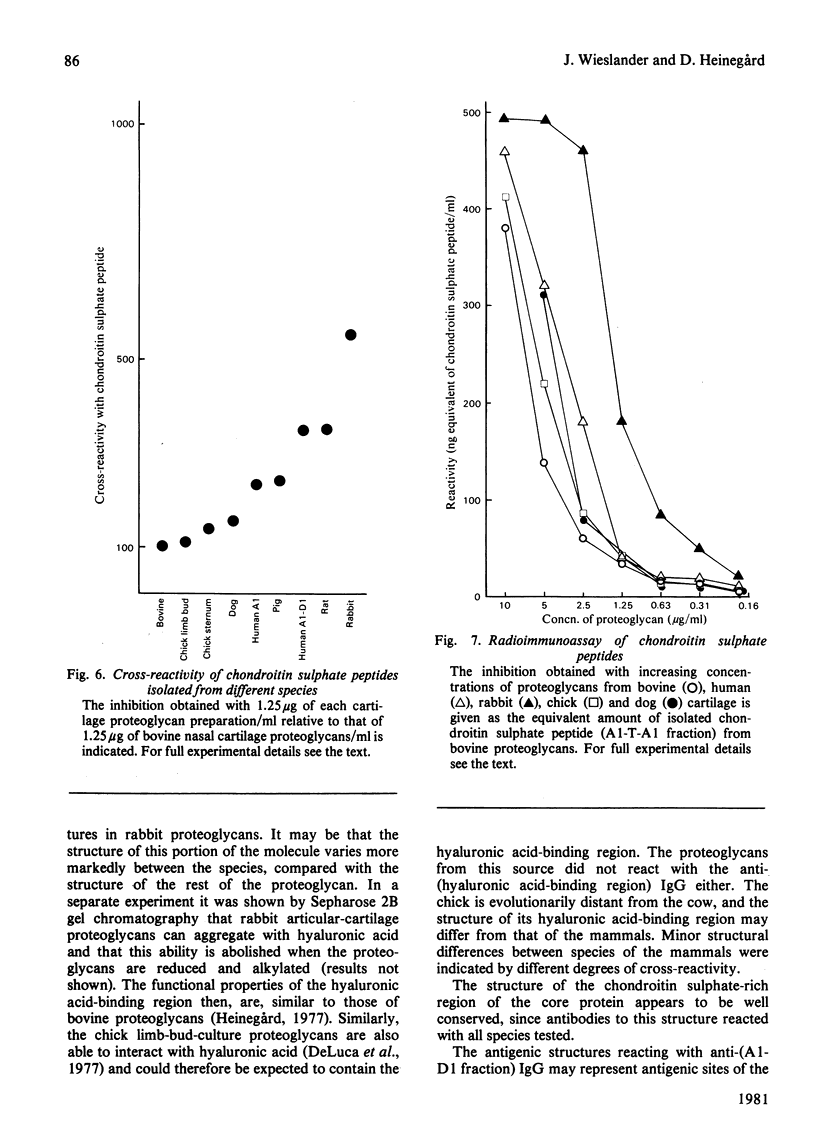
Abstract
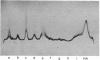
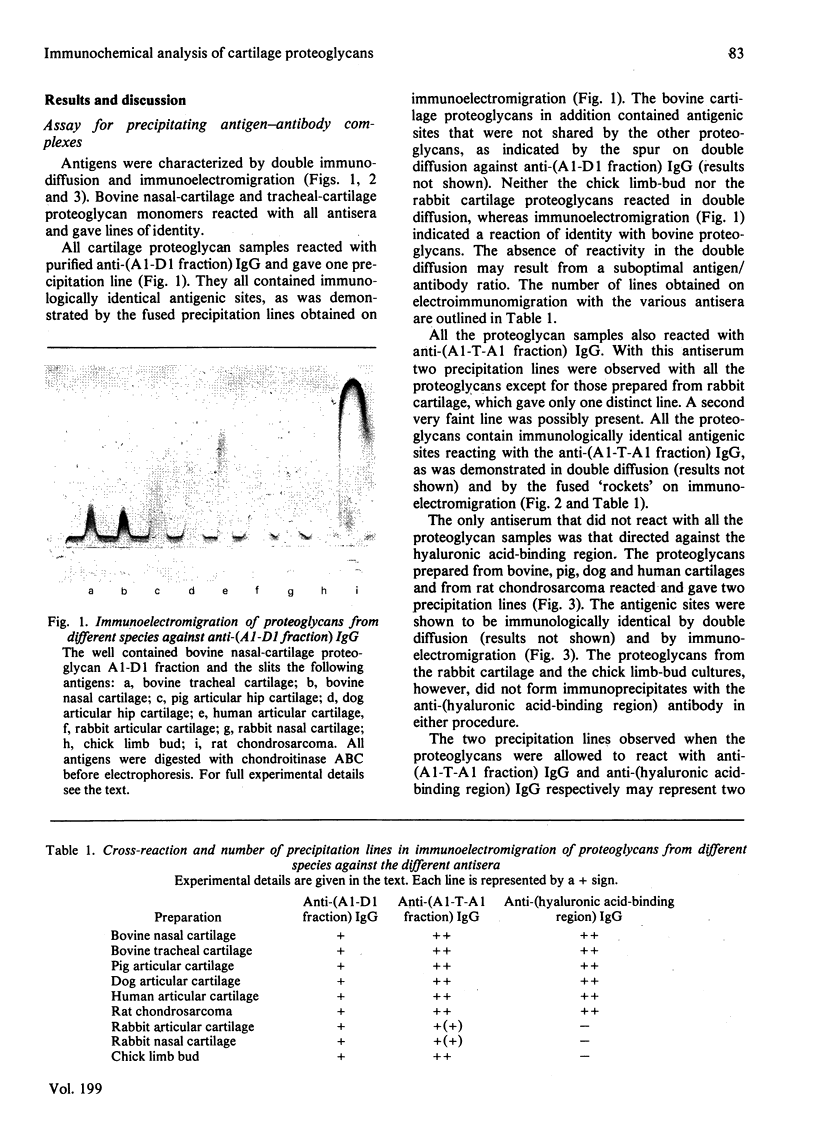
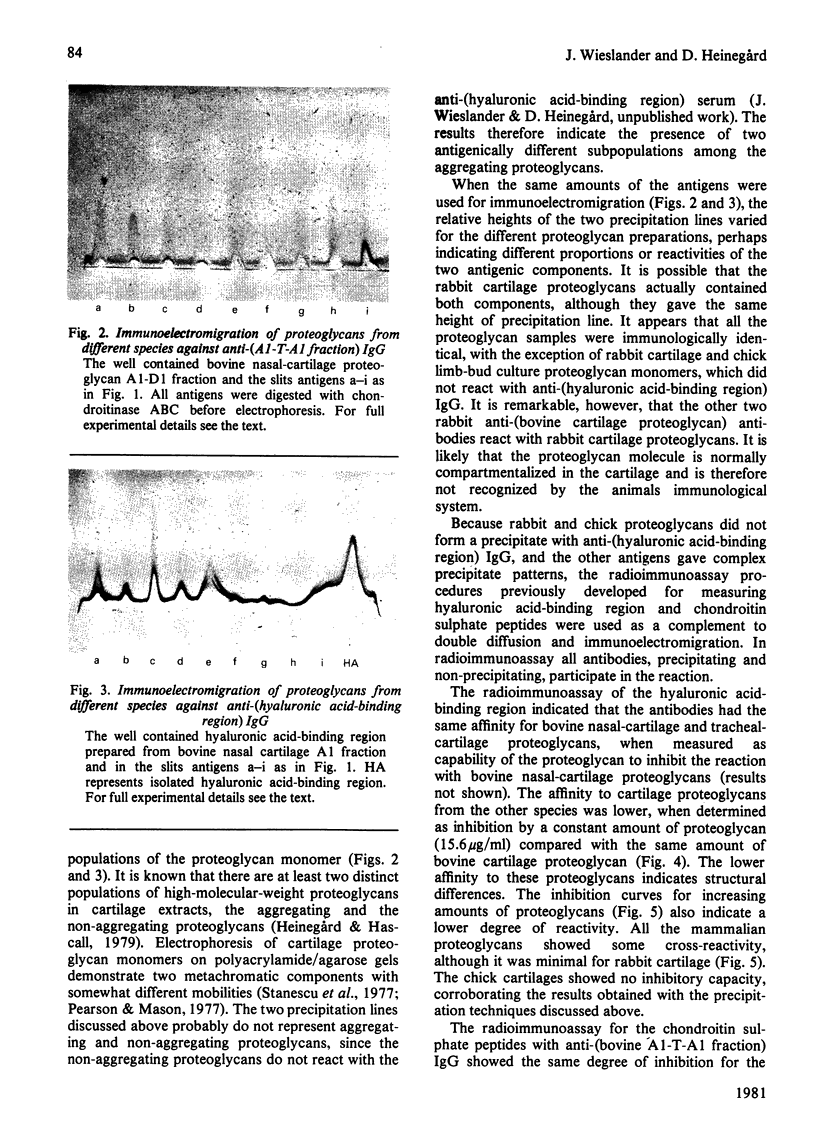
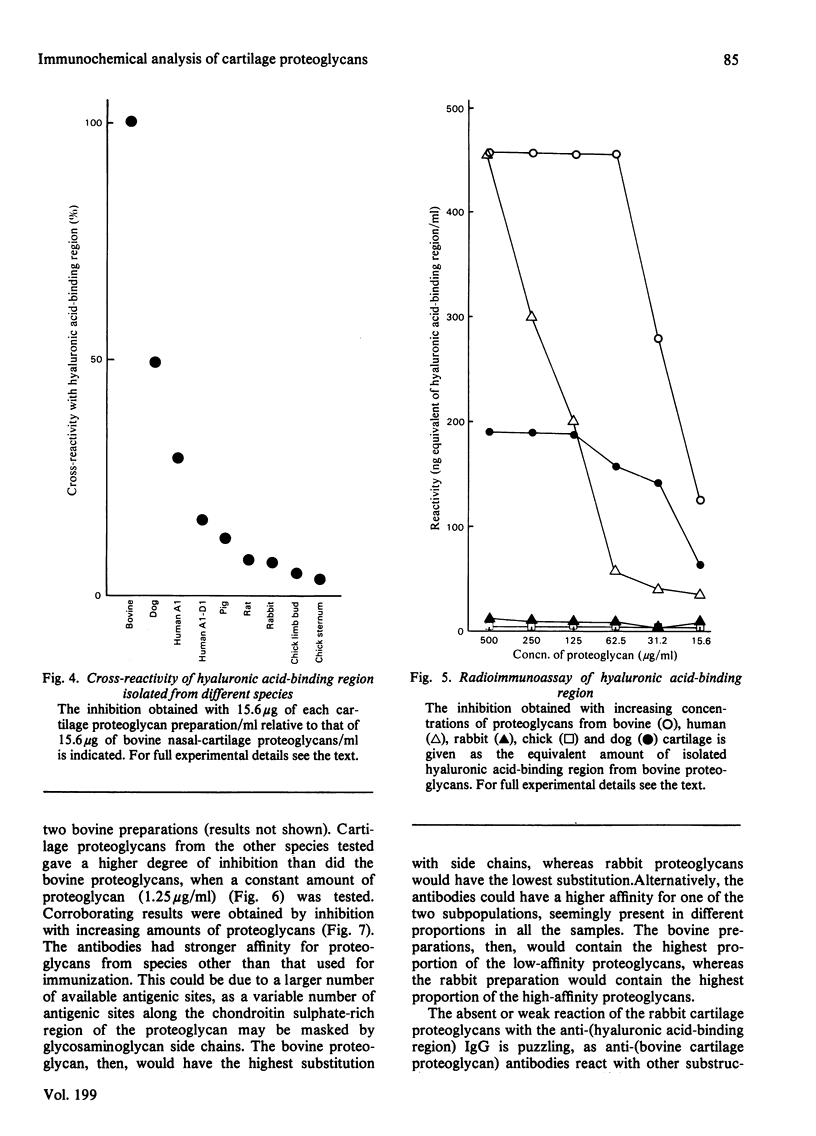
Antibodies directed against whole bovine nasal-cartilage proteoglycan and against the hyaluronic acid-binding region and chondroitin sulphate peptides from the same molecule were used in immunodiffusion and immunoelectromigration experiments. Proteoglycans from bovine nasal and tracheal cartilage showed immunological identity, with all three antisera. Proteoglycans from pig hip articular cartilage, dog hip articular cartilage, human tarsal articular cartilage and rat chondrosarcoma reacted with all the antisera and showed immunological identity with the corresponding structures isolated from bovine nasal-cartilage proteoglycans. In contrast, proteoglycans from rabbit articular cartilage, rabbit nasal cartilage and cultured chick limb buds did not react with the antibodies directed against the hyaluronic acid-binding region, though reacting with antibodies raised against whole proteoglycan monomer and against chondroitin sulphate peptides. All the proteoglycans gave two precipitation lines with the anti-(chondroitin sulphate peptide) antibodies. Similarly, the proteoglycans reacting with the anti-(hyaluronic acid-binding region) antibodies gave two precipitation lines. The results indicate the presence of at least two populations of aggregating proteoglycan monomers in cartilage. The relative affinity of the antibodies for