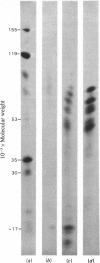
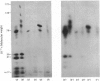
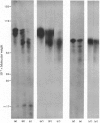
Abstract
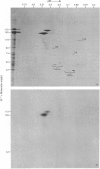
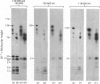
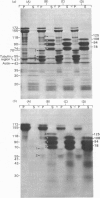
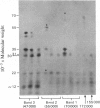
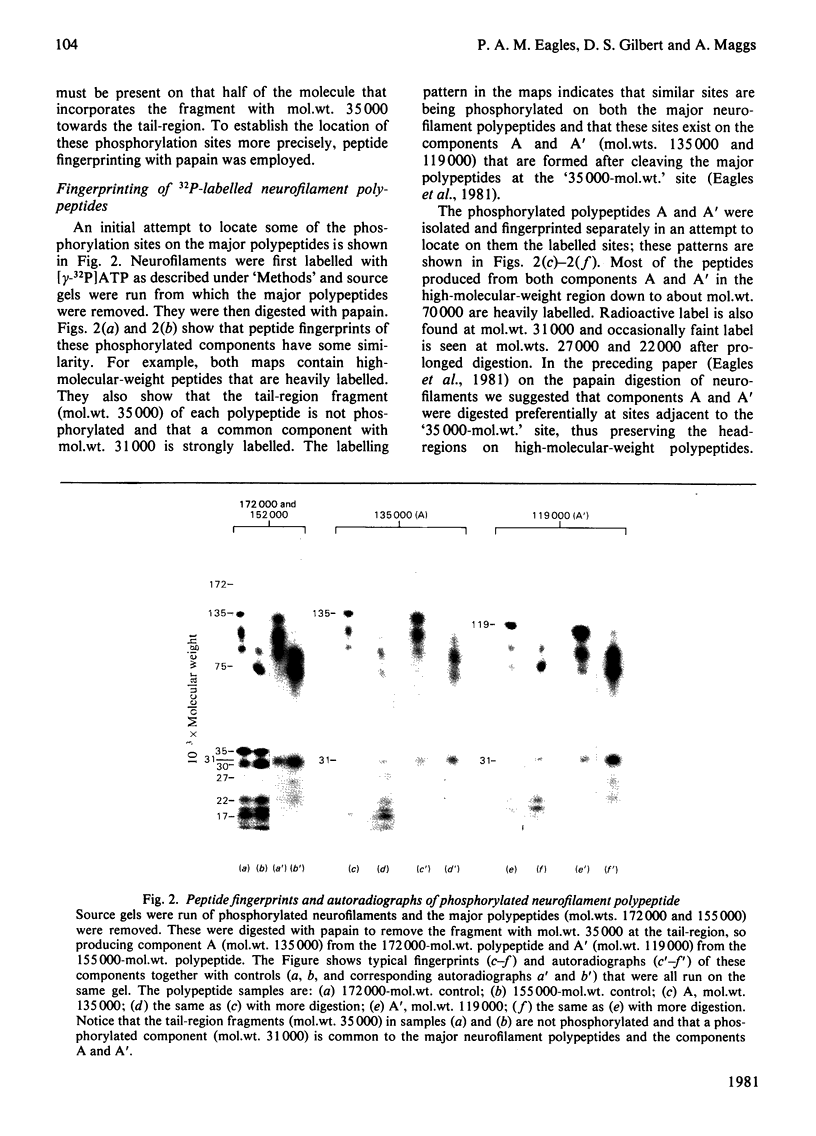
1. When axoplasm is incubated with [32P]Pi the main phosphorylated components are the neurofilament polypeptides. 2. Activation with Ca2+ of the proteinase present in axoplasm causes degradation of these neurofilaments and the peptides produced by this reaction have been analysed by fingerprinting. 3. Fingerprinting shows that initially the Ca2+-activated proteinase cleaves the neurofilament polypeptides at three major sites producing polypeptides with mol.wts. 70,000, 50,000 and 47,000. 4. These polypeptides sediment with filaments, originate from the tail-region of the molecule and contain a little radioactive label. 5. As these polypeptides are produced, other polypeptides that come from the head-region of the molecule are liberated as soluble products that contain the bulk of the radioactivity. 6. Fingerprinting therefore shows that at least two regions on the molecule are phosphorylated and that the major one is located towards the head-end of the polypeptides.
