear diseases such as audiogram which enables measuring of sound frequency. Remote-fitting hearing aids or cochlear implants (CIs) have also been studied. The otoscope, a manual medical instrument conventionally employed by physicians for examining the external ear canal and tympanic membrane, has undergone technological advancements. The telemedicine-enabled otoscope represents the fusion of telemedicine features into this basic tool. Equipped with digital enhancements, it allows for the instantaneous remote sharing of otoscopic images and videos between healthcare professionals and patients. These new techniques have the advantage over the older methods as they provide better visualization and magnification.[8]
In light of the increasing importance of telemedicine-enabled otoscopes (TEO) in the field of healthcare, it becomes essential to conduct a systematic review and meta-analysis to assess the effectiveness and overall impact of this technology. By conducting a comprehensive evaluation of the existing literature, this study aims to evaluate the effectiveness of the TEO.
MATERIALS AND METHODS
The reporting of this systematic review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) standards. Additionally, the review was officially registered with PROSPERO under the registration number CRD42020175123.
Eligibility criteria
The PICO criteria for inclusion encompassed individuals within the community experiencing various ear infections or disorders. The intervention involved the use of telemedicine-enabled otoscopes by community or primary health workers, and this was compared to the standard ear examination procedures, typically conducted by ENT specialists or other clinicians using a traditional otoscope. The assessment focused on evaluating outcomes in terms of both efficacy and the quality of images produced.
Exclusion criteria
Studies describing methodological methods without results, letters to the editor, comments, suggestions, reviews, and any other work not directly connected to the research subject were excluded. Studies that did not evaluate the proposed outcomes were also disregarded.
Information sources
A thorough exploration of the PubMed, Cochrane, and Embase databases was carried out, encompassing data available until June 2021. The combination of synonyms for telemedicine, otoscope, ear diseases, health care, and the targeted outcomes were combined to form the search strategies at various databases
Study selection
The studies were reviewed by two independent researchers in accordance with the protocol’s description, and the conflicts were detected by the Rayyan tool for systematic literature reviews and they were solved by a third reviewer. Two separate reviewers independently gathered data from the studies and assessed the combined sensitivity and specificity values. The agreement value (kappa value) was also calculated among the TEO and standard otoscope. In addition to this, the quality of the photo taken from the TEO was analyzed.
Data items
The following variables were extracted for analysis: Investigator (Physician/Health Community Health Worker), agreement between the conventional otoscope and TEO, sensitivity, specificity, the usability of the device, and photo quality of the images.
Risk of bias in individual studies
The assessment of bias risk in each study was based on the patient selection and diagnostic test parameters, employing the JBI Critical Appraisal Checklist for Diagnostic Test Accuracy Studies. Any discrepancies that arose were resolved through discussions and mutual agreement among the authors.
Statistical analyses
The statistical analyses were conducted using Meta-DiSc statistical software version 1.4, developed by the Unit of Clinical Biostatistics team at Ramón y Cajal Hospital in Madrid, Spain. A bivariate random effects model was used to pool the various diagnostic parameters. The Diagnostic Odds Ratio (DOR) serves as a comprehensive measure of diagnostic performance, indicating the degree to which the odds of having a disease are higher for individuals with a positive test result compared to those with a negative test result. Summary Receiver Operator Characteristics (SROC) curves were generated to explore the interplay between sensitivity and specificity. To assess publication bias, Deeks’ funnel plot asymmetry test was employed. This involved plotting the logarithm of the diagnostic odds ratio (DOR) against the inverse of the square root of the effective sample size (ESS). A statistically significant result was considered when P < 0.05.
RESULTS
Initially, 1278 records were identified, with 419 duplicates removed. After scrutinizing titles and abstracts, 800 records were excluded, leaving 59 studies for a comprehensive full-text review. Ultimately, 12 studies met the criteria for inclusion in the review. The complete study selection process is depicted in the detailed PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) flow diagram for clarity [Figure 1].
Figure 1.
