Abstract
Mouse mammary tumor virus (MMTV) superantigens (vSAgs) can undergo intercellular transfer in vivo and in vitro such that a vSAg can be presented to T cells by major histocompatibility complex (MHC) class II proteins on antigen-presenting cells (APCs) that do not express the superantigen. This process may allow T-cell activation to occur prior to viral infection. Consistent with these findings, vSAg produced by Chinese hamster ovary (CHO) cells was readily transferred to class II IE and IA (H-2k and H-2d) proteins on a B-cell lymphoma or mouse splenocytes. Fixed class II-expressing acceptor cells were used to demonstrate that the vSAg, but not the class II proteins, underwent intercellular transfer, indicating that vSAg binding to class II MHC could occur directly at the cell surface. Intercellular transfer also occurred efficiently to splenocytes from endogenous retrovirus-free mice, indicating that other proviral proteins were not involved. Presentation of vSAg7 produced by a class II-negative, furin protease-deficient CHO variant (FD11) was unsuccessful, indicating that proteolytic processing was a requisite event and that proteolytic activity could not be provided by an endoprotease on the acceptor APC. Furthermore, vSAg presentation was effected using cell-free supernatant from class II-negative, vSAg-positive cells, indicating that a soluble molecule, most likely produced by proteolytic processing, was sufficient to stimulate T cells. Because the membrane-proximal endoproteolytic cleavage site in the vSAg (residues 68 to 71) was not necessary for intercellular transfer, the data support the notion that the carboxy-terminal endoproteolytic cleavage product is an active vSAg moiety.
Activation of T cells by mouse mammary tumor virus (MMTV) superantigens (vSAgs) is essential for viral transmission (for a review see reference 1). This activation is mediated via interaction of the vSAgs with class II major histocompatibility complex (MHC) proteins on antigen-presenting cells (APCs) and the variable region of the β chain of the T-cell receptor. The vSAgs are produced as glycosylated type II integral membrane proteins that require endoproteolytic maturation to activate T cells (11). In CHO cells, proteolytic processing is effected by furin, a member of a family of endoproteases known as protein convertases (PCs) (17), and results in the generation of one or more proteolytic products. An 18-kDa carboxy-terminal proteolytic cleavage product (p18) has been demonstrated to associate on the cell surface of B cells both with an amino-terminal vSAg proteolytic cleavage product and with the class II MHC protein IAk (23, 24). Although similar in function to the well-characterized bacterial superantigens, the vSAgs and the bacterial SAgs bear no genetic resemblance, and the structure of the vSAgs and details of their interactions with the class II MHC proteins have not been resolved.
Another feature of vSAgs is their capacity to undergo intercellular transfer. vSAgs will not stimulate T cells in the absence of class II MHC proteins (2), and vSAg intercellular transfer was first observed in mixed bone marrow reconstituted chimeric mice where the donor cells expressed separately a proviral SAg or an appropriate class II MHC protein (14, 19). Intercellular vSAg transfer was evidenced by the ability of the vSAg expressed in the mixed bone marrow chimeric mice to effect intrathymic deletion of reactive T cells. Evidence that vSAg-expressing CD8 T cells, which do not express class II proteins, could induce T-cell deletion in vivo also provided evidence for intercellular transfer (21). Intercellular transfer has also been demonstrated to occur in vitro by coculture of vSAg-reactive T cells with mixtures of independently transfected vSAg and class II MHC-expressing APCs (4). Intercellular transfer of the vSAgs may act during infection by exogenous virus to allow activation of T cells independent of or prior to infection of B cells and/or other APCs.
The vSAgs are integral membrane proteins and therefore unlikely to undergo intercellular transfer in the absence of posttranslational processing that would eliminate membrane tethering. An appealing hypothesis has been that vSAg intercellular transfer is facilitated by the proteolytic generation of a soluble vSAg protein (e.g., p18) (24, 25). Evidence for intercellular transfer of such a moiety would indicate that the regions necessary for interaction of the vSAg with the MHC proteins and the T-cell receptor reside therein. Here, we both confirm and extend a previous study that has demonstrated intercellular transfer of a soluble vSAg in vitro. We provide a formal demonstration that the vSAg, but not class II MHC, undergoes intercellular transfer, and that vSAg binding to class II MHC occurs at the cell surface. It is also shown that intercellular transfer requires furin-dependent proteolytic processing. The data suggest that all regions required for superantigen activity, including the interaction with both the class II MHC proteins and the T-cell receptor, reside on a vSAg carboxy-terminal proteolytic fragment.
MATERIALS AND METHODS
Animals.