Full text
PDF

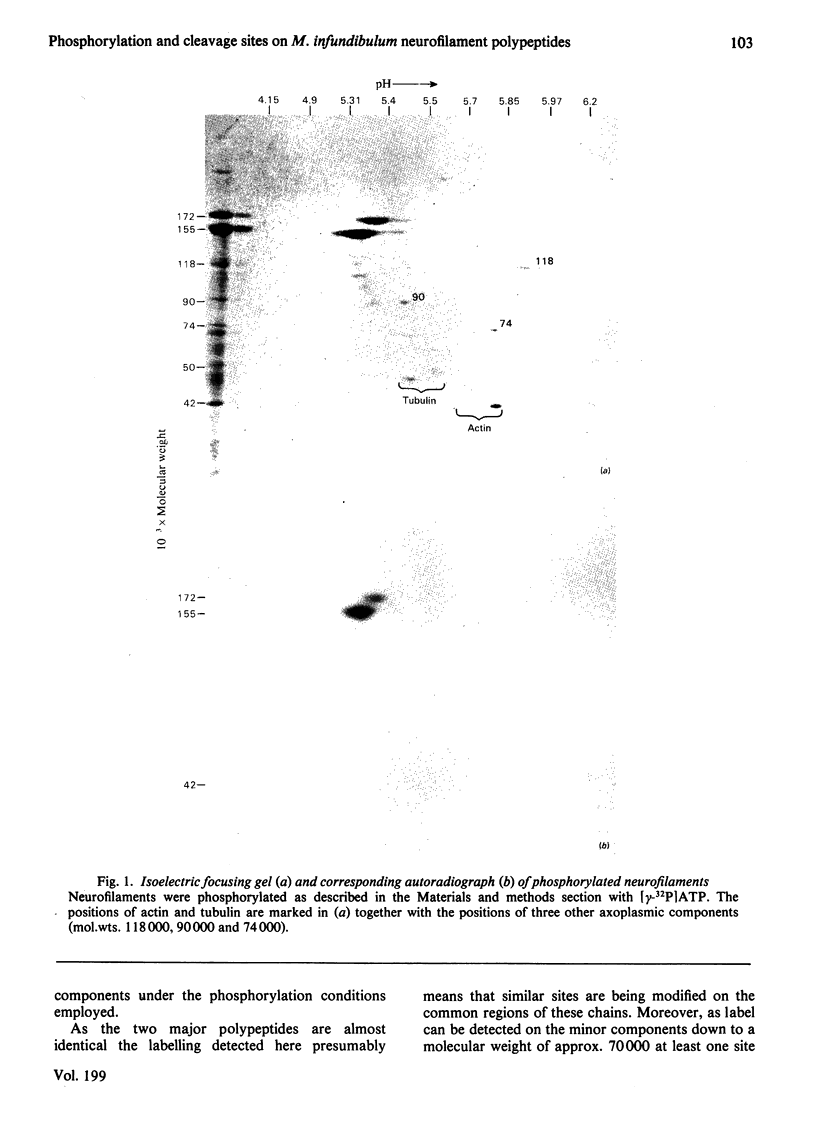

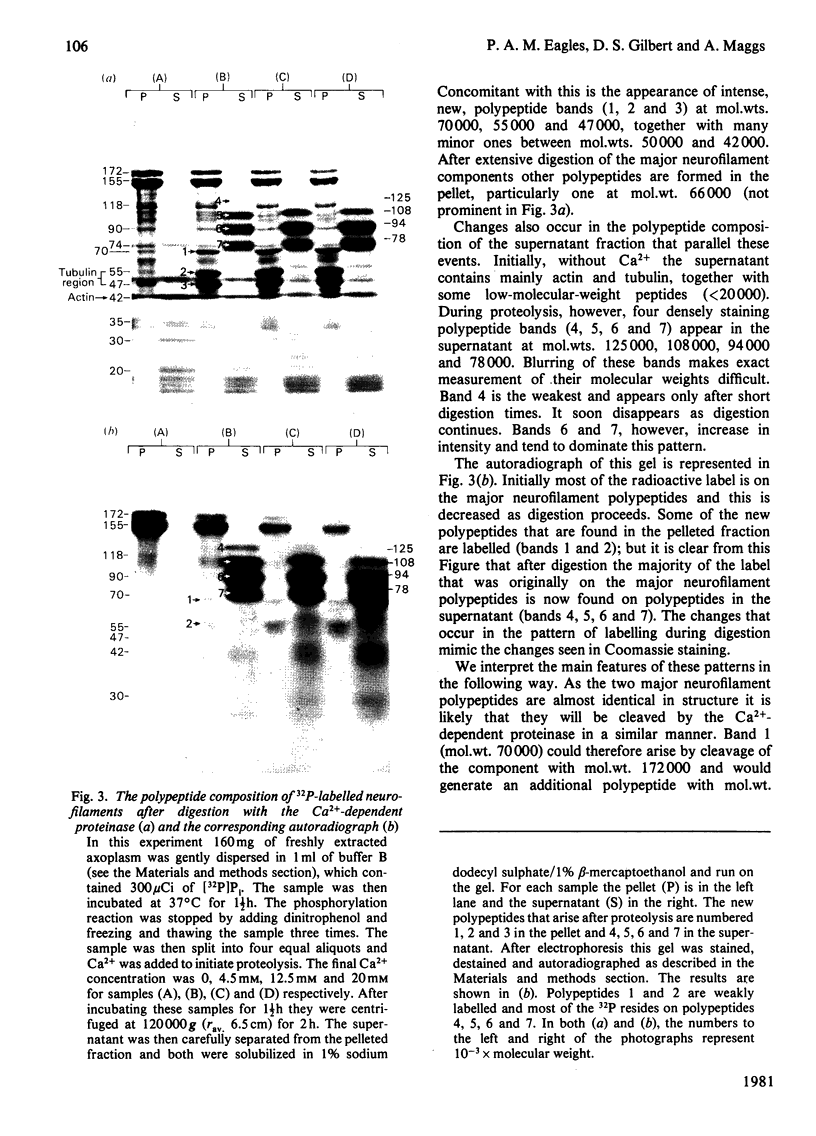
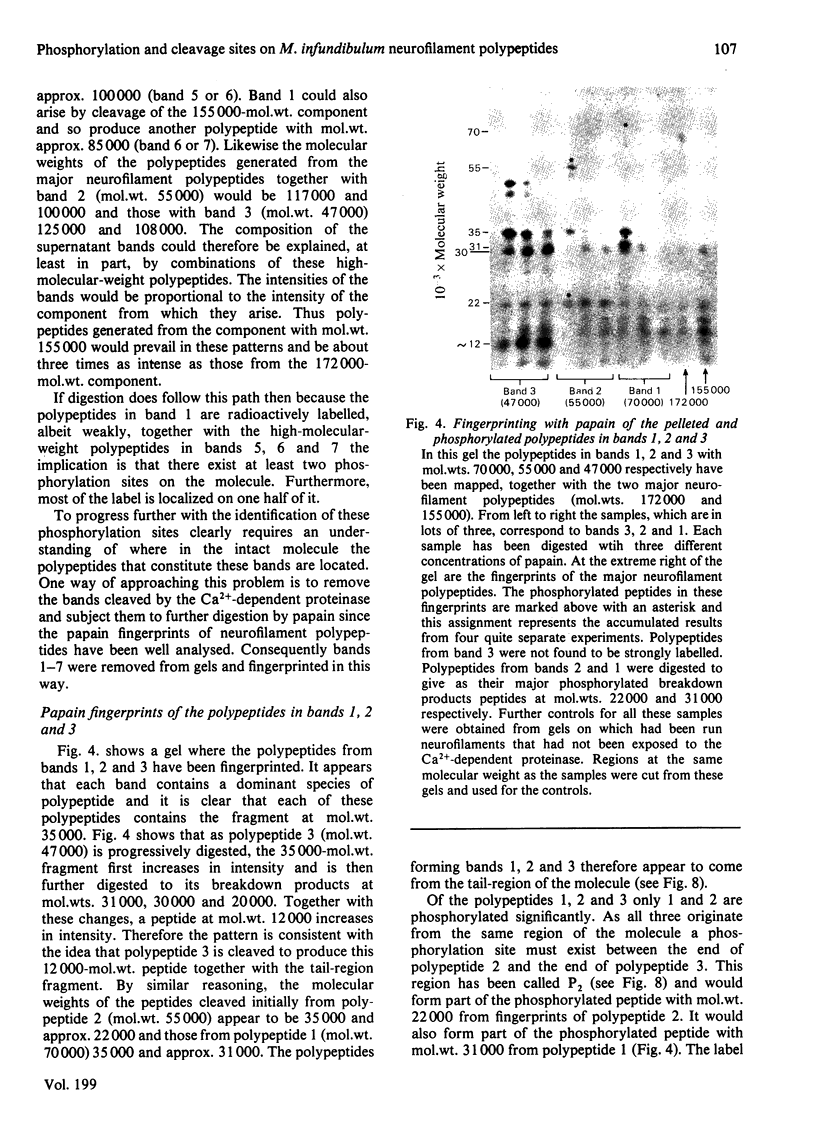
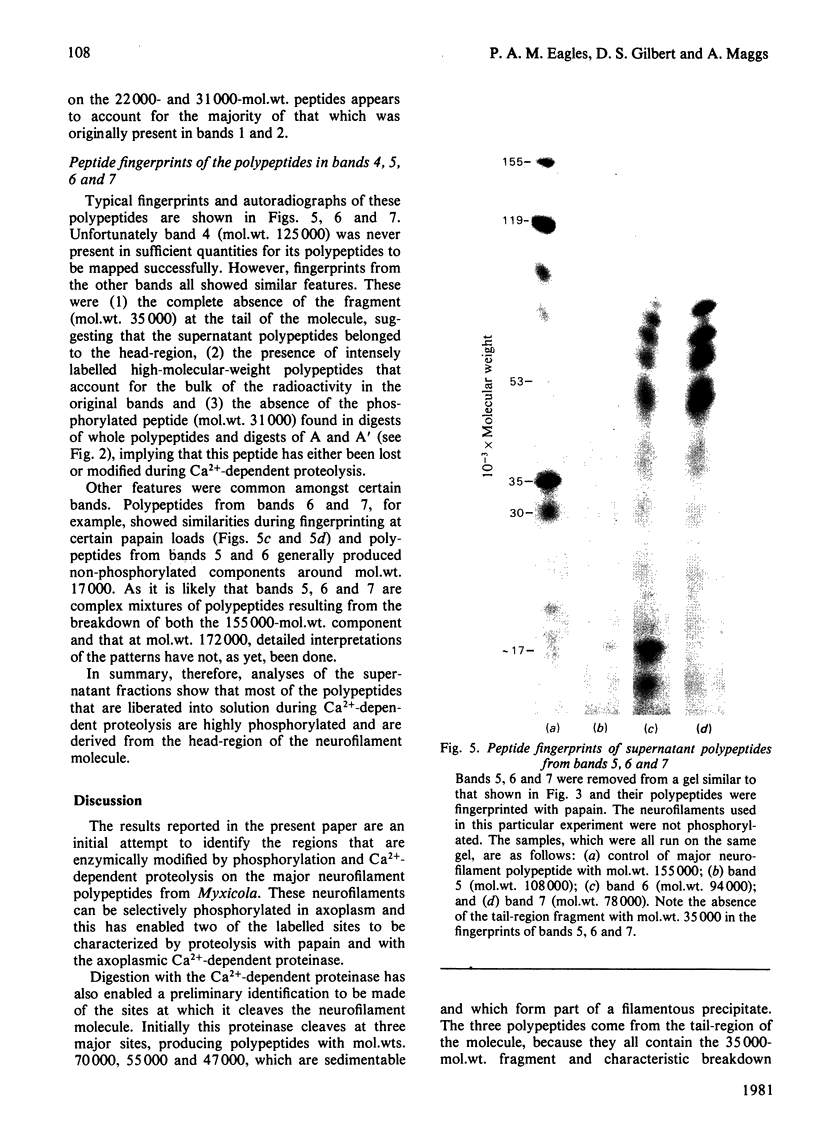
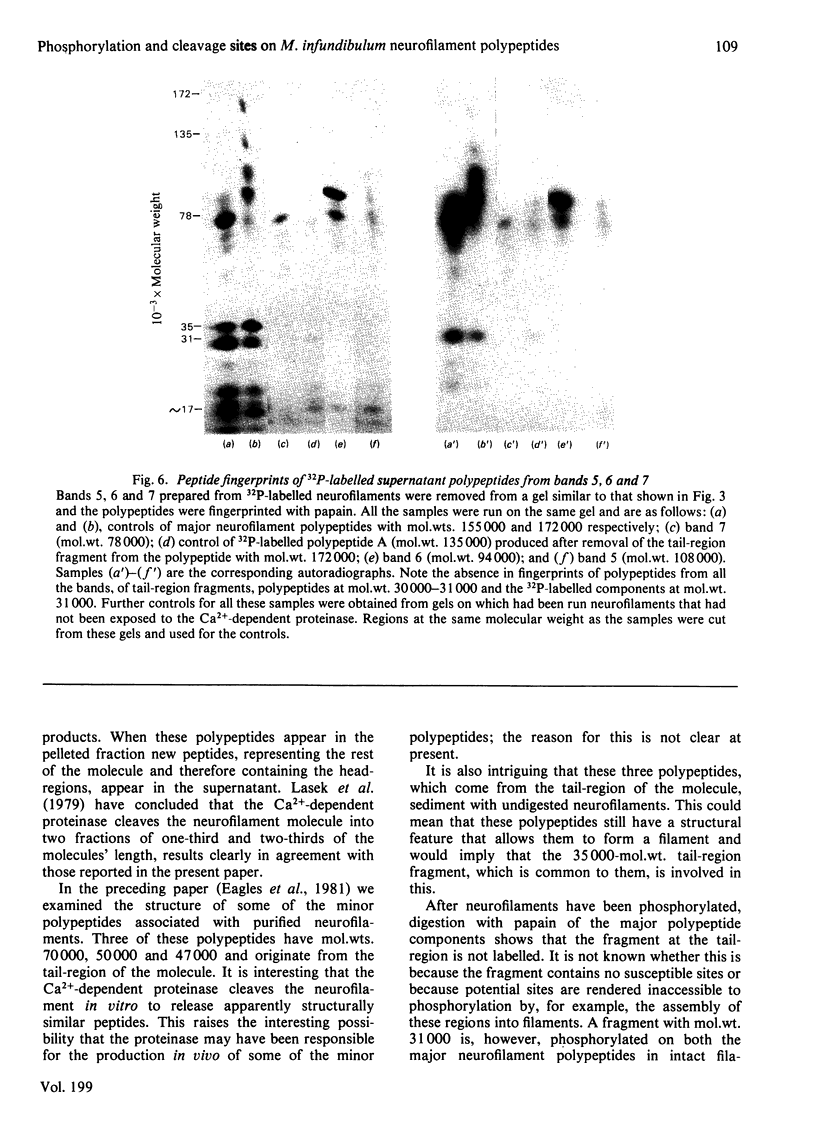
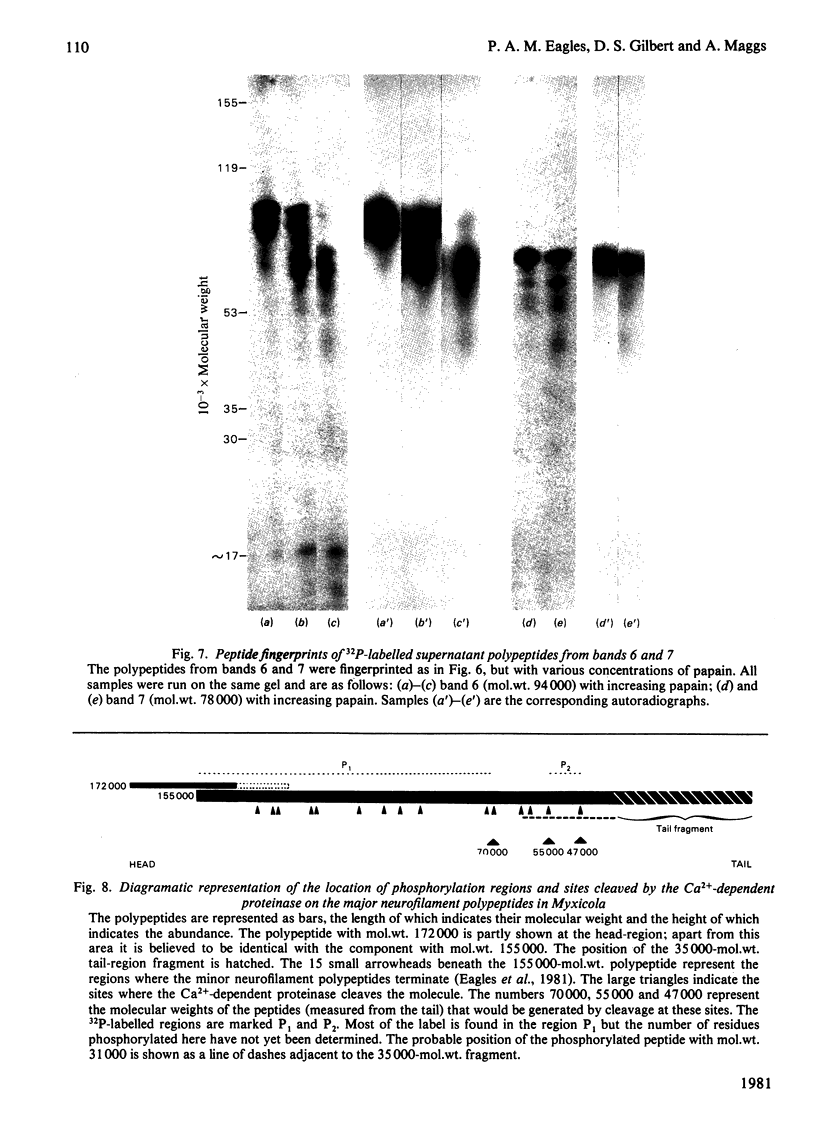

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eagles P. A., Gilbert D. S., Maggs A. Neurofilament structure and enzymic modification. Biochem Soc Trans. 