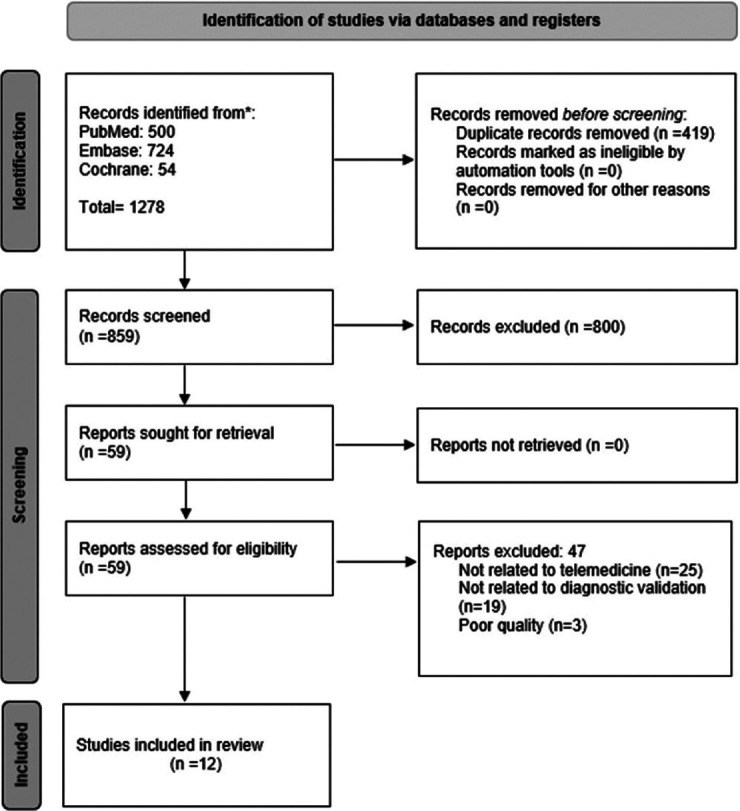
PRISMA Flow Diagram for Study Selection
Characteristics of the studies
Out of the 12 studies, ten studies were hospital-based and two studies were community-based. Sensitivity and specificity data were available in 10 studies, whereas agreement data were available in 2 studies [Table 1]. There were exclusively five studies where a physician was the investigator, and four studies were a community health worker (CHW) was the investigator. There were two studies were both separately investigated the patients, and their values were analyzed separately. The CHW included healthcare facilitators, parents, and field health workers. In adults, 5 studies were carried out; in children, 6 studies were carried out; and 1 study was carried out in both children and adults.
Table 1:
Characteristics for all included studies (n=12)
| Author Year | Study Settinga | Country | Investigator | Total Participants | Age Group | Sensitivity Data | Specificity Data | Kappa Value Data | Devices |
|---|---|---|---|---|---|---|---|---|---|
| Schuster-bruce et al. 2021[13] | HosB | UK | Physician | 40 | Adults | Y | Y | N | TYMPA smartphone system v1 |
| Gupta et al. 2019[15] | HosB | India | Physician | 33 | Adults | Y | Y | N | ENTraview Smartphone (Manufactured by Medtronic) |
| Mandavia et al. 2018[8] |
HosB | Nepal | Physician | 52 | Adults & Children | Y | Y | Y | Cupris otoscope connected to an iPhone 5s |
| Samantha et al. 2018[14] |
CB | South Africa | Community Health Worker | 73 | Children | Y | Y | N | DE500 Firefly digital video otoscope |
| Shah et al. 2018[17] | HosB | USA | Both | 40 | Children | N | N | Y | CellScope Oto (CSO) (Cellscope, Inc, San Francisco, CA) attached to iPhone 5 |
| Bhavna et al. 2018[19] | HosB | India | Physician | 50 | Adults | Y | Y | N | Smartphone otoscope (brand not mentioned) |
| Lundberg et al. 2017[10] |
HosB | South Africa | Community Health Worker | 140 | Children | Y | Y | Y | Dino-Lite Pro Earscope connected to Laptop |
| Moshtaghi et al. 2017[18] |
HosB | USA | Community Health Worker | 57 | Adults | Y | Y | N | CellScope Oto (CSO) (Cellscope, Inc, San Francisco, CA) attached to iPhone 5 |
| Richards et al. 2015[16] | HosB | USA | Physician | 51 | Children | N | N | Y | CellScope Oto (CSO) (Cellscope, Inc, San Francisco, CA) attached to iPhone |
| Lundberg et al. 2014[11] |
HosB | South Africa | Community Health Worker | 140 | Children | Y | Y | N | Dino-Lite Pro Earscope connected to Laptop |
| Biagio et al. 2013[9] | HosB | South Africa | Both | 60 | Adults | Y | Y | N | Welch Allyn (Skaneateles Falls, NY) digital MacroView videootoscope (model WA-23920-Set) |
| Eikelboom et al. 2004[12] |
CB | Australia | Physician | 66 | Children | Y | Y | N | MedRX video-otoscope (MedRx Inc., Seminole, FL, USA) |