cartilage proteoglycans and proteoglycan substructures from various species was determined by radioimmunoassay. The affinity of the anti-(hyaluronic acid-binding region) antibodies for the proteoglycans decreased in the order bovine, dog, human and pig cartilage. Rat sternal-cartilage and rabbit articular-cartilage proteoglycans reacted weakly, whereas chick limb-bud and chick sternal-cartilage proteoglycans did not react. In contrast, the affinity of antibodies to chondroitin sulphate peptides for proteoglycans increased in the order bovine cartilage, chick limb bud and chick sternal cartilage, dog cartilage, rat chondrosarcoma, human cartilage, pig cartilage, rat sternal cartilage and rabbit cartilage.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon R., Neurath H. An immunological approach to the study of evolution of trypsins. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1323–1328. doi: 10.1073/pnas.64.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter E., Muir H. The antigenicity of cartilage proteoglycans: the relationship of the antigenic determinants present in Smith degraded and intact proteoglycans. Biochim Biophys Acta. 1972 Sep 15;279(2):276–281. doi: 10.1016/0304-4165(72)90143-2. [DOI] [PubMed] [Google Scholar]
- De Luca S., Heinegård D., Hascall V. C., Kimura J. H., Caplan A. I. Chemical and physical changes in proteoglycans during development of chick limb bud chondrocytes grown in vitro. J Biol Chem. 1977 Oct 10;252(19):6600–6608. [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. The specific interaction of hyaluronic acid with cartillage proteoglycans. Biochim Biophys Acta. 1972 Sep 15;279(2):401–405. doi: 10.1016/0304-4165(72)90160-2. [DOI] [PubMed] [Google Scholar]