Inbred mice used as a source of splenocytes were bred at the Wadsworth Center, NYS Department of Health, under institutional guidelines for animal care and use. The MMTV-negative mice (16) were obtained from the laboratory of Philippa Marrack and John Kappler at the Howard Hughes Medical Institute, Denver, Colo.
Cell lines and APCs.
All cells were cultured in complete tumor medium as described previously (11). Transfection, drug selection, T-cell stimulation assays, and flow cytometry were also performed as described previously (11). All of the T-cell hybridomas and most APCs used in this study have been described previously (11). The CHO/S7 and FD/S7 vSAg7 transfectants were generated by transfection of CHO and FD11 cells, respectively, with the vSAg7 expression plasmid pSRαSAg7, as described previously (11). Splenocytes were obtained after passage of tissue through a 100-μm nylon mesh, and erythrocytes were removed using 150 mM ammonium chloride–0.73 mM potassium phosphate. T cells were depleted using the anti-Thy1.2 antibody HO13.4 and rabbit complement; the remaining splenocytes were washed with Hanks balanced salt solution (HBSS) and then treated with mitomycin C at a final concentration of 25 μg/ml in HBSS. Cells were fixed by incubation in 0.05% glutaraldehyde in HBSS for 30 s, 1 volume of 0.2 M lysine in HBSS was added immediately, and the cells were washed in HBSS.
IL-2 assays.
Interleukin-2 (IL-2) production from T-cell hybridomas was measured using the IL-2 dependent cell line HT-2. HT-2 cell viability was quantitated by [3H]thymidine incorporation as described previously (22). All assays were performed in triplicate. The data were analyzed for statistical significance as described previously (22).
Supernatant transfer.
CHO/S7 cells were harvested using trypsin-EDTA (0.25% trypsin and 1 mM EDTA in HBSS), washed with HBSS, and incubated in complete tumor medium (11) at a concentration of 0.5 × 107 to 1.0 × 107/ml at 37° for 2 h. The cells were centrifuged at 1,000 × g, and the supernatant was removed and passed through a 0.2-μm-pore-size filter prior to use in the assays.
RESULTS
Intercellular transfer of vSAg7.
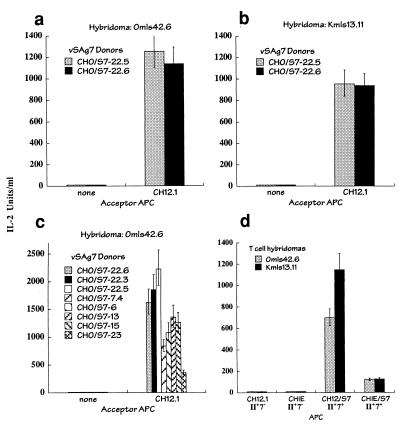
To assess superantigen intercellular transfer, vSAg7 (derived from the MMTV-7 provirus) was expressed in class II-negative CHO (CHO/S7) cells. The vSAg7 transfectants (vSAg donor cells) were incubated with the class II-positive B-cell lymphoma CH12.1 (acceptor APC) and the vSAg7-reactive T-cell hybridomas Omls42.6 and Kmls13.11 (responder cells; both Vβ6 [11]). The T cells responded readily to vSAg7 presented in the three-cell culture but did not respond to the vSAg7 transfectants in the absence of the class II-positive acceptor APCs (Fig. 1). Although vSAg7 is expressed at relatively low levels on the CHO transfectants (Fig. 2a), intercellular transfer was nevertheless quite efficient, as IL-2 production engendered in the three-cell culture often approached that obtained when the vSAg was produced in the class II-expressing APCs (referred to here as endogenous presentation) (Fig. 1d). However, the transferred vSAg7 was not detected on the acceptor cells by flow cytometry (data not shown), which indicated that very low levels of vSAg could nevertheless promote strong T-cell responses.
FIG. 1.
Intercellular transfer of vSAg7. (a and b) T-cell recognition of vSAg7 after intracellular transfer. The T-cell hybridomas Omls42.6 (a) and Kmls13.11 (b) (both Vβ6), were incubated with class II-negative, vSAg7-positive CHO cells (CHO/S7; vSAg7 donors) alone (none) or with the class II-positive, vSAg7-negative murine B-cell lymphoma CH12.1 (acceptor APC). T-cell responsiveness was indicated by IL-2 production, as determined by measurement of [3H]thymidine incorporation by HT-2 cells. (c) Intercellular transfer to CH12.1 of vSAg7 produced by several independently isolated CHO cell vSAg7 transfectants. (d) T-cell recognition of endogenously expressed vSAg presented by class II-expressing APCs. vSAg7 was expressed in CH12.1 (CH12/S7) and class II-positive CHO (CHIE/S7) cells and was used to stimulate the indicated T-cell hybridomas. CH12.1 and CHIE are the vSAg7-negative parent cells. Class II (II) and vSAg7 (7) expression on the cell lines is indicated. The error bars indicate upper and lower 90% confidence intervals obtained from IL-2 assays performed in triplicate.