aStudy setting: HosB=Hospital Based, CB=Community Based
Sensitivity, specificity, and agreement
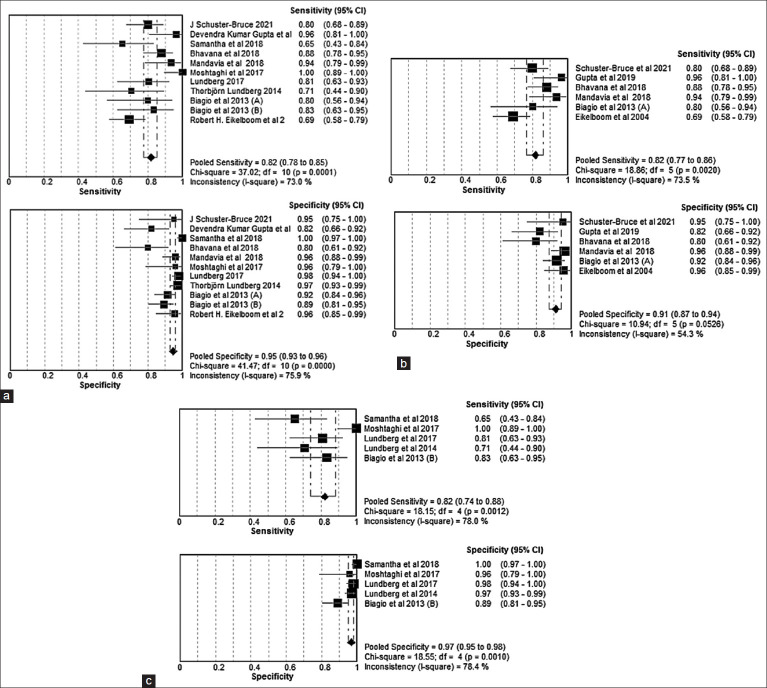
Our analysis included 11 studies presented the sensitivity and specificity of TEOs. The overall combined sensitivity of TEOs was determined to be 82% (CI 78–85%), while the overall combined specificity was found to be 95% (CI 93–96%) [Figure 2a]. Among these studies, six focused on the sensitivity and specificity of TEO when used by physicians. The pooled sensitivity of physicians employing TEO was 84% (CI 79–88%), and the pooled specificity was 91% (CI 87–94%) [Figure 2b]. Additionally, five studies explored the sensitivity and specificity of TEO when operated by community health workers (CHWs). The pooled sensitivity of CHWs using TEO was determined to be 80% (CI 72–87%), with a pooled specificity of 97% (confidence interval: 95–98%) [Figure 2c]. The average agreement, calculated from six studies with kappa values, was 0.72, indicating a moderate agreement between traditional otoscopes and telemedicine-enabled otoscopes. Agreement (k = 0.74) observed was strong among physicians, while agreement (k = 0.42) was weaker among parents/guardians in the study conducted by Shah et al. [Table 2].[17] The study by Shah et al. also highlighted that agreement might be influenced by the presence of wax, which parents/guardians found challenging to remove easily.
Figure 2.
(a) Sensitivity and Specificity of Telemedicine-Enabled Otoscope Overall,(b) Sensitivity and Specificity of Telemedicine-Enabled Otoscope Physician, (c) Sensitivity and Specificity of Telemedicine-Enabled Otoscope CHW
Table 2:
Agreement between the traditional otoscope and telemedicine-enabled otoscope
| Sl No | Authors & Year of Publication | Investigator | Total Participants | Kappa Value |
|---|---|---|---|---|
| 1. | John R. Richards et al. 2015 (A)[16] | Resident Physician | 51 | 0.74 |
| 2. | John R. Richards et al. 2015 (B)[16] | Attending Physician | 51 | 0.86 |
| 3. | Mandavia et al. 2018[8] | Physician | 52 | 0.95 |
| 4. | Bhavana et al. 2018[19] | Physician | 50 | 0.67 |
| 5. | Shah 2018 (A)[17] | Community Health Worker | 40 | 0.42 |
| 6. | Shah 2018 (B)[17] | Physician | 40 | 0.74 |
Diagnostic performance
The evaluation of the test’s diagnostic performance was most effectively conducted through the use of diagnostic odds ratios [Figure 3] and Summary Receiver Operator Characteristics (SROC) curves. [Figure 4]. CHW showed the highest Area under curve (AUC) (0.96 SE: 0.02) followed by overall (0.95 SE: 0.01) and physician (0.94 SE: 0.01) handled telemedicine-enabled otoscope.
Figure 3.
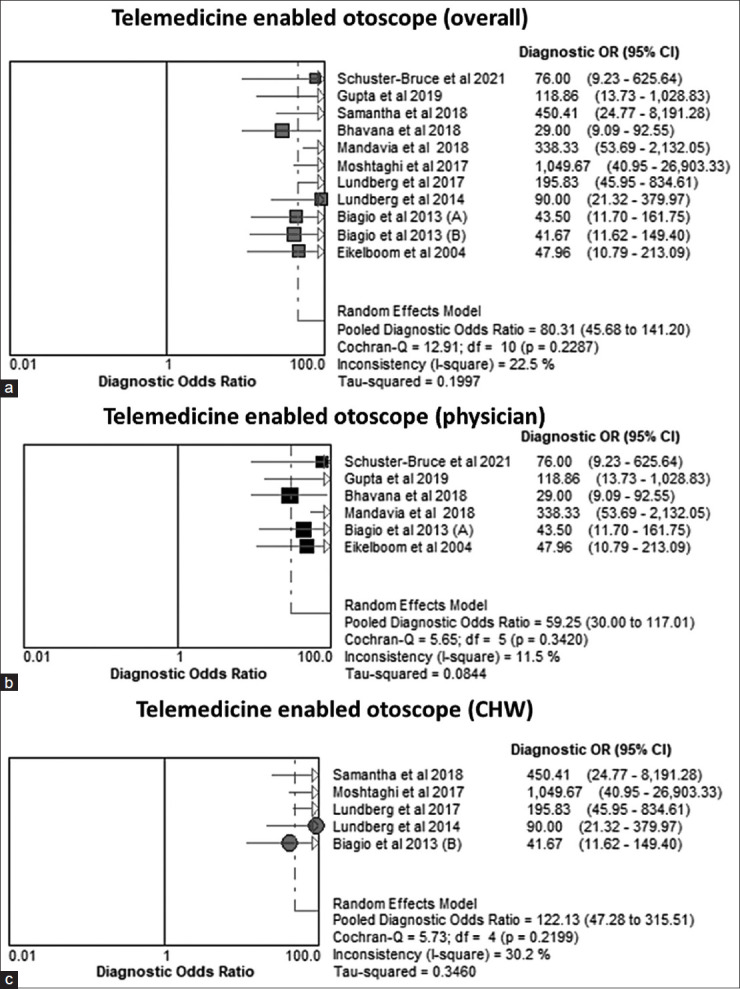
Diagnostic Odds Ratio (DOR) of Telemedicine-Enabled Otoscope (a) Overall, (b) Physician, (c) CHW
Figure 4.
