Abstract
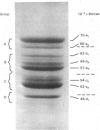
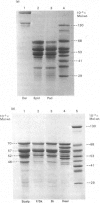
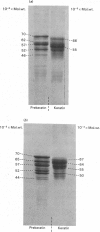
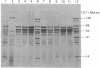
The polypeptide-chain components of human epidermal prekeratin and keratin were analysed by high-resolution SDS (sodium dodecyl sulphate)/polyacrylamide-gradient-gel electrophoresis. Size heterogeneity existed amongst prekeratin components and at least ten polypeptides, in the molecular-weight range 46,000-70,000, were observed in 0.1 M-citric acid/sodium citrate buffer (pH 2.65) extracts of scale epidermis. Prekeratin from scalp pilosebaceous ducts was identical with that from the contiguous epidermis, and no prekeratin was found in extracts of scale dermis. Prekeratin from plantar epidermis contained additional polypeptide chains, but only slight anatomical variation existed between the non-callus sites examined. Keratin differed from prekeratin in at least two major respects: (a) many major components did not co-electrophorese on high-resolution SDS/polyacrylamide slab gels, and (b) keratin, but not prekeratin, required denaturing and reducing conditions for extraction. Keratin extracted from scale epidermis after complete removal of prekeratin was identical with forearm stratum-corneum keratin. Palmar and plantar keratin contained additional polypeptide chains and had a different size distribution compared with forearm and scalp keratin components. Modification of prekeratin components to produce the keratin polypeptide profile occurred during epidermal differentiation, and these changes appeared to take place in the granular-layer region of the epidermis.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Nikaido K. Two-dimensional gel electrophoresis of membrane proteins. Biochemistry. 1976 Feb 10;15(3):616–623. doi: 10.1021/bi00648a026. [DOI] [PubMed] [Google Scholar]
- BRENTANI M., BRENTANI R., RAW I. ROLE OF NUCLEOLAR RIBONUCLEIC ACID IN INCORPORATION OF RIBOSOMAL AMINO ACID. Nature. 1964 Mar 14;201:1130–1130. doi: 10.1038/2011130a0. [DOI] [PubMed] [Google Scholar]
- Baden H. P., Kubilus J., Argyris T. S. Modification of polypeptide composition in keratinocyte fibrous protein. J Invest Dermatol. 1980 Nov;75(5):383–387. doi: 10.1111/1523-1747.ep12523622. [DOI] [PubMed] [Google Scholar]
- Baden H. P., Lee L. D. Fibrous protein of human epidermis. J Invest Dermatol. 1978 Aug;71(2):148–151. doi: 10.1111/1523-1747.ep12546905. [DOI] [PubMed] [Google Scholar]
- Baden H. P., Lee L. D., Kubilus J. The fibrous proteins of stratum corneum. J Invest Dermatol. 1976 Nov;67(5):573–576. doi: 10.1111/1523-1747.ep12541671. [DOI] [PubMed] [Google Scholar]
- Brody I. Ultrastructure of the fibrous substance in the keratinocytes of the epidermis in healthy individuals. J Cutan Pathol. 1979 Oct;6(5):333–346. doi: 10.1111/j.1600-0560.1979.tb01157.x. [DOI] [PubMed] [Google Scholar]
- Dale B. A., Holbrook K. A., Steinert P. M. Assembly of stratum corneum basic protein and keratin filaments in macrofibrils. Nature. 1978 Dec 14;276(5689):729–731. doi: 10.1038/276729a0. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Appelhans B., Schmid E., Freudenstein C., Osborn M., Weber K. The organization of cytokeratin filaments in the intestinal epithelium. Eur J Cell Biol. 1979 Aug;19(3):255–268. [PubMed] [Google Scholar]
- Franke W. W., Weber K., Osborn M., Schmid E., Freudenstein C. Antibody to prekeratin. Decoration of tonofilament like arrays in various cells of epithelial character. Exp Cell Res. 1978 Oct 15;116(2):429–445. doi: 10.1016/0014-4827(78)90466-4. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980 Apr;19(4):1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. Multiple keratins of cultured human epidermal cells are translated from different mRNA molecules. Cell. 1979 Jul;17(3):573–582. doi: 10.1016/0092-8674(79)90265-4. [DOI] [PubMed] [Google Scholar]
- Gilmartin M. E., Culbertson V. B., Freedberg I. M. Phosphorylation of epidermal keratins. J Invest Dermatol. 1980 Sep;75(3):211–216. doi: 10.1111/1523-1747.ep12522887. [DOI] [PubMed] [Google Scholar]
- Kellum R. E. Isolation of human sebaceous glands. Arch Dermatol. 1966 May;93(5):610–612. [PubMed] [Google Scholar]
