Abstract
Introduction:
Serotonergic psychedelics and ketamine produce rapid and long-lasting symptomatic relief in multiple psychiatric disorders. Evidence suggests that despite having distinct molecular targets, both drugs may exert therapeutic benefit via their pro-neuroplastic effects. Following treatment with ketamine or serotonergic psychedelics, patients are reported to be more open to behavioral change, which is leveraged for psychotherapy-assisted reframing of narratives of the self. This period of enhanced behavioral change is postulated to be supported by a post-treatment window of enhanced neural plasticity, but evidence for such “metaplastic” effects is limited. In this study, we tested for neural plasticity and metaplasticity in murine hippocampus.
Methods:
Brain slices were obtained from C57BL/6J mice 24 h after treatment (intraperitoneal injection) with saline, ketamine, or the serotonergic psychedelic 2,5-dimethoxy-4-iodoamphetamine (DOI). Extracellular fiber volleys (FVs) and field excitatory postsynaptic potentials (fEPSPs) were recorded in stratum radiatum of CA1 in response to stimulation of Schaffer collateral fibers before and after induction of short-term potentiation (STP) and long-term potentiation (LTP).
Results:
Before LTP induction, responses differed across treatment groups (F2,67 = 5.407, p = 0.00665), with fEPSPs enhanced in slices from DOI-treated animals (p = 0.0182), but not in ketamine-treated animals (p = 0.9786), compared with saline. There were no treatment effects on LTP (F2,56 = 0.6, p = 0.516), but there were on STP (F2,56 = 4.409, p = 0.0167), with enhanced STP in DOI-treated animals (p = 0.0352), but not in ketamine-treated (p = 0.9999) animals, compared with saline. A presynaptic component to the mechanism for the DOI effects was suggested by (1) significantly enhanced FV amplitudes (F2,61 = 3.17, p = 0.049) in DOI-treated animals (p = 0.0457), but not in ketamine-treated animals, compared with saline (p = 0.8677); and (2) enhanced paired pulse ratios (F2,61 = 3.581, p = 0.0339) in slices from DOI-treated animals (p = 0.0257), but not in ketamine-treated animals (p = 0.4845), compared with saline.
Conclusions:
DOI, but not ketamine, induced significant neuroplastic and metaplastic effects at hippocampal CA1 synapses 24 h after treatment, likely in part via a presynaptic mechanism.
Keywords: electrophysiology, DOI, metaplasticity, hippocampus, plasticity, ketamine
Introduction
Ketamine and serotonergic psychedelics ameliorate symptoms of multiple psychiatric disorders in patient populations1,2 and modify behavioral phenotypes in rodent tasks dependent on neuronal circuitry relevant to these disorders.3–6 In patients administered these drugs, improvements in symptoms are linked to enhanced openness and psychological flexibility.7–12 Unlike existing medical therapies, the effects of these treatments are rapid and persistent, continuing well beyond their plasma exposure time course;13 the therapeutic effects of serotonergic psychedelics last several-fold longer than those of ketamine.14–16
The underlying neural mechanisms of serotonergic psychedelics and ketamine is a dissociative anesthetic that blocks both N-methyl-d-aspartate (NMDA) receptors and hyperpolarization-activated cyclic nucleotide-gated channels.17 Serotonergic psychedelics exert their behavioral effects in humans primarily via agonism at 5-HT2A receptors.18,19 Because ketamine and serotonergic psychedelics have distinct molecular targets in the brain and produce distinct effects on perception and cognition, the mechanisms underlying their antidepressant activity action likely overlap only in part.20–26
Long-term behavioral effects in human subjects are presumed to reflect neuroplastic changes.27–29 There is considerable evidence from preclinical models for rapidly induced, persistent neuroplastic effects of ketamine and serotonergic psychedelics in medial prefrontal cortex and hippocampus and that this plasticity underlies changes in behavioral phenotypes. These neuroplastic changes include increases in dendritic spine density and size,3,30–38 enhanced neurogenesis in the hippocampus,39–43 and increased expression of plasticity-associated genes such as cfos and Sgk1 in neocortex and hippocampus.44–50 Plasticity has also been observed in the nucleus accumbens.51 These drug-induced structural and functional changes represent clear evidence of acutely induced, long-lasting neural plasticity. Furthermore, these changes associate with modifications in behavioral phenotype in tasks assessing psychiatric disease-relevant processes.3,30,38,51
In psychedelic-assisted psychotherapy, postdosing integration sessions are designed to leverage improved cognitive flexibility and new openness to change in perspectives and self-narratives in the days and weeks following treatment.52 This enhanced behavioral plasticity is postulated to arise when serotonergic psychedelics and ketamine open a long-lasting window of neuroplasticity that outlasts its clearance from the brain.51,53,54 In this model, the neural basis for this enhanced plasticity would be acutely induced and persistent neural changes that manifest post-acutely as lowered threshold for, or enhanced magnitude of, synaptic plasticity in neural circuits following treatment. This phenomenon is referred to as “metaplasticity”55,56 and is distinct from, although possibly dependent on, neuroplastic changes summarized above. To date, support for this model has been demonstrated in medial prefrontal cortex (mPFC) and nucleus accumbens,38,51 but not yet in hippocampus.