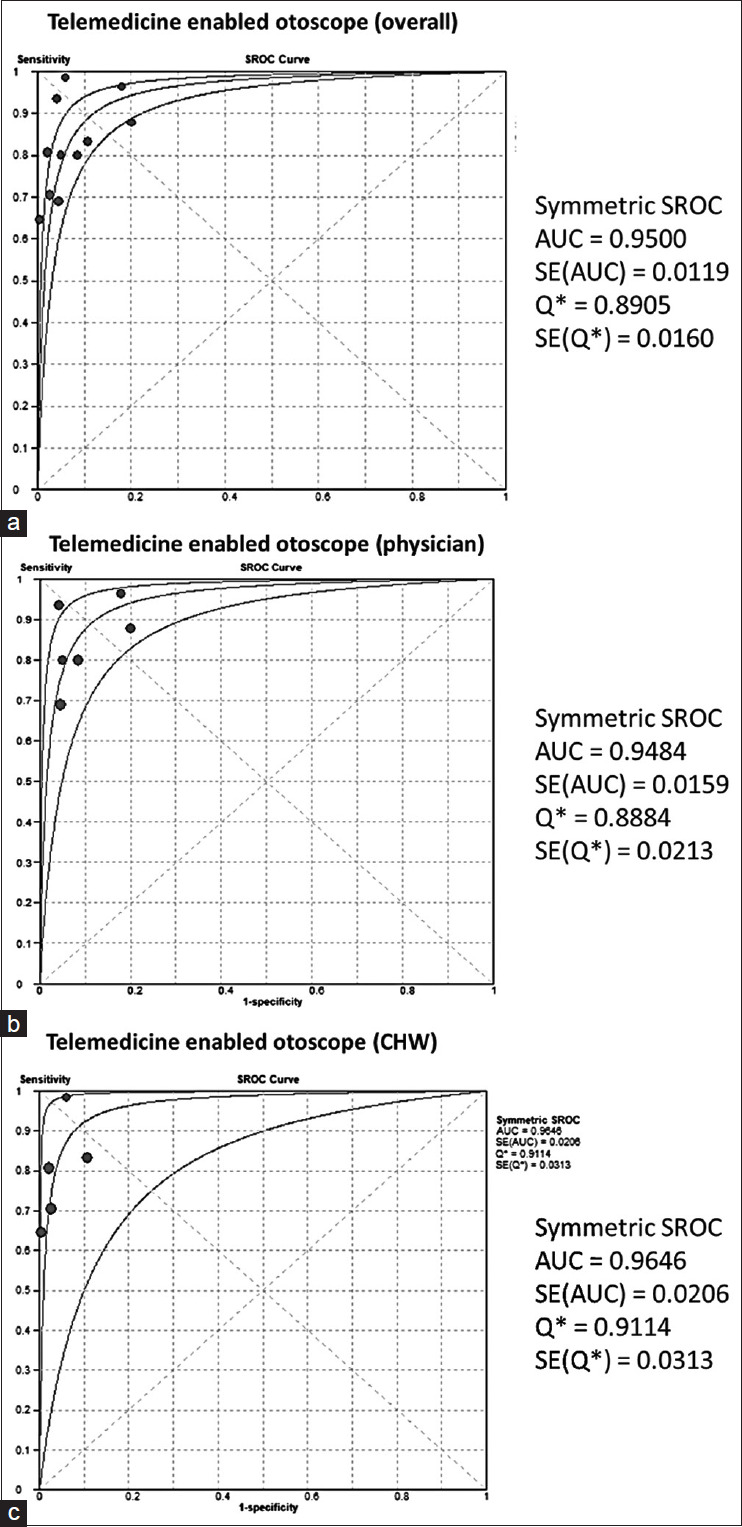
Summary Receiver Operator Characteristics (SROC) of Telemedicine-Enabled Otoscope (a) Overall, (b) Physician, (c) CHW
Devices used
A total of nine telemedicine-enabled devices were used for capturing and analyzing images of the ear. Out of the devices, three devices (Welch Allyn, Dino-Lite Pro Earscope, MedRX video-otoscope)[9,10,11,12] were specifically made for the purpose of performing video otoscopy. These required a separate laptop connectivity with proper software for capturing and storing of the images. The pooled sensitivity and specificity of these specific devices were 76% (CI 68–83%) and 94% (CI 89–97%) respectively. Three devices (TYMPA smartphone system v1, DE500 Firefly digital video otoscope, ENTraview Smartphone)[13,14,15] were specialized smartphone otoscopes that were built only for this purpose with an inbuilt camera and other additional hardware requirements. The pooled sensitivity and specificity of these specific smartphone devices were 83% (CI 61–98%) and 94% (CI 81–100%), respectively. Two accessory devices (Cupris otoscope, CellScope Oto)[8,16,17,18] were used with an iPhone device for capturing images. These accessories can be attached to any supported smartphone device with specific software. The pooled sensitivity and specificity of these accessory devices were 94% (CI 79–100%) and 97% (CI 95–98%), respectively. Only one study used no accessory smartphone[19] whose sensitivity and specificity were 88% (CI 81–94%) and 80% (CI 72–87%), respectively.