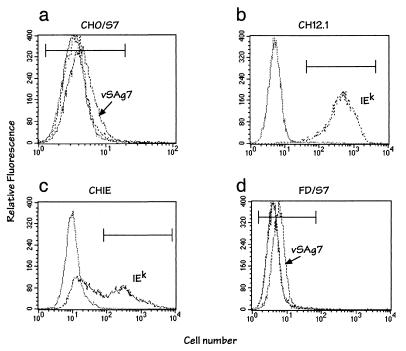
FIG. 2.
Surface expression of vSAg7 and class II MHC proteins. Cell lines were analyzed by flow cytometry for vSAg7 (dashed lines) and/or class II IEk (dotted lines). Background fluorescence obtained using the control secondary reagent (phycoerythrin-streptavidin) is indicated (solid lines). vSAg7 and IEk expression was detected using biotinylated VS7 and fluorescein isothiocyanate-conjugated 14-4-4, respectively, as described previously (11). FD/S7 is a furin-deficient CHO cell variant (6) that was transfected with vSAg7. Mean channel fluorescence values of the cells within the markers on the histogram were as follows: CHO/S7 (clone 22.6) control, 4.01; VS7, 4.97; 14-4-4, 3.98; CH12.1 control, 7.11; 14-4-4, 589; CHIE (clone 13.2) control, 11.05; 14-4-4, 565; FD/S7 (clone 2.1) control, 4.68; VS7, 7.85; 14-4-4, 6.45. vSAg7 expression was not observed on CHIE or CH12.1 cells (data not shown). The partial loss of class II expression observed in the CHIE cells (c) was not characteristic of this cell line.
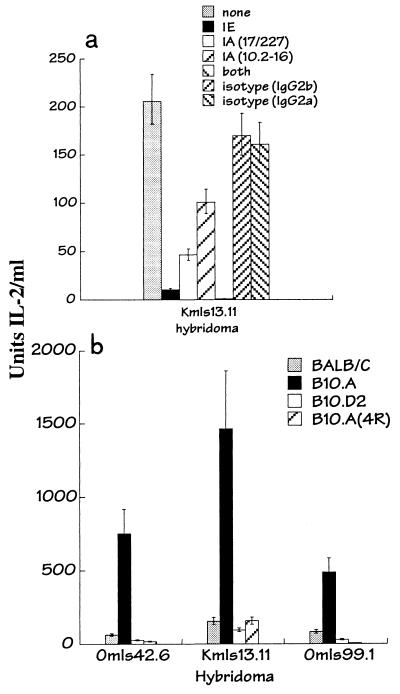
Presentation of the transferred vSAg7 to T cells was class II MHC dependent, because antibodies directed at either IEk or IAk expressed on the CH12.1 acceptor cells inhibited T-cell responses (Fig. 3a). Inhibition of transfer was more effective using anti-IEk antibodies, consistent with previous observations that IEk is a better presenter of vSAgs than IAk (10). Incubation with both IE and IA antibodies completely blocked presentation of the transferred vSAg to T cells.
FIG. 3.
Presentation of transferred vSAg7 by class II MHC. (a) IL-2 production by T-cell hybridomas was measured after culture with the vSAg7 donor cell line CHO/S7 and the acceptor APC CH12.1 without antibodies (none) or in the presence of the following antibodies, as indicated: anti-class II IEk (14-4-4), anti-class II IAk (17/227 or 10.2-16), both anti-IE and anti-IA (10.2.16), or isotype-matched control antibodies (immunoglobulins G2a and G2b). (b) T-cell hybridomas were incubated with the vSAg7 donor cell CHO/S7 and splenocytes obtained from the following mouse strains: BALB/c (IAd IEd), (B10.A (IAk IEk), and B10.D2 (IAd IEd) B10.A(4R) (IAk). IL-2 production was determined as for Fig. 1.
To further evaluate the role of class II MHC during intercellular transfer and to test the capacity of normal APCs to accept vSAg7 transfer, the vSAg7 donor cells were incubated with vSAg7-negative, class II-positive spleen cells. Splenocytes from BALB/c (H-2d), B10.A (H-2k), B10.D2 (H-2d), and the MHC recombinant strain B10.A(4R) (which expresses only IAk) all presented the transferred vSAg (Fig. 3b). Presentation by IEk was significantly better than that by both IAk [Fig. 3b, compare B10.A with B10.A(4R)] and class II H-2d. These findings recapitulate the hierarchy of class II presentation of endogenously expressed vSAg7 by class II MHC (10, 14) and demonstrate that normal APCs can present the transferred vSAg in vitro.
vSAg, but not class II IEk, underwent intercellular transfer.
