Abstract
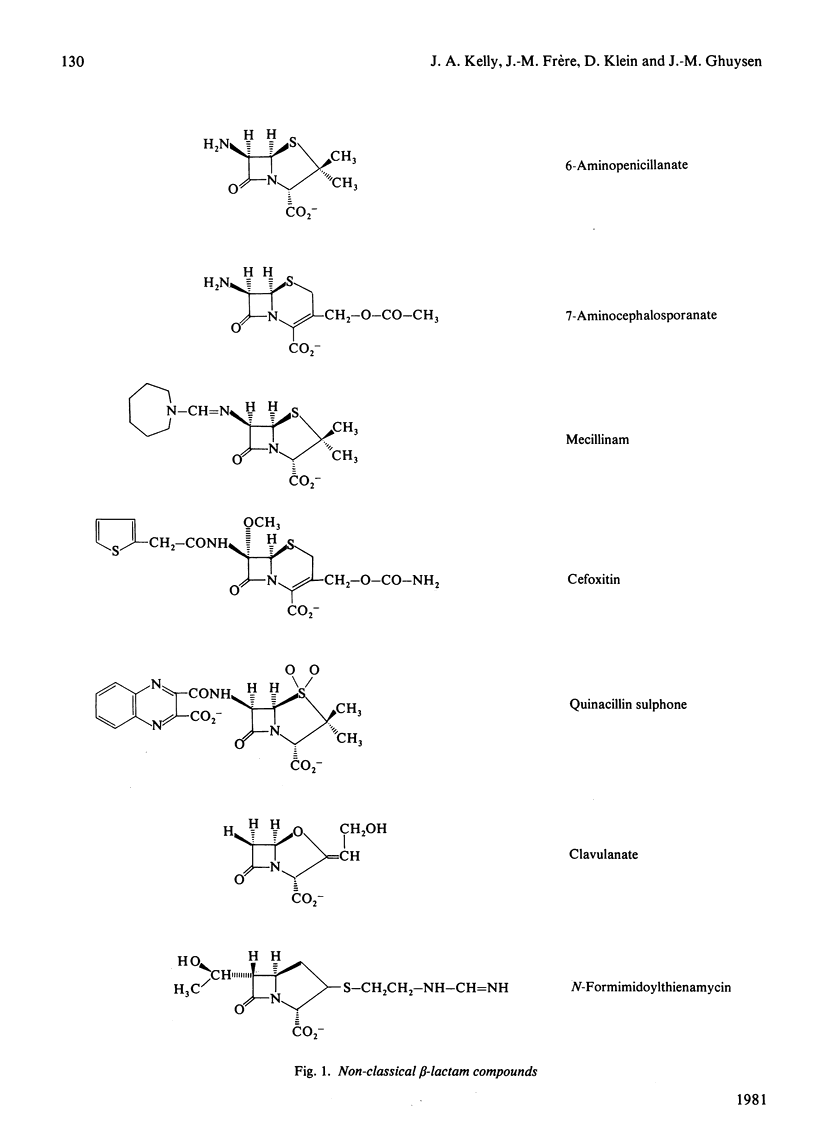
Streptomyces albus G secretes a Zn2+-containing D-alanyl-D-alanine peptidase. Streptomyces R61 and Actinomadura R39 secrete D-alanyl-D-alanine-cleaving serine peptidases. The effect of non-classical beta-lactam antibiotics on these three model enzymes has been studied. Mecillinam, cefoxitin, quinacillin, quinacillin sulphone, clavulanate and N-formimidoylthienamycin have no effect on the Zn2+-containing enzyme. 6-Amino-penicillanic acid slowly inactivates this enzyme and 7-aminocephalosporanic acid behaves as a reversible inhibitor. Cefoxitin and N-formimidoylthienamycin are potent anti-bacterial agents; they effectively inactivate the serine R39 enzyme and, to a lesser extent, the serine R61 enzyme. All the other beta-lactam compounds tested, including mecillinam, are slow inactivators of these serine enzymes. The intermediates formed between 6-aminopenicillanic acid and the R61 and R39 enzymes are long- and short-lived respectively, whereas those formed between 7-aminocephalosporanic acid and the same R61 and R39 enzymes are short- and long-lived respectively. Breakdown of the short-lived intermediates thus obtained gives rise to several ninhydrin-positive degradation products. The intermediates formed between clavulanate and the serine enzymes are long-lived. With the R39 enzyme, the inactivated complex formed in a first step undergoes subsequent monomolecular rearrangement to give rise to a second species exhibiting a high absorbance at 273 nm.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cartwright S. J., Coulson A. F. A semi-synthetic penicillinase inactivator. Nature. 1979 Mar 22;278(5702):360–361. doi: 10.1038/278360a0. [DOI] [PubMed] [Google Scholar]
- Dideberg O., Charlier P., Dupont L., Vermeire M., Frere J. M., Ghuysen J. M. The 4.5 A resolution structure analysis of the exocellular DD-carboxypeptidase of Streptomyces albus G. FEBS Lett. 1980 Aug 11;117(1):212–214. doi: 10.1016/0014-5793(80)80947-1. [DOI] [PubMed] [Google Scholar]
- Dideberg O., Joris B., Frere J. M., Ghuysen J. M., Weber G., Robaye R., Delbrouck J. M., Roelandts I. The exocellular DD-carboxypeptidase of Streptomyces albus G: a metallo (Zn2+) enzyme. FEBS Lett. 1980 Aug 11;117(1):215–218. doi: 10.1016/0014-5793(80)80948-3. [DOI] [PubMed] [Google Scholar]
- Duez C., Frère J. M., Geurts F., Ghuysen J. M., Dierickx L., Delcambe L. The exocellular DD-carboxypeptidase-endopeptidase from Streptomyces albus G. Purification and chemical properties. Biochem J. 1978 Dec 1;175(3):793–800. doi: 10.1042/bj1750793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duez C., Frère J. M., Klein D., Noël M., Ghuysen J. M., Delcambe L., Dierickx L. The exocellular beta-lactamase of Streptomyces albus G. Purification, properties and comparison with the exocellular DD-carboxypeptidase. Biochem J. 1981 Jan 1;193(1):75–82. doi: 10.1042/bj1930075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duez C., Joris B., Frère J. M., Ghuysen J. M., Van Beeumen J. The penicillin-binding site in the exocellular DD-carboxypeptidase-transpeptidase of Actinomadura R39. Biochem J. 1981 Jan 1;193(1):83–86. doi: 10.1042/bj1930083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J., Belasco J. G., Charnas R. L., Khosla S., Knowles J. R. Beta-lactamase inactivation by mechanism-based reagents. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):309–319. doi: 10.1098/rstb.1980.0048. [DOI] [PubMed] [Google Scholar]
- Frere J., Ghuysen J., Degelaen J., Loffet A., Perkins H. R. Fragmentation of benzylpenicillin after interaction with the exocellular DD-carboxypeptidase-transpeptidases of Streptomyces R61 and R39. Nature. 1975 Nov 13;258(5531):168–170. doi: 10.1038/258168a0. [DOI] [PubMed] [Google Scholar]