Quality of the studies
The JBI Critical Appraisal Checklist for Diagnostic Test Accuracy Studies rated each study as having a low risk of bias. No articles were excluded based on the quality assessment [Supplementary Table 1].
Supplementary Table 1:
Quality Appraisal of the included studies using JBI Critical Appraisal Checklist for Diagnostic Test Accuracy Studies
| Component | Question | Mandavia et al. 2018 | Schuster- Bruce et al. 2021 | Samantha et al. 2018 | Biagio et al. 2013 | Richards et al. 2015 | Shah et al. 2018 | Lundberg et al. 2017 | Guptaet al. 2019 | Lundberg et al. 2014 | Bhavna et al. 2018 | Moshtaghi et al. 2017 | Eikelboom et al. 2004 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PATIENT SELECTION | 1. Was a consecutive or random sample of patients enrolled? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 2. Was a case-control design avoided? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| 3. Did the study avoid inappropriate exclusions? | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | U | U | |
| INDEX TEST | 4. Were the index test results interpreted without knowledge of the results of the reference standard? | Y | Y | Y | Y | Y | Y | Y | U | Y | U | Y | U |
| 5. If a threshold was used, was it prespecified? | NA | NA | Y | NA | NA | NA | NA | Y | NA | NA | NA | NA | |
| 6. Is the reference standard likely to correctly classify the target condition? | Y | Y | U | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| 7. Were the reference standard results interpreted without knowledge of the results of the index test? | Y | U | U | Y | Y | Y | Y | Y | Y | U | Y | U | |
| 8. Was there an appropriate interval between the index test and reference standard? | Y | Y | Y | U | Y | U | Y | Y | Y | U | U | Y | |
| 9. Did all patients receive the same reference standard? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| 10. Were all patients included in the analysis? | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
where, Y=Yes N=No U=Unclear NA=Not Applicable
The Deek’s funnel plot assessing the publication bias revealed no association among the log Diagnostic Odds Ratio (DOR) and the inverse of the square root of the effective sample size. This lack of correlation suggests the absence of publication bias in the study [Figure 5].
Figure 5.
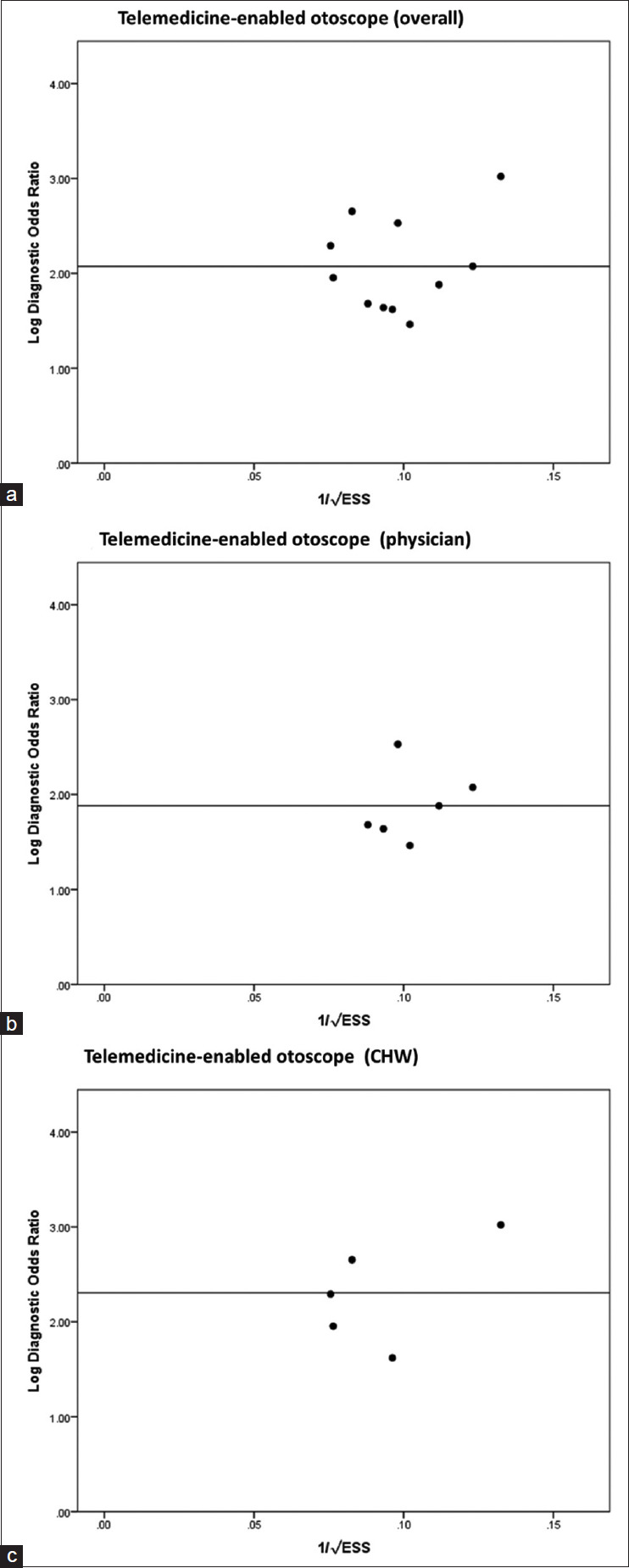
Funnel Plot for Telemedicine-Enabled Otoscope (a) Overall, (b) Physician, (c) CHW
DISCUSSION
The overall sensitivity of the telemedicine-enabled otoscope is regarded as acceptable but with excellent specificity values. The sensitivity among physicians was higher than the CHWs, but the specificity was found to be higher and excellent among the CHWs. Various devices are being manufactured with different technologies. Otoscope devices attached to smartphones such as iPhones were found to have better sensitivity and specificity than other devices. The smartphone camera being used as an otoscope had the lowest sensitivity and specificity. The statement can be challenged as the brand of this smartphone was not mentioned, and there was only one study regarding such devices.
Telemedicine-enabled otoscopes can break down geographical barriers, allowing patients in remote or underserved areas to receive specialized ear care without the need for extensive travel. Our study findings of suitable sensitivity and excellent specificity among the CHWs enable the device to increase service capacity and provide a pathway for improving access to health care in low-resource settings. The CHW would require adequate training who work as facilitators between a patient and an ENT specialist or a higher medical facility. A study in Arizona showed that proper training can enable CHW as a facilitator for tele-audiology patients and sites.[20] Due to the good sensitivity and specificity of the TEO, ear testing may be more widely available, particularly in low- and middle-income countries. Similar results were discovered for smartphone self-test audiometry, which was carried out in low-income community clinics.[21]
This can be used for ear screening in school as found in the study conducted in South Africa by a facilitator.[14] School teachers can also be trained for screening via TEO. Similar findings were also found in a study conducted in south India, where teachers were trained for screening for ear diseases among school children.[22] In this study, internet connectivity and the performance of facilitators were found to be important factors that influence the results.