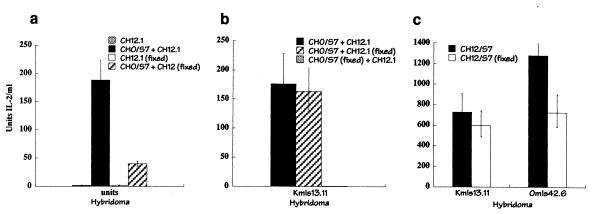
Previous in vivo and in vitro studies did not eliminate the formal possibility that the vSAg responses resulted from transfer of class II MHC proteins from the class II-expressing cells to the vSAg-expressing cells. To address this possibility, experiments were performed using vSAg7- or class II-presenting cells that had been fixed with glutaraldehyde. Significant vSAg intercellular transfer was observed when fixed class II-positive CH12 cells were incubated with nonfixed vSAg7-expressing cells (Fig. 4). In contrast, fixation of the vSAg-expressing cells did not allow presentation by nonfixed CH12 cells (Fig. 4b). Presentation of vSAg7 endogenously produced in CH12 cells was largely unaffected by fixation (Fig. 4c). These experiments demonstrated that the vSAg, but not the class II protein, underwent intercellular transfer and that class II MHC binding occurred directly at the cell surface of the APC.
FIG. 4.
Fixed cells presented both transferred and endogenous vSAg7. The T-cell hybridomas Omls42.6 (a) and Kmls13.11 (b) were incubated with combinations of untreated or glutaraldehyde-treated CHO/S7 donor cells and CH12.1 APCs, as indicated, and IL-2 production was measured. The cells were fixed, where indicated, with 0.05% glutaraldehyde. (c) T cells were incubated with fixed or nonfixed vSAg7 class II-positive CH12/S7 cells to measure endogenous vSAg7 presentation.
Intercellular transfer did not require other MMTV proteins.
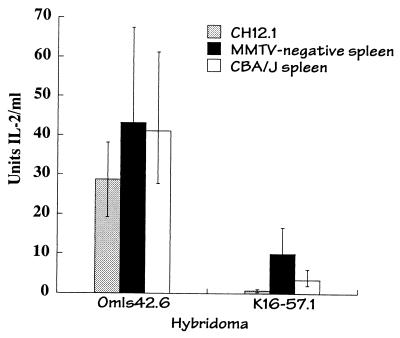
Although transfer was readily observed with CH12.1 as the acceptor cell, transfer was not observed when the class II-positive CHO transfectant CHIE was used as an acceptor cell (data not shown). To address whether this may have reflected a requirement for other provirus-encoded MMTV proteins to facilitate intracellular transfer, experiments were performed using splenocytes obtained from a mammary tumor provirus-free mouse strain (16). Intercellular transfer of vSAg7 to the MMTV provirus free splenocytes was observed (Fig. 5), indicating that additional MMTV proteins were not required for vSAg intercellular transfer. Similar levels of T-cell stimulation were detected using splenocytes obtained from the genetically related strain CBA/J (H-2k), which express endogenous vSAg7, suggesting that presentation of endogenous and transferred vSAg7 by the splenocytes was equally efficient. Further studies suggested that the failure of the CHIE cells to present the transferred vSAg was due to an overall lower efficiency of T-cell activation (data not shown).
FIG. 5.
Other MMTV proteins were not required to facilitate vSAg7 transfer. Presentation of vSAg7 (produced in CHO/S7 cells) by CH12.1 or by splenocytes obtained from an MMTV endogenous provirus negative mouse (MMTV negative, H-2k [16]) was measured. Endogenous presentation of vSAg7 to T cells by splenocytes obtained from an Mtv-7 provirus-positive H-2k mouse (CBA/J) is also shown. K16.57.1 is a vSAg7-responsive Vβ8.1 T-cell hybridoma. The overall lower levels of IL-2 production observed in this and some other experiments was likely due to variability in T-cell hybridoma responses.
Furin-dependent proteolytic processing was required for vSAg presentation.