1980 Oct;8(5):484–487. doi: 10.1042/bst0080484. [DOI] [PubMed] [Google Scholar]
- Eagles P. A., Gilbert D. S., Maggs A. The polypeptide composition of axoplasm and of neurofilaments from the marine worm Myxicola infundibulum. Biochem J. 1981 Oct 1;199(1):89–100. doi: 10.1042/bj1990089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D. S., Newby B. J. Neurofilament disguise, destruction and discipline. Nature. 1975 Aug 14;256(5518):586–589. doi: 10.1038/256586a0. [DOI] [PubMed] [Google Scholar]
- Lasek R. J., Krishnan N., Kaiserman-Abramof I. R. Identification of the subunit proteins of 10-nm neurofilaments isolated from axoplasm of squid and Myxicola giant axons. J Cell Biol. 1979 Aug;82(2):336–346. doi: 10.1083/jcb.82.2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pant H. C., Shecket G., Gainer H., Lasek R. J. Neurofilament protein is phosphorylated in the squid giant axon. J Cell Biol. 1978 Aug;78(2):R23–R27. doi: 10.1083/jcb.78.2.r23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runge M. S., el-Maghrabi M. R., Claus T. H., Pilkis S. J., Williams R. C., Jr A MAP-2-stimulated protein kinase activity associated with neurofilaments. Biochemistry. 1981 Jan 6;20(1):175–180. doi: 10.1021/bi00504a029. [DOI] [PubMed] [Google Scholar]
- Schlaepfer W. W., Micko S. Calcium-dependent alterations of neurofilament proteins of rat peripheral nerve. J Neurochem. 1979 Jan;32(1):211–219. doi: 10.1111/j.1471-4159.1979.tb04530.x. [DOI] [PubMed] [Google Scholar]