With rapid access to images and consultations, healthcare providers can offer prompt recommendations, potentially preventing complications or worsening of ear conditions. The ability to remotely examine a patient’s ear provides a valuable tool for triaging cases, determining the necessity of in-person visits, and facilitating timely interventions. It can screen a larger audience depending on the battery capacity of the attached smartphone. Power Banks and spare batteries can be carried out to solve the issue of battery capacity.[8] Various programs have also been started in a setting of a limited infrastructure for screening and treatment of hearing loss and are having an impact on developing counties like India and Bangladesh. In India, this can be a part of the National Programme for Prevention and Control of Deafness (NPPCD) which has the objective of preventing avoidable hearing loss on account of disease or injury.[23]
Telemedicine-enabled otoscopes can also serve as educational tools for medical students and can also enable practitioners to guide patients through self-examinations and promote ear health awareness. The study assessing the effect of smartphone otoscope in undergraduate medical training found that it enables the learners to better observe and recognize middle ear pathology and has the ability to increase learning abilities through otoscopy.[13]
The diagnostic ability of a telemedicine-enabled otoscope depends on its image quality, as well as the skills of the healthcare provider interpreting the images. When used correctly by trained professionals, telemedicine-enabled otoscopes have proven to be highly effective in diagnosing common ear conditions such as ear infections, wax buildup, and even eardrum abnormalities. A study comparing four digital otoscopes found that image quality varies in terms of image quality.[24] A study at the University of California found that the CSO image quality was equivalent or superior to a traditional otoscope.[25] The study in Western Australia found that not just the images but comprehensive clinical history data are equally important in making correct diagnoses and providing management advice.[12] The study conducted at All India Institute of Medical Science (AIIMS) Patna found that the efficacy of visualization of the external canal and tympanic membrane is 97% and 96.7%, respectively.[19] According to a study done at an otolaryngology clinic in the United States, practitioners removed cerumen when it was necessary before taking the image; this could be difficult for patients who are unable to do it themselves or for primary care doctors who might not have the necessary tools to perform cerumen debridement before taking an SEO image.[18] The study also discovered that the effectiveness of the device may be diminished if patients and family members do not recognize the tympanic membrane. According to a study conducted at a telemedicine clinic in South Africa, facilitator-taken video-otoscopic images (23.4%) were deemed to be of poorer quality when compared to otolaryngologist-taken images (15.0%).[9] The study suggested taking multiple photographs to avoid re-examination of patients. Lundberg et al.[10] revealed a gradual improvement in images over time as a result of the investigators experience. A study found the images of tympanic membranes after a tympanostomy tube captured via video otoscope images of after are comparable to an in-person microscopic examination.[26]
A variety of healthcare settings can implement the telemedicine-enabled otoscope. It can be implemented in India at the primary healthcare level by ASHAs who are the community health workers among the people. It can also be used in camp-mode screening campaigns which will enable to provide specialized diagnoses of ear diseases. Medical treatment of the diagnosed persons needs to be kept in mind before implementing on a larger scale. A proper referral mechanism must be in place for patients who require treatment at higher facilities. It can also be used for follow-up for patients who have undergone surgery where visualization of the ear anatomy at various timelines is required. It shall decrease the out-of-pocket expenditure as well as save time.