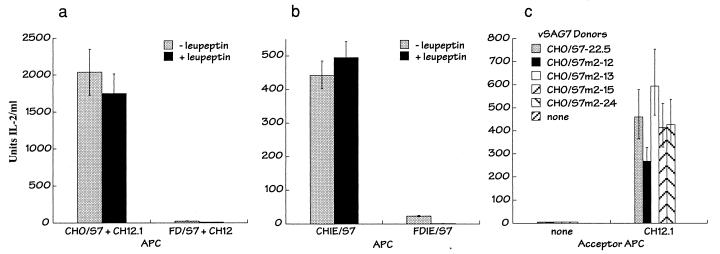
The PC furin has been demonstrated to mediate endoproteolytic processing of vSAgs in vitro and in vivo (13, 11), and furin-dependent processing is required for T-cell activation of vSAg7 in CHO cells (11). To determine if furin was also required for transfer and/or presentation of the transferred vSAg, vSAg7 was expressed, in the absence of class II, in the furin-deficient CHO cell line FD11 (FD/S7) (Fig. 2) (6). Activation of T cells upon transfer of vSAg from the furin-deficient cells was approximately 80-fold lower than that obtained using the furin-positive CHO cells (Fig. 6a). Moreover, treatment of the furin-deficient cells with leupeptin, which has previously been shown to abrogate the residual presentation of vSAg7 by the furin-deficient class II-positive transfectant FDIE/S7, completely blocked the activity of the transferred vSAg from the furin-deficient class II-negative cells (Fig. 6a and b). Thus, furin-dependent proteolytic processing was a requisite step in vSAg7 transfer from CHO donor cells.
FIG. 6.
Intercellular transfer required donor cell proteolytic processing. (a) IL-2 production from the T-cell hybridoma Omls42.6 after incubation with the acceptor APC CH12.1 and either the furin-positive, vSAg7-positive donor cell line CHO/S7 or the furin-negative, vSAg7-positive donor cell line FD/S7, in the absence or presence of the protease inhibitor leupeptin. Leupeptin has been shown previously to abrogate the residual vSAg7 presentation observed using furin-deficient APCs (11). IL-2 production observed using FD/S7 in the presence of leupeptin was at background levels. No IL-2 production was observed using the vSAg7 donor cell line FD/S7 in the absence of a class II-positive acceptor APC (not shown). (b) Endogenous vSAg7 presentation by the class II-positive vSAg7-expressing cell lines CHIE/S7 (furin positive) and FDIE/S7 (furin negative). The APCs were incubated with the T-cell hybridoma Omls42.6 in the presence of absence of leupeptin, as indicated. (c) The furin endoproteolytic cleavage site at positions 68 to 71 in vSAg7 was not required for intercellular transfer. Wild-type vSAg7 donor cells or CHO transfectants that expressed vSAg7 mutant proteins that lacked a PC recognition site at positions 68 to 71 (vSAg7m2 [22]) were incubated with the hybridoma Omls42.6 alone (none) or with the acceptor APC CH12.1, and IL-2 production was measured.
Proteolytic processing of vSAg7 at positions 168 to 171 was shown to be required for vSAg activity when expressed in CHO cells (22). In contrast, furin processing at the conserved membrane-proximal cleavage site in vSAg7 (positions 68 to 71) was found to be inessential for activation of T cells by class II-positive APCs (22). Because the furin recognition site at positions 68 to 71 is, with one exception, conserved in all known vSAgs (23), it was considered that proteolytic processing at this position might be required for intercellular transfer, even though it was not required for endogenous presentation. To test this possibility, a previously described vSAg7 variant, vSAg7m2 (22), which lacks the PC processing site at positions 68 to 71, was expressed in class II-negative CHO cells and examined for its ability to undergo intercellular transfer. Four independent vSAg7m2 transfectants readily mediated vSAg7 transfer in vitro (Fig. 6), indicating that processing at this position was not required for intercellular transfer. Similar studies showed that the dibasic residues at positions 193 to 194 in vSAg7 were also not required for transfer (data not shown). The data from Fig. 6 therefore suggest that proteolytic processing at the furin recognition site at positions 168 to 171, but not at positions 68 to 71, was required for intercellular transfer.
Transfer of a soluble vSAg.