- Heinegård D. K., Hascall V. C. Characteristics of the nonaggregating proteoglycans isolated from bovine nasal cartilage. J Biol Chem. 1979 Feb 10;254(3):927–934. [PubMed] [Google Scholar]
- Heinegård D., Axelsson I. Distribution of keratan sulfate in cartilage proteoglycans. J Biol Chem. 1977 Mar 25;252(6):1971–1979. [PubMed] [Google Scholar]
- Heinegård D. Extraction, fractionation and characterization of proteoglycans from bovine tracheal cartilage. Biochim Biophys Acta. 1972 Nov 28;285(1):181–192. doi: 10.1016/0005-2795(72)90190-0. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Heinegård D. Polydispersity of cartilage proteoglycans. Structural variations with size and buoyant density of the molecules. J Biol Chem. 1977 Mar 25;252(6):1980–1989. [PubMed] [Google Scholar]
- Keiser H., DeVito J. Immunochemical studies of fragments of bovine nasal cartilage proteoglycan subunit. Connect Tissue Res. 1974;2(4):273–282. doi: 10.3109/03008207409152256. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. II. Prog Allergy. 1962;6:30–154. doi: 10.1159/000313795. [DOI] [PubMed] [Google Scholar]
- Oegema T. R., Jr, Hascall V. C., Dziewiatkowski D. D. Isolation and characterization of proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1975 Aug 10;250(15):6151–6159. [PubMed] [Google Scholar]
