Abstract
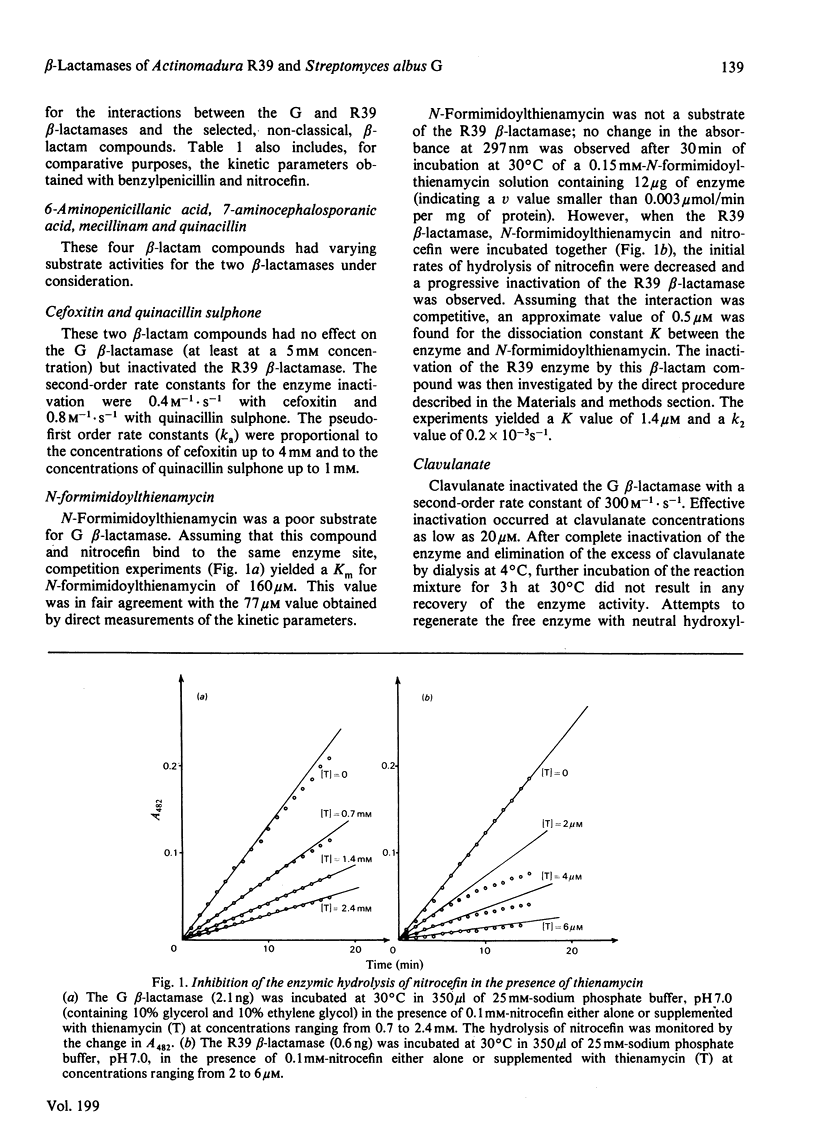
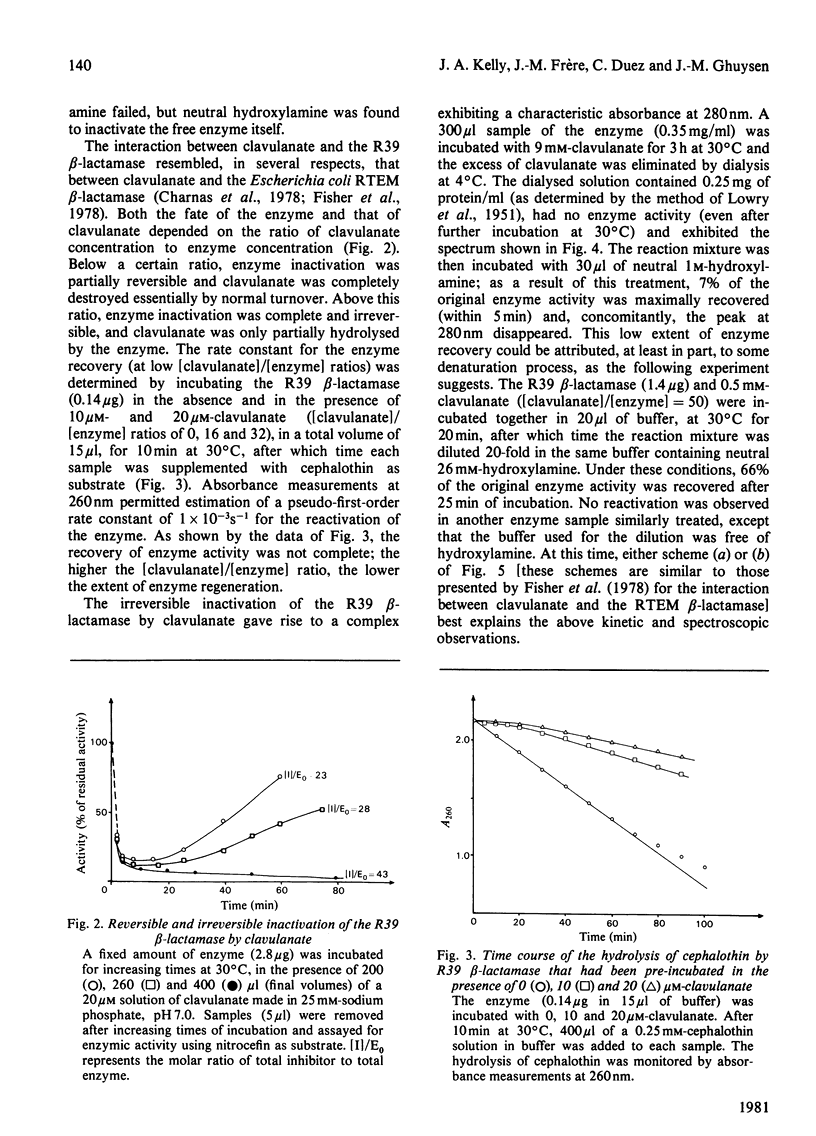
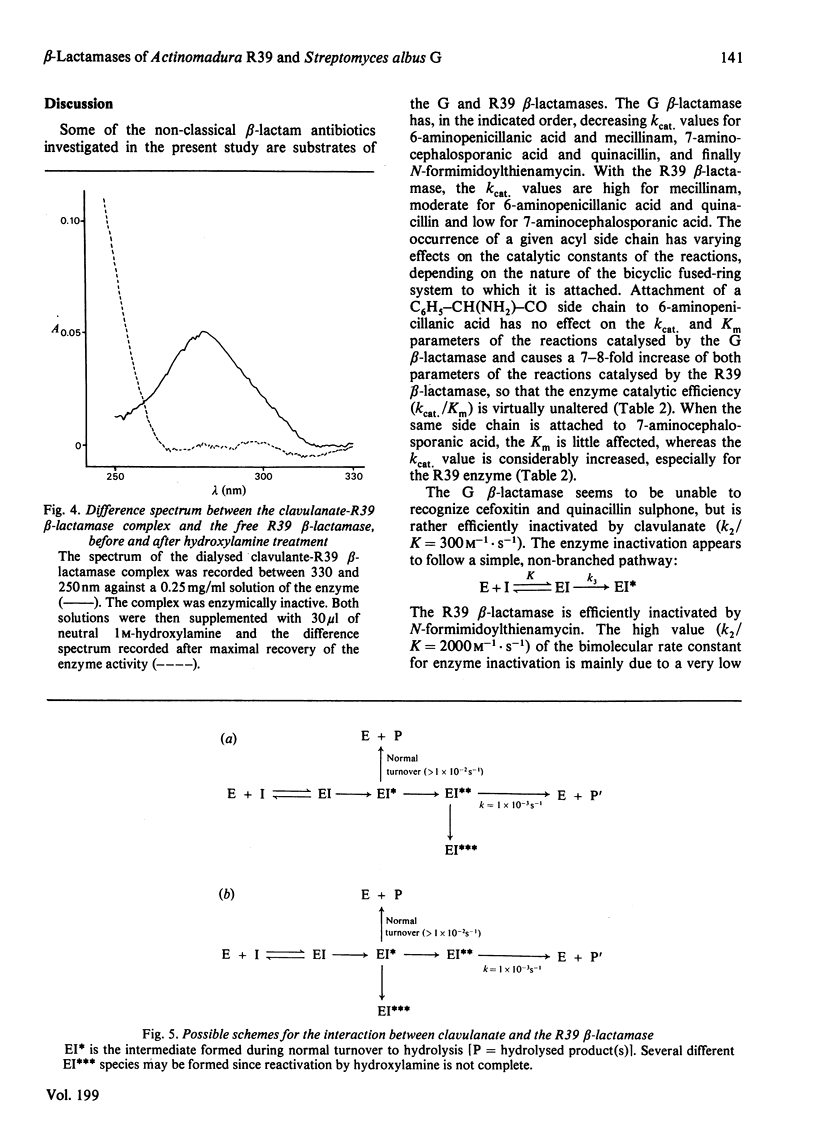
6-Aminopenicillanic acid, 7-aminocephalosporanic acid, mecillinam and quinacillin have varying substrate activities for both the R39 beta-lactamase (excreted by Actinomadura R39) and the G beta-lactamase (excreted by Streptomyces albus G). Cefoxitin and quinacillin sulphone are not recognized by the G beta-lactamase and are weak inactivators of the R39 beta-lactamase. N-Formimidoylthienamycin is a poor substrate for the G beta-lactamase and a potent inactivator of the R39 beta-lactamase. The high value of the bimolecular rate constant for enzyme inactivation is mainly due to a very low dissociation constant (1 microM). Clavulanate is an inactivator of both G and R39 beta-lactamases. The reaction with this latter enzyme is a branched pathway where normal turnover and permanent enzyme inactivation occur concomitantly. Between 28 and 43 molecules of clavulanate are hydrolysed before one of them has the opportunity to inactivate one molecule of enzyme.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cartwright S. J., Coulson A. F. Active site of staphylococcal beta-lactamase. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):370–372. [PubMed] [Google Scholar]
- Charnas R. L., Fisher J., Knowles J. R. Chemical studies on the inactivation of Escherichia coli RTEM beta-lactamase by clavulanic acid. Biochemistry. 1978 May 30;17(11):2185–2189. doi: 10.1021/bi00604a025. [DOI] [PubMed] [Google Scholar]