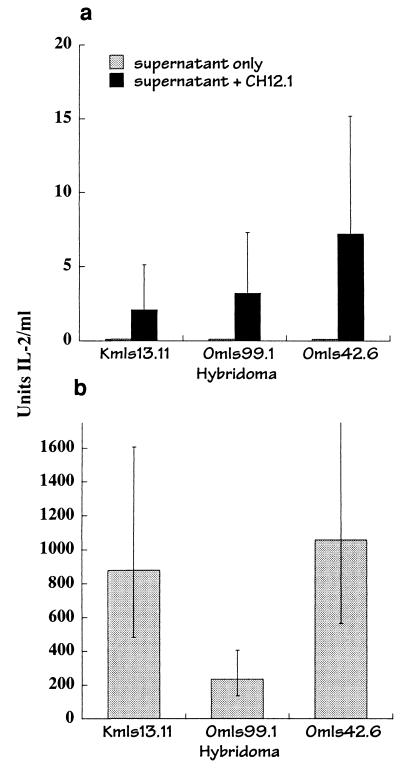
Although reported previously (4), in our hands transfer was not observed when the vSAg7 donor and class II-expressing acceptor cells were separated by a semipermeable membrane (data not shown). It is possible that a relatively high local concentration of the vSAg might be required to observe intercellular transfer, and this was not readily achieved under our conditions. To further explore the possibility that a soluble vSAg underwent transfer, supernatant was obtained after culture of 0.5 × 107 to 1.0 × 107 CHO/S7 cells/ml in medium for 2 to 4 h, and the supernatant was filtered through a cell-impermeable membrane and tested for its capacity to stimulate IL-2 production from T-cell hybridomas in the presence of CH12 acceptor cells. Detectable T-cell activation was observed upon transfer of supernatant from the vSAg7-expressing cells (Fig. 7a), although levels of IL-2 production were much lower than those observed in the coculture experiments (Fig. 7b). Attempts to biochemically characterize the transferred vSAg have not yet been successful. These data nevertheless provide clear evidence that a soluble superantigen was transferred from the vSAg donor cells.
FIG. 7.
Transfer of a soluble superantigen. (a) Supernatant was obtained after culture of CHO/S7 cells in medium for 2 h, followed by filtration through a 0.2-μm-pore-size filter. The supernatant was added at 2 × to 4 × 106 cell equivalents/ml to the T-cell hybridomas alone (supernatant only) or to the T-cell hybridomas and the acceptor APC CH12.1. IL-2 production was determined as described for Fig. 1. (b) Responses of the hybridomas used in for panel a, demonstrating transfer of vSAg7 from CHO/S7 to CH12.1 cells after coculture.
DISCUSSION
Although the vSAgs are synthesized as membrane-bound glycoproteins, this study demonstrates that a functional form of the vSAg can undergo intercellular transfer in vitro and thus confirms and extends the previous in vivo and in vitro studies that demonstrated vSAg intercellular transfer (4, 14, 19). In our studies, intercellular transfer occurred readily from vSAg7-expressing CHO cells to the B-cell lymphoma cells, and to normal spleen cells, and presentation of the transferred vSAg to T cells was inhibited, as expected, by MHC class II antibodies. Although the transferred vSAg was not detectable on the cell surface of the acceptor APCs, presentation to T cells was nevertheless quite efficient, because levels of IL-2 production in some cases approached that obtained when the vSAg was expressed endogenously. These data suggest that relatively few vSAg molecules can stimulate a strong T-cell response, much like that observed for conventional peptide antigens, where as few as 100 peptide molecules are sufficient for T-cell activation (8). The efficiency of intercellular transfer also suggests that vSAg intercellular transfer may be a common or even requisite event during viral infection and concomitant T-cell activation.
In the previous in vitro and in vivo studies, the possibility that intercellular transfer was due to transfer not of the vSAg but of class II proteins was not ruled out. This was addressed in the present studies using cell fixation. Glutaraldehyde-fixed APCs were capable of presenting to T cells vSAg7 that had been produced by unfixed vSAg7 donor cells. Moreover, vSAg intercellular transfer did not occur when the vSAg-expressing cells were fixed. Because transfer of class II proteins or vSAgs was unlikely to occur from fixed cells, the data indicate that the vSAg protein was the transferred moiety. Moreover, the presentation of vSAg7 by fixed APCs indicated that vSAg association with the class II proteins occurred at the cell surface. Thus, vSAg7-class II binding did not require endocytosis and presentation via the conventional class II antigen presentation pathway (3), and so this association did not require accessory molecules, such as H-2 DM, that are typically required for MHC presentation of conventional peptide ligands (12).
No differences were observed in the hierarchy of class II MHC presentation when vSAg7 was expressed endogenously or upon intercellular transfer. These data suggest that the mode of class II binding of the transferred vSAg is similar or identical to that of endogenously expressed vSAgs, and they demonstrate that vSAgs can bind to stable class II MHC proteins, as has been suggested previously (7).
vSAgs are detected on the cell surface of APCs in a processed form (25), and so it is likely that a proteolytic fragment of the vSAg undergoes intercellular transfer. Furin-deficient CHO cells, which do not express detectable processed vSAg7 (11), were poor vSAg7 donors, and when transfer experiments were performed in the presence of the protease inhibitor leupeptin, the residual transfer and/or presentation of vSAg7 by these cells was completely abolished. These findings suggest that proteolytic processing is required for intercellular transfer. However, one cannot rule out that in the absence of processing the vSAg undergoes intercellular transfer, but the transferred vSAg is not in a form that can be presented to T cells. However, the failure to observe presentation of the unprocessed vSAg indicates that furin or other PCs known to be present on the surface of the acceptor cells (18) were incapable of effecting proteolytic activation. The data are thus consistent with the interpretation that intercellular transfer first requires that the vSAg be proteolytically processed.