There is still room for more research, but the studies in this review have shown how video otoscopy can improve otological care in a variety of situations. It is difficult to get to definitive results because of the numerous devices utilized as assessors, the diverse study methods, and the training technique. A few challenges which can be corrected include the requirement of accurate interpretation of otoscope images by a specialist from the images clicked as there may be technical limitations among the community health worker to provide the same. Inconsistent internet connections or device malfunctions can hinder real-time examinations and communication between patients and practitioners. Few devices had the capability of storing information in the device locally and then transmitting the same via the internet when connectivity improves. This phenomenon should be present in all devices to unveil the actual potential of this device that is to provide services in hard to reach areas and populations. Some conditions might require tactile assessment or additional tests that cannot be performed remotely.
There are several strengths for our review. Firstly, this is the first systematic review study to focus on the subgroup analysis of the physician and community health workers. As both are required for implementing this device for the prevention of ear diseases at various levels of healthcare. We also analyzed according to the type of device which shall help the policymakers in planning and decision making.
Our review has a few limitations which we would like to recognize. We included only peer-revied articles in English, which could have introduced publication bias and inadvertently exclude valuable research conducted in other languages, potentially limiting the comprehensiveness and diversity of perspectives within the review’s findings. The heterogeneity in the pooled figures was generally high. We performed a sensitivity analysis, but the outcome was similar which may be due to a lesser number of studies.
CONCLUSIONS
The introduction of hearing screening using TEO represents a notable accomplishment. To tackle the shortage of skilled ENT professionals at primary health care level in India, the reinforcement of care through telemedicine emerges as a viable strategy to bridge healthcare workforce gap. This approach holds the potential to significantly enhance the accessibility of hearing and ear services, encompassing evaluation, public outreach, and fundamental care. Leveraging smartphone attachments and technological advancements can empower community health workers to conduct effective screenings. However, it is imperative to ensure that primary healthcare practitioners undergo proper training in the utilization of TEO. Training sessions should be extended to physicians and other individuals responsible for capturing images to ensure the effective implementation of TEO.
Financial support and sponsorship
This work was supported by Health Technology Assessment in India, Department of Health Research, Ministry of Health and Family Welfare, Govt. of India.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Haile LM, Kamenov K, Briant PS, Orji AU, Steinmetz JD, Abdoli A, et al. Hearing loss prevalence and years lived with disability, 1990–2019: Findings from the Global Burden of Disease Study 2019. Lancet. 2021;397:996–1009. doi: 10.1016/S0140-6736(21)00516-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis AC, Hoffman HJ. Hearing loss: Rising prevalence and impact. Bull World Health Organ. 2019;97:646. doi: 10.2471/BLT.19.224683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varshney S. Deafness in India. Indian J Otol. 2016;22:73–6. [Google Scholar]
- 4.Galhotra A, Sahu P. Challenges and solutions in implementing hearing screening program in India. Indian J Community Med. 2019;44:299–302. doi: 10.4103/ijcm.IJCM_73_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Sample Survey of Estimation of Out-of-School Children in the Age 6-13 in India. SRI. 2014. Available from: https://www.education.gov.in/sites/upload_files/mhrd/files/upload_document/National-Survey-Estimation-School-Children-Draft-Report.pdf. [Last accessed on 2023 Aug 23]
- 6.Verma RR, Konkimalla A, Thakar A, Sikka K, Singh AC, Khanna T. Prevalence of hearing loss in India. Natl Med J India. 2021;34:216–22. doi: 10.25259/NMJI_66_21. [DOI] [PubMed] [Google Scholar]
- 7.Bitar H, Alismail S. The role of eHealth, telehealth, and telemedicine for chronic disease patients during COVID-19 pandemic: A rapid systematic review. Digit Health. 2021;7:20552076211009396. doi: 10.1177/20552076211009396. doi: 10.1177/20552076211009396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandavia R, Lapa T, Smith M, Bhutta MF. A cross-sectional evaluation of the validity of a smartphone otoscopy device in screening for ear disease in Nepal. Clin Otolaryngol. 2018;43:31–8. doi: 10.1111/coa.12898. [DOI] [PubMed] [Google Scholar]