- Dideberg O., Joris B., Frere J. M., Ghuysen J. M., Weber G., Robaye R., Delbrouck J. M., Roelandts I. The exocellular DD-carboxypeptidase of Streptomyces albus G: a metallo (Zn2+) enzyme. FEBS Lett. 1980 Aug 11;117(1):215–218. doi: 10.1016/0014-5793(80)80948-3. [DOI] [PubMed] [Google Scholar]
- Duez C., Frère J. M., Klein D., Noël M., Ghuysen J. M., Delcambe L., Dierickx L. The exocellular beta-lactamase of Streptomyces albus G. Purification, properties and comparison with the exocellular DD-carboxypeptidase. Biochem J. 1981 Jan 1;193(1):75–82. doi: 10.1042/bj1930075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duez C., Joris B., Frère J. M., Ghuysen J. M., Van Beeumen J. The penicillin-binding site in the exocellular DD-carboxypeptidase-transpeptidase of Actinomadura R39. Biochem J. 1981 Jan 1;193(1):83–86. doi: 10.1042/bj1930083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J., Belasco J. G., Khosla S., Knowles J. R. beta-Lactamase proceeds via an acyl-enzyme intermediate. Interaction of the Escherichia coli RTEM enzyme with cefoxitin. Biochemistry. 1980 Jun 24;19(13):2895–2901. doi: 10.1021/bi00554a012. [DOI] [PubMed] [Google Scholar]