- Pearson J. P., Mason R. M. The stability of bovine nasal cartilage proteoglycans during isolation and storage. Biochim Biophys Acta. 1977 Jun 23;498(1):176–188. doi: 10.1016/0304-4165(77)90098-8. [DOI] [PubMed] [Google Scholar]
- Prieels J. P., Poortmans J., Dolmans M., Léonis J. Immunological cross-reactions of alpha-lactalbumins from different species. Eur J Biochem. 1975 Jan 15;50(3):523–527. doi: 10.1111/j.1432-1033.1975.tb09892.x. [DOI] [PubMed] [Google Scholar]
- Stanescu V., Maroteaux P., Sobczak E. Proteoglycan populations of baboon (Papio papio) articular cartilage. Biochem J. 1977 Apr 1;163(1):103–109. doi: 10.1042/bj1630103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbuch M., Audran R. The isolation of IgG from mammalian sera with the aid of caprylic acid. Arch Biochem Biophys. 1969 Nov;134(2):279–284. doi: 10.1016/0003-9861(69)90285-9. [DOI] [PubMed] [Google Scholar]
- Sugahara K., Dorfman A. Effect of reduction and alkylation on the antigenicity of cartilage proteoglycans. Biochem Biophys Res Commun. 1979 Aug 28;89(4):1193–1199. doi: 10.1016/0006-291x(79)92134-x. [DOI] [PubMed] [Google Scholar]
- Wieslander J., Heinegárd D. Immunochemical analysis of cartilage proteoglycans. Radioimmunoassay of the molecules and the substructures. Biochem J. 1980 Jun 1;187(3):687–694. doi: 10.1042/bj1870687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieslander J., Heinegård D. Immunochemical analysis of cartilage proteoglycans. Antigenic determinants of substructures. Biochem J. 1979 Apr 1;179(1):35–45. doi: 10.1042/bj1790035. [DOI] [PMC free article] [PubMed] [Google Scholar]