- Frieden C. Kinetic aspects of regulation of metabolic processes. The hysteretic enzyme concept. J Biol Chem. 1970 Nov 10;245(21):5788–5799. [PubMed] [Google Scholar]
- Frère J. M., Duez C., Ghuysen J. M., Vandekerkhove J. Occurrence of a serine residue in the penicillin-binding site of the exocellular DD-carboxy-peptidase-transpeptidase from Streptomyces R61. FEBS Lett. 1976 Nov;70(1):257–260. doi: 10.1016/0014-5793(76)80770-3. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Geurts F., Ghuysen J. M. The exocellular DD-carboxypeptidase-endopeptidase of Streptomyces albus G. Interaction with beta-lactam antibiotics. Biochem J. 1978 Dec 1;175(3):801–805. doi: 10.1042/bj1750801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frère J. M., Ghuysen J. M., Iwatsubo M. Kinetics of interaction between the exocellular DD-carboxypeptidase-transpeptidase from Streptomyces R61 and beta-lactam antibiotics. A choice of models. Eur J Biochem. 1975 Sep 15;57(2):343–351. doi: 10.1111/j.1432-1033.1975.tb02307.x. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Ghuysen J. M., Perkins H. R., Nieto M. Molecular weight and amino acid composition of the exocellular DD-carboxypeptidase-transpeptidase of Streptomyces R61. Biochem J. 1973 Nov;135(3):463–468. doi: 10.1042/bj1350463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frère J. M., Moreno R., Ghuysen J. M. Molecular weight, amino acid composition and physicochemical properties of the exocellular DD-carboxypeptidase-transpeptidase of Streptomyces R39. Biochem J. 1974 Oct;143(1):233–240. doi: 10.1042/bj1430233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuad N., Frère J. M., Ghuysen J. M., Duez C., Iwatsubo M. Mode of interaction between beta-lactam antibiotics and the exocellular DD-carboxypeptidase--transpeptidase from Streptomyces R39. Biochem J. 1976 Jun 1;155(3):623–629. doi: 10.1042/bj1550623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuysen J. M., Frère J. M., Leyh-Bouille M., Coyette J., Dusart J., Nguyen-Distèche M. Use of model enzymes in the determination of the mode of action of penicillins and delta 3-cephalosporins. Annu Rev Biochem. 1979;48:73–101. doi: 10.1146/annurev.bi.48.070179.000445. [DOI] [PubMed] [Google Scholar]
- Johnson K., Duez C., Frère J. M., Ghuysen J. M. Beta-lactamases (Actinomycetes species). Methods Enzymol. 1975;43:687–698. doi: 10.1016/0076-6879(75)43134-2. [DOI] [PubMed] [Google Scholar]
- Nieto M., Perkins H. R., Frère J. M., Ghuysen J. M. Fluorescence and circular dichroism studies on the Streptomyces R61 DD-carboxypeptidase-transpeptidase. Penicillin binding by the enzyme. Biochem J. 1973 Nov;135(3):493–505. doi: 10.1042/bj1350493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H. Description and classification of the newer cephalosporins and their relationships with the established compounds. J Antimicrob Chemother. 1979 Nov;5(6):635–671. doi: 10.1093/jac/5.6.635. [DOI] [PubMed] [Google Scholar]
- Perkins H. R., Nieto M., Frére J. M., Leyh-Bouille M., Ghuysen J. M. Streptomyces DD-carboxypeptidases as transpeptidases. The specificity for amino compounds acting as carboxyl acceptors. Biochem J. 1973 Apr;131(4):707–718. doi: 10.1042/bj1310707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilf W., Frère P., Frère J. M., Martin H. H., Ghuysen J. M., Adriaens P., Meesschaert B. Interaction between penicillin and the DD-carboxypeptidase of the unstable L-form of Proteus mirabilis strain 19. Eur J Biochem. 1978 Apr 17;85(2):325–330. doi: 10.1111/j.1432-1033.1978.tb12242.x. [DOI] [PubMed] [Google Scholar]
- Thatcher D. R. The partial amino acid sequence of the extracellular beta-lactamase I of Bacillus cereus 569/H. Biochem J. 1975 May;147(2):313–326. doi: 10.1042/bj1470313. [DOI] [PMC free article] [PubMed] [Google Scholar]