We investigated the effects of ketamine and 2,5-dimethoxy-4-iodoamphetamine (DOI) on neural plasticity and metaplasticity in the hippocampus of mice. DOI is a serotonergic psychedelic that is more selective for the 5-HT2A receptor in comparison to tryptamine psychedelics (e.g., psilocybin) and is known to result in increased glutamate neurotransmission, similar to other serotonergic psychedelics.57–59 Evidence suggests that signaling deficits in hippocampus, mPFC, and amygdala associate with symptoms of depression and anxiety.60–62 In particular, an overarching psychological framework for the mechanisms of these drugs' therapeutic effects is grounded in an altered narrative of the self, which would likely involve autobiographical memory formation and retrieval via the hippocampus.63 Consistent with this model, previous studies have shown that both serotonergic psychedelics and ketamine strengthen hippocampal CA1 synapses in rodent models.30,64 In this study, we investigated synaptic plasticity and metaplasticity in the hippocampus 24 h after administration of DOI and ketamine.
Methods
All experimental protocols conformed to the American Physiological Society/National Institutes of Health guidelines and were approved by the University of Wisconsin Animal Care and Use Committee.
Animals
Male and female C57BL/6J mice (n = 55 mice; 31 females; after exclusion: n = 51; 28 females), 12–20 weeks old, were bred in house (F2 generation) or ordered from Jackson Labs. Mice were maintained on a 12:12 light–dark cycle (light on at 06:00) with ad libitum food and water. All animals were housed in groups until drug administration.
Drugs
Aliquots of (±)-DOI hydrochloride (Millipore Sigma) and ketamine hydrochloride (100 mg/mL <0.10 mg/mL benzethonium chloride added as a preservative; Pfizer) diluted in 0.9% sodium chloride were filtered through a 0.22 μm pore membrane and stored at −80°C. Pairs of animals were randomly assigned to a drug treatment, and the experimenter was blinded to the treatment condition during the experiment and analysis. Intraperitoneal injections (DOI: 1 mg/kg, ketamine: 10 mg/kg; DOI, ketamine, 0.9% saline: 5 mL/kg total volume) were administered ∼24 h in advance of dissections, and animals were placed into a new cage after drug administration.
The chosen dosage of DOI has been shown to elicit a robust head twitch response, a behavioral marker for hallucinogenic effects,65,66 and consistent behavioral effects.67,68 For ketamine, 10 mg/kg is commonly used in preclinical models of psychiatric disorders and has demonstrated robust and long-lasting neural and behavioral changes at this dose.69 At higher doses, ketamine begins to have anesthetic effects,70 which have been suggested to arise from mechanisms distinct from those supporting the antidepressant effects seen at lower doses.71
Brain slice preparation
Approximately 24 h after drug administration, dorsal hippocampal slices were prepared as previously described.72 Briefly, animals were deeply anesthetized with 3% isoflurane and then decapitated. Brains were quickly extracted from the skull and placed into ice-cold “cutting artificial cerebrospinal fluid” (cutting artificial cerebrospinal fluid [ACSF]; see below for details) saturated with carbogen (95% O2/5% CO2). The cerebellum was cutoff at ∼15-degree caudo-rostral angle to create an “off coronal” cutting plane for slicing. The brain was then glued caudal side down onto a metal stage, mounted into the vibratome (Model 7000smz-2 vibratome, 80 Hz, 0.11 mm/s; Campden Instruments), and submerged in ice-cold cutting ACSF.