- Fisher J., Charnas R. L., Knowles J. R. Kinetic studies on the inactivation of Escherichia coli RTEM beta-lactamase by clavulanic acid. Biochemistry. 1978 May 30;17(11):2180–2184. doi: 10.1021/bi00604a024. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Duez C., Ghuysen J. M., Vandekerkhove J. Occurrence of a serine residue in the penicillin-binding site of the exocellular DD-carboxy-peptidase-transpeptidase from Streptomyces R61. FEBS Lett. 1976 Nov;70(1):257–260. doi: 10.1016/0014-5793(76)80770-3. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Geurts F., Ghuysen J. M. The exocellular DD-carboxypeptidase-endopeptidase of Streptomyces albus G. Interaction with beta-lactam antibiotics. Biochem J. 1978 Dec 1;175(3):801–805. doi: 10.1042/bj1750801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frère J. M., Ghuysen J. M., Iwatsubo M. Kinetics of interaction between the exocellular DD-carboxypeptidase-transpeptidase from Streptomyces R61 and beta-lactam antibiotics. A choice of models. Eur J Biochem. 1975 Sep 15;57(2):343–351. doi: 10.1111/j.1432-1033.1975.tb02307.x. [DOI] [PubMed] [Google Scholar]
- Fuad N., Frère J. M., Ghuysen J. M., Duez C., Iwatsubo M. Mode of interaction between beta-lactam antibiotics and the exocellular DD-carboxypeptidase--transpeptidase from Streptomyces R39. Biochem J. 1976 Jun 1;155(3):623–629. doi: 10.1042/bj1550623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuysen J. M., Frère J. M., Leyh-Bouille M., Coyette J., Dusart J., Nguyen-Distèche M. Use of model enzymes in the determination of the mode of action of penicillins and delta 3-cephalosporins. Annu Rev Biochem. 1979;48:73–101. doi: 10.1146/annurev.bi.48.070179.000445. [DOI] [PubMed] [Google Scholar]