Furin-dependent processing of vSAg7 in CHO cells has been observed to occur at or near two consensus furin recognition sites (residues 68 to 71 and 168 to 171). Proteolytic processing at the former site was unnecessary, because a mutant vSAg7 that lacked a furin recognition motif at positions 68 to 71 (22) underwent intracellular transfer efficiently. These data, along with the apparent requirement for furin-dependent processing, suggest that the active vSAg is a carboxyl-terminal proteolytic fragment. This interpretation suggests that all of the sites necessary for interaction of the vSAg with both the class II protein and the T-cell receptor are encoded on a carboxy-terminal vSAg proteolytic fragment (residues 171 to 321). It is possible that the vSAg amino-terminal proteolytic processing product may serve to facilitate intracellular transport of the active vSAg or to perform yet uncharacterized roles in viral pathogenesis. Thus, the vSAgs, although produced as integral membrane proteins, may function in their active form in a manner equivalent to the bacterial superantigens, which are produced as small soluble proteins that freely associate with APCs in vivo.
vSAg7 activity could be transferred to class II-positive acceptor cells using cell-free supernatant from vSAg7 donor cells, indicating that cell-to-cell contact was not required for transfer. Although T-cell stimulation was relatively inefficient, these experiments used vSAg obtained after only 2 h of culture and are therefore not directly comparable to those performed under conditions of continuous culture. These findings are consistent, however, with the previous study that demonstrated inefficient vSAg transfer when donor and acceptor cells were separated by a cell-impermeable membrane (4). The inefficiency may be due to the apparent instability of the vSAgs (9). This instability may act to limit the transfer of the vSAg during viral infection to only closely associated APCs.
It is possible that vSAg intercellular transfer is an important facet of MMTV infection. Retroviruses typically require cycling cells for productive infection (15, 26), and so the MMTV may facilitate transfer of the vSAg to noninfected resting B cells. In this model, uninfected B cells that presented transferred vSAgs, upon activation by T cells, would become targets for infection. However, in one study, activation of resting B cells in vivo with lipopolysaccharide did not enhance infection by MMTVs (5), suggesting that resting, not activated, B cells were targets of viral infection. Alternatively, vSAg intercellular transfer may contribute to pathogenesis during viral infection of class II-negative cells such as T lymphocytes (20).
ACKNOWLEDGMENTS
We thank Donal Murphy and William Lee for critical reviews of the manuscript and for the B10 congenic mice, and we thank the Wadsworth Center Immunology Core Facility and the Computational Molecular Biology and Statistics Core Facility.
This work was supported by Public Health Service grant CA69710-02.
REFERENCES
- 1.Beutner U, McLellan B, Kraus E, Huber B T. Lack of MMTV superantigen presentation in MHC class II-deficient mice. Cell Immunol. 1996;168:141–147. doi: 10.1006/cimm.1996.0060. [DOI] [PubMed] [Google Scholar]
- 2.Cresswell P. Assembly, transport, and function of MHC class II molecules. Annu Rev Immunol. 1995;12:259–293. doi: 10.1146/annurev.iy.12.040194.001355. [DOI] [PubMed] [Google Scholar]
- 3.Delcourt M, Thibodeau J, Denis F, Sekaly R-P. Paracrine transfer of mouse mammary tumor virus superantigen. J Exp Med. 1997;185:471–480. doi: 10.1084/jem.185.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finke D, Mortezavi L, Acha-Orbea H. Preactivation of B lymphocytes does not enhance mouse mammary tumor virus infection. J Virol. 1998;72:7688–7691. doi: 10.1128/jvi.72.9.7688-7691.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon V M, Klimpel K R, Arora N, Henderson M A, Leppla S H. Proteolytic activation of bacterial toxins by eukaryotic cells is performed by furin and by additional cellular proteases. Infect Immun. 1995;63:82–87. doi: 10.1128/iai.63.1.82-87.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grigg M E, McMahon C W, Morkowski S, Rudensky A Y, Pullen A M. Mtv-1 superantigen trafficks independently of major histocompatibility complex class II directly to the B-cell surface by the exocytic pathway. J Virol. 1998;72:2577–2588. doi: 10.1128/jvi.72.4.2577-2588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harding C V, Unanue E R. Quantitation of antigen-presenting cell MHC class II/peptide complexes necessary for T-cell stimulation. Nature. 1990;346:574–576. doi: 10.1038/346574a0. [DOI] [PubMed] [Google Scholar]