Coronal slices (400 μm) containing hippocampus were hemisected and incubated at 33°C for 30 min in carbogen-saturated “recording” ACSF. Slices were then allowed to come to room temperature (∼24°C) for 60 min before transfer to recording chambers for electrophysiology. Cutting ACSF consisted of (in mM): 127 NaCl, 1.88 KCl, 1.21 KH2PO4, 26 NaHCO3, 10 glucose, 2.5 sodium ascorbate, 5 kynurenic acid, 1.44 MgSO4, 11 MgCl2, and 2.17 CaCl2. Recording ACSF consisted of (in mM): 127 NaCl, 1.88 KCl, 1.21 KH2PO4, 26 NaHCO3, 10 glucose, 1.44 MgSO4, and 2.17 CaCl2. All solutions were buffered to pH 7.3–7.4 when saturated with 95% O2/5% CO2 and had a recorded osmolality between 294 and 297 mOsm/kgH2O.
Data collection
Brain slices were placed in a submersion-style recording chamber with carbogen-saturated ACSF flowing at a rate of 3.0 ml/min (Minipuls 3; Gilson, Inc., Middleton, WI) and maintained at 30°C (TC-344C Automatic Temperature Controller; Warner Instruments, CT). Slices were placed upon an elevated mesh netting to allow for perfusion of both surfaces, with nylon-strung platinum harps to anchor slices onto the netting. To measure fiber volleys (FVs) and field excitatory postsynaptic potentials (fEPSPs), tungsten recording electrodes (100 kΩ; World Precision Instruments, Sarasota, FL) were inserted at a depth of 70–150 μm into the stratum radiatum of hippocampus. Concentric bipolar stimulating electrodes (200 kΩ; World Precision Instruments) were placed ∼1 mm away in stratum radiatum to stimulate Schaffer collateral inputs to CA1 pyramidal cells.
fEPSP stimulus–response (S-R) curves were obtained to determine the intensity that elicited the half-maximal fEPSP slope. Stimulation values ranged from 40 to 500 μA. Bipolar stimuli (0.2 ms per polarity, separated by 0.1 ms) were delivered via constant-current stimulus isolator units (Multi Channel Systems STG 4004; MCS, Reutlingen, Germany). The intensity producing the half-maximal fEPSP slope was chosen for long-term potentiation (LTP) protocols.
During LTP induction protocols, test stimuli were delivered at 0.05 Hz until a stable 30-min baseline period was achieved (defined as a <10% shift in fEPSP slope). The theta-burst stimulation (TBS) protocol to induce LTP consisted of 10 trains stimuli delivered at 5 Hz, with each train consisting of four stimulus pulses delivered at 100 Hz. Responses were then recorded for 60 additional minutes following TBS.
Recordings were obtained using the pClamp 10 software (Molecular Devices, San Jose, CA). Data were amplified × 1000, filtered between 1 Hz and 20 kHz using a Microelectrode AC Amplifier (Model 1800; A-M Systems, Everett, WA), and digitized at 40 kHz (National Instruments, Austin, TX).
Data Analysis
Data preprocessing
Evoked responses from each slice (n = 89 slices from 55 animals) were visually inspected for the following exclusion criteria: FV amplitude >30% of the fEPSP amplitude at the half-maximal intensity or peak fEPSP amplitude <0.5 mV. Additional criteria for exclusion from the LTP analysis were emergence of a population spike during the 30-min baseline recording and baseline instability, which may have indicated poor tissue health or temperature or mechanical fluctuations in the bath. In total, n = 72 slices from 51 animals (38 slices from female animals) were used in the S-R analysis and n = 60 slices from 44 animals (29 slices from female animals) for the LTP analysis. The peak amplitude (mV) of the presynaptic FV and the maximum rising slope of the fEPSP (mV/ms) were calculated in the Clampfit software using the analyze/statistics function with optional smoothing (five samples, 0.3 ms).