- 9.Biagio L, Swanepoel DW, Adeyemo A, Hall III JW, Vinck B. Asynchronous video-otoscopy with a telehealth facilitator. Telemed J E Health. 2013;19:252–8. doi: 10.1089/tmj.2012.0161. [DOI] [PubMed] [Google Scholar]
- 10.Lundberg T, De Jager LB, Swanepoel DW, Laurent C. Diagnostic accuracy of a general practitioner with video-otoscopy collected by a health care facilitator compared to traditional otoscopy. Int J Pediatr Otorhinolaryngol. 2017;99:49–53. doi: 10.1016/j.ijporl.2017.04.045. [DOI] [PubMed] [Google Scholar]
- 11.Lundberg T, Biagio L, Laurent C, Sandström H, Swanepoel DW. Remote evaluation of video-otoscopy recordings in an unselected pediatric population with an otitis media scale. Int J Pediatr Otorhinolaryngol. 2014;78:1489–95. doi: 10.1016/j.ijporl.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Eikelboom RH, Mbao MN, Coates HL, Atlas MD, Gallop MA. Validation of tele-otology to diagnose ear disease in children. Int J Pediatr Otorhinolaryngol. 2005;69:739–44. doi: 10.1016/j.ijporl.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Schuster-Bruce JR, Ali A, Van M, Rogel-Salazar J, Ofo E, Shamil E. A randomised trial to assess the educational benefit of a smartphone otoscope in undergraduate medical training. Eur Arch Otorhinolaryngol. 2021;278:1799–804. doi: 10.1007/s00405-020-06373-1. [DOI] [PubMed] [Google Scholar]
- 14.Govender SM, Mars M. Assessing the efficacy of asynchronous telehealth-based hearing screening and diagnostic services using automated audiometry in a rural South African school. S Afr J Commun Disord. 2018;65:e1–9. doi: 10.4102/sajcd.v65i1.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta DK, Pati S, Singh S, Roy R, Chugh R, Goyal S, et al. Efficacy of android based mobile device as a screening tool for hearing loss in quiet and noisy environments. J Evol Med Dent Sci. 2019;8:2577–81. [Google Scholar]
- 16.Richards JR, Gaylor KA, Pilgrim AJ. Comparison of traditional otoscope to iPhone otoscope in the pediatric ED. Am J Emerg Med. 2015;33:1089–92. doi: 10.1016/j.ajem.2015.04.063. [DOI] [PubMed] [Google Scholar]
- 17.Shah MU, Sohal M, Valdez TA, Grindle CR. iPhone otoscopes: Currently available, but reliable for tele-otoscopy in the hands of parents? Int J Pediatr Otorhinolaryngol. 2018;106:59–63. doi: 10.1016/j.ijporl.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Moshtaghi O, Sahyouni R, Haidar YM, Huang M, Moshtaghi A, Ghavami Y, et al. Smartphone-enabled otoscopy in neurotology/otology. Otolaryngol Head Neck Surg. 2017;156:554–8. doi: 10.1177/0194599816687740. [DOI] [PubMed] [Google Scholar]
- 19.Bhavana K, Ahmad M, Sharma P. Smartphone Otoscopy Sans Attachment: A Paradigm Shift in Diagnosing Ear Pathologies. OTO Open. 2018:2. doi: 10.1177/2473974X18786496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coco L, Piper R, Marrone N. Feasibility of community health workers as teleaudiology patient-site facilitators: A multilevel training study. Int J Audiol. 2021;60:663–76. doi: 10.1080/14992027.2020.1864487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandström J, Swanepoel D, Laurent C, Umefjord G, Lundberg T. Accuracy and reliability of smartphone self-test audiometry in community clinics in low income settings: A comparative study. Ann Otol Rhinol Laryngol. 2020;129:578–84. doi: 10.1177/0003489420902162. [DOI] [PubMed] [Google Scholar]
- 22.Monica SD, Ramkumar V, Krumm M, Raman N, Nagarajan R, Venkatesh L. School entry level tele-hearing screening in a town in South India–Lessons learnt. Int J Pediatr Otorhinolaryngol. 2017;92:130–5. doi: 10.1016/j.ijporl.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 23.Angral S, Varshney S, Aanand P, Raj R. Tele otology in India: Last 10 years—A scopic review. Indian J Otolaryngol Head Neck Surg. 2022;74(Suppl 3):3776–88. doi: 10.1007/s12070-021-02546-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tötterman M, Jukarainen S, Sinkkonen ST, Klockars T. A comparison of four digital otoscopes in a teleconsultation setting. Laryngoscope. 2020;130:1572–6. doi: 10.1002/lary.28340. [DOI] [PubMed] [Google Scholar]
- 25.Sahyouni R, Moshtaghi O, Rajaii R, Tran DK, Bustillo D, Huang M, et al. Evaluation of an iPhone otoscope in a neurotrauma clinic and as an adjunct to neurosurgical education. Insights Neurosurg. 2016;1:4. doi: 10.21767/2471-9633.10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patricoski C, Kokesh J, Ferguson AS, Koller K, Zwack G, Provost E, et al. A comparison of in-person examination and video otoscope imaging for tympanostomy tube follow-up. Telemed J E Health. 2003;9:331–44. doi: 10.1089/153056203772744653. [DOI] [PubMed] [Google Scholar]