- 8.Krummenacher C, Diggelmann H. The mouse mammary tumor virus long terminal repeat encodes a 47kDa glycoprotein with a short half-life in mammalian cells. Mol Immunol. 1993;30:1151–1157. doi: 10.1016/0161-5890(93)90133-v. [DOI] [PubMed] [Google Scholar]
- 9.MacDonald H R, Glasebrook A L, Schneider R, Lees R K, Pircher H, Pedrazzini T, Kanagawa O, Nicolas J F, Howe R C, Zinkernagel R M, Hengartner H. T-cell reactivity and tolerance to Mlsa-encoded antigens. Immunol Rev. 1989;107:89–108. doi: 10.1111/j.1600-065x.1989.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 10.Mix D, Winslow G M. Proteolytic processing activates a viral superantigen. J Exp Med. 1996;184:1549–1554. doi: 10.1084/jem.184.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris P, Shaman J, Attaya M, Amaya M, Goodman S, Bergman C, Monaco J J, Mellins E. An essential role for HLA-DM in antigen presentation by class II major histocompatibility molecules. Nature. 1994;368:551–554. doi: 10.1038/368551a0. [DOI] [PubMed] [Google Scholar]
- 12.Park C G, Jung M-Y, Choi Y, Winslow G M. Proteolytic processing is required for viral superantigen activity. J Exp Med. 1994;181:1899–1904. doi: 10.1084/jem.181.5.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pullen A M, Marrack P, Kappler J W. The T-cell repertoire is heavily influenced by tolerance to polymorphic self-antigens. Nature. 1988;335:796–801. doi: 10.1038/335796a0. [DOI] [PubMed] [Google Scholar]
- 14.Roe T, Reynolds T, Yu G, Brown P O. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 1993;12:2099–2108. doi: 10.1002/j.1460-2075.1993.tb05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scherer M T, Ignatowicz L, Pullen A, Kappler J, Marrack P. The use of mammary tumor virus (Mtv)-negative and single-Mtv mice to evaluate the effects of endogenous viral superantigens on the T cell repertoire. J Exp Med. 1995;182:1493–1504. doi: 10.1084/jem.182.5.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seidah N G, Chrétien M. Eukaryotic protein processing: endoproteolysis of precursor proteins. Curr Opin Biotechnol. 1997;8:602–607. doi: 10.1016/s0958-1669(97)80036-5. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro J, Sciaky N, Lee J, Bosshart H, Angeletti R H. Localization of endogenous furin in cultured cell lines. J Histochem Cytochem. 1997;45:3–12. doi: 10.1177/002215549704500102. [DOI] [PubMed] [Google Scholar]
- 18.Speiser D E, Schneider R, Hengartner H, MacDonald H R, Zinkernagel R M. Clonal deletion of self-reactive T cells in irradiation bone marrow chimeras and neonatally tolerant mice. Evidence for intercellular transfer of Mlsa. J Exp Med. 1989;170:595–600. doi: 10.1084/jem.170.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waanders G A, Shakhov A N, Held W, Karapetian O, Acha-Orbea H, MacDonald H R. Peripheral T cell activation and deletion induced by transfer of lymphocyte subsets expressing endogenous or exogenous mouse mammary tumor virus. J Exp Med. 1993;177:1359–1366. doi: 10.1084/jem.177.5.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webb S R, Sprent J. Induction of neonatal tolerance to Mlsa antigens by CD8+ T cells. Science. 1990;248:1643–1646. doi: 10.1126/science.1973003. [DOI] [PubMed] [Google Scholar]
- 21.Winslow G M, Cronin T, Mix D, Reilly M. Redundant proteolytic activation of a viral superantigen. Mol Immunol. 1998;35:897–903. doi: 10.1016/s0161-5890(98)00088-1. [DOI] [PubMed] [Google Scholar]
- 22.Winslow G M, Kappler J, Marrack P. Structural features of MMTV superantigens. In: Leung D, Huber B, Schlievert P, editors. Superantigens: structure, biology, and relevance to human disease. New York, N.Y: Marcel Dekker; 1997. pp. 37–60. [Google Scholar]
- 23.Winslow G M, Marrack P, Kappler J W. Processing and major histocompatibility complex binding of the MTV7 superantigen. Immunity. 1994;1:23–34. doi: 10.1016/1074-7613(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 24.Winslow G M, Scherer M T, Kappler J W, Marrack P. Detection and biochemical characterization of the mouse mammary tumor virus 7 superantigen (Mls-1a) Cell. 1992;71:719–730. doi: 10.1016/0092-8674(92)90549-r. [DOI] [PubMed] [Google Scholar]
- 25.Zack J A, Arrigo S J, Weitsman S R, Go A S, Haislip A, Chen I S Y. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]