- Kubilus J., MacDonald M. J., Baden H. P. Epidermal proteins of cultured human and bovine keratinocytes. Biochim Biophys Acta. 1979 Jun 19;578(2):484–492. doi: 10.1016/0005-2795(79)90178-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambin P. Reliability of molecular weight determination of proteins by polyacrylamide gradient gel electrophoresis in the presence of sodium dodecyl sulfate. Anal Biochem. 1978 Mar;85(1):114–125. doi: 10.1016/0003-2697(78)90281-6. [DOI] [PubMed] [Google Scholar]
- Lavker R. M., Matoltsy A. G. Formation of horny cells: the fate of cell organelles and differentiation products in ruminal epithelium. J Cell Biol. 1970 Mar;44(3):501–512. doi: 10.1083/jcb.44.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. D., Kubilus J., Baden H. P. Intraspecies heterogeneity of epidermal keratins isolated from bovine hoof and snout. Biochem J. 1979 Jan 1;177(1):187–196. doi: 10.1042/bj1770187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoltsy A. G. Desmosomes, filaments, and keratohyaline granules: their role in the stabilization and keratinization of the epidermis. J Invest Dermatol. 1975 Jul;65(1):127–142. doi: 10.1111/1523-1747.ep12598093. [DOI] [PubMed] [Google Scholar]
- Matoltsy A. G. Keratinization. J Invest Dermatol. 1976 Jul;67(1):20–25. doi: 10.1111/1523-1747.ep12512473. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rice R. H., Green H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope: activation of the cross-linking by calcium ions. Cell. 1979 Nov;18(3):681–694. doi: 10.1016/0092-8674(79)90123-5. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Green H. The cornified envelope of terminally differentiated human epidermal keratinocytes consists of cross-linked protein. Cell. 1977 Jun;11(2):417–422. doi: 10.1016/0092-8674(77)90059-9. [DOI] [PubMed] [Google Scholar]
- Schweizer J., Goerttler K. Synthesis in vitro of keratin polypeptides directed by mRNA isolated from newborn and adult mouse epidermis. Eur J Biochem. 1980 Nov;112(2):243–249. doi: 10.1111/j.1432-1033.1980.tb07200.x. [DOI] [PubMed] [Google Scholar]
- Skerrow D., Hunter I. Protein modifications during the keratinization of normal and psoriatic human epidermis. Biochim Biophys Acta. 1978 Dec 20;537(2):474–484. doi: 10.1016/0005-2795(78)90532-9. [DOI] [PubMed] [Google Scholar]
- Skerrow D., Matoltsy A. G., Matoltsy M. N. Isolation and characterization of the helical regions of epidermal prekeratin. J Biol Chem. 1973 Jul 10;248(13):4820–4826. [PubMed] [Google Scholar]
- Skerrow D. The structure of prekeratin. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1311–1316. doi: 10.1016/0006-291x(74)90457-4. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W. Postsynthetic modifications of mammalian epidermal alpha-keratin. Biochemistry. 1979 Dec 11;18(25):5664–5669. doi: 10.1021/bi00592a022. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W. The polypeptide composition of bovine epidermal alpha-keratin. Biochem J. 1975 Dec;151(3):603–614. doi: 10.1042/bj1510603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W., Wantz M. L. Characterization of the keratin filament subunits unique to bovine snout epidermis. Biochem J. 1980 Jun 1;187(3):913–916. doi: 10.1042/bj1870913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W., Zimmerman S. B. Self-assembly of bovine epidermal keratin filaments in vitro. J Mol Biol. 1976 Dec 15;108(3):547–567. doi: 10.1016/s0022-2836(76)80136-2. [DOI] [PubMed] [Google Scholar]
- Steinert P. M. Structure of the three-chain unit of the bovine epidermal keratin filament. J Mol Biol. 1978 Jul 25;123(1):49–70. doi: 10.1016/0022-2836(78)90376-5. [DOI] [PubMed] [Google Scholar]
- Steinert P. M. The extraction and characterization of bovine epidermal alpha-keratin. Biochem J. 1975 Jul;149(1):39–48. doi: 10.1042/bj1490039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. T., Green H. Keratin filaments of cultured human epidermal cells. Formation of intermolecular disulfide bonds during terminal differentiation. J Biol Chem. 1978 Mar 25;253(6):2053–2060. [PubMed] [Google Scholar]
- Sun T. T., Shih C., Green H. Keratin cytoskeletons in epithelial cells of internal organs. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2813–2817. doi: 10.1073/pnas.76.6.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viac J., Schmitt D., Staquet M. J., Thivolet J., Ortonne J. P., Bustamante R. Binding specificity of guinea pig anti-alpha-keratin polypeptide sera on human keratinocytes: comparison of their receptors with those of human epidermal cytoplasmic antibodies. Acta Derm Venereol. 1980;60(3):189–196. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]