- Hill H. A., Sammes P. G., Waley S. G. Active sites of beta-lactamases from Bacillus cereus. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):333–344. doi: 10.1098/rstb.1980.0050. [DOI] [PubMed] [Google Scholar]
- Johnson K., Duez C., Frère J. M., Ghuysen J. M. Beta-lactamases (Actinomycetes species). Methods Enzymol. 1975;43:687–698. doi: 10.1016/0076-6879(75)43134-2. [DOI] [PubMed] [Google Scholar]
- Kelly J. A., Frère J. M., Klein D., Ghuysen J. M. Interaction between non-classical beta-lactam compounds and the Zn2+-containing G and serine R61 and R39 D-alanyl-D-alanine peptidases. Biochem J. 1981 Oct 1;199(1):129–136. doi: 10.1042/bj1990129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott-Hunziker V., Waley S. G., Orlek B. S., Sammes P. G. Penicillinase active sites: labelling of serine-44 in beta-lactamase I by 6beta-bromopenicillanic acid. FEBS Lett. 1979 Mar 1;99(1):59–61. doi: 10.1016/0014-5793(79)80248-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Waxman D. J., Yocum R. R., Strominger J. L. Penicillins and cephalosporins are active site-directed acylating agents: evidence in support of the substrate analogue hypothesis. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):257–271. doi: 10.1098/rstb.1980.0044. [DOI] [PubMed] [Google Scholar]