Stimulus–response curves
Sigmoidal model fits were applied to fEPSP S-R data obtained from each slice using the function fit() in MATLAB, with the following function , where RMax is the asymptotic response, M is the slope, and S50% is the stimulus intensity eliciting half maximal response. Hypothesis testing of stimulus–response data was performed using one-way analysis of variance (ANOVA). Effects of treatment (saline, DOI, and ketamine) were assessed on the fit parameters (RMax, M, and S50%). Once significance was established, Tukey's Honest Significant Difference (HSD) test was used for post hoc comparisons.
FV S-R data were not well-fit by sigmoid curves. Instead, maximum FV amplitudes from each slice were compared across drug groups in a one-way ANOVA in R (v4.2.2; R Core Team, 2022). Significant differences were subsequently tested with Tukey's HSD for post hoc comparisons.
Measures of short- and long-term plasticity
Two measures were used to characterize short-term potentiation (STP). First, the magnitude of the normalized fEPSP slope was measured during the period t = 0–5 min and normalized by the average of the last 10 min of the baseline period. Second, the decay of the fEPSP slope time series from t = 0 to 60 min was measured by applying a biexponential fit using the fit() function in Matlab. Because only the overall time course of this decay was of interest, and because data from a small number (7%) of experiments were better fit by monoexponential decays, the time course of decay was characterized as the weighted time constant , where ai and are the amplitudes and time constants of the two exponential components, respectively. The magnitude of LTP was measured as the fEPSP slope averaged over the time window t = 50–60 min post-TBS, normalized by the average of the last 10 min of the baseline period.
Hypothesis testing for STP and LTP was performed using one-way analysis of variance in R (v4.2.2; R Core Team, 2022). We compared the effect of drug condition (Saline, DOI, and Ketamine) on the mean fEPSP slope in the first 5 min post-TBS and on τWt (for short-term plasticity), and on mean fEPSP slope over the last 10 min post-TBS (for LTP).
Analysis of paired pulse ratio
To investigate the contributions of presynaptic mechanisms to observed treatment effects, paired pulse ratios (PPRs) of fEPSPs were assessed.73 PPRs were assessed from the first two stimuli of the first train of the TBS inducing LTP (interstimulus interval = 10 ms), calculated as the ratio of the second to the first fEPSP slope. Thus, a PPR >1 indicates paired pulse facilitation. The data were compared across drug groups in a one-way analysis of variance (ANOVA) in R (v4.2.2; R Core Team, 2022). Significant differences were subsequently tested with Tukey's HSD for post hoc comparisons.
Results
Pretreatment with DOI increases excitability of hippocampal CA1 synapses
Stimulation of Schaffer collateral inputs to stratum radiatum in CA1 evoked FVs and fEPSPs with amplitudes and time courses typical of this experimental preparation72 (Fig. 1). Example fEPSP S-R curves recorded in slices obtained from saline-, DOI-, and ketamine-treated animals are shown in Figure 1, and averaged results for each condition are shown in Figure 2A.
FIG. 1.
Picture of slice and example fEPSP S-R curves and fEPSP traces for each treatment group. (A) An image of the dorsal hippocampal slices, electrodes and areas are marked. The figures in (B) show single slice examples of S-R curves recorded in stratum radiatum in CA1 in response to Schaffer collateral stimulation at varying stimulation intensities (40–500 μA) before LTP induction. Sigmoidal curve fits are superimposed. Averaged traces for each experiment are shown as insets. fEPSP, field excitatory postsynaptic potential; LTP, long-term potentiation; S-R, stimulus–response.
FIG. 2.
Slices from DOI-treated animals exhibited increased excitability compared with ketamine and saline. (A) Averaged fEPSP S-R curves across all slices for each drug condition before TBS induction. (B) fEPSP S-R curve fit parameters for each drug condition pre-TBS. Each symbol is one individual slice. Box plots show median, upper and lower quartile ranges, and data range excluding outliers. DOI-treated animals had slopes that were significantly greater compared with saline (p = 0.0182) and ketamine (p = 0.0136). There were no differences observed between treatment groups for RMax or S50% (RMax: F2,67 = 1.55, p = 0.222; S50%: F2,67 = 2.76, p = 0.0706). *p < 0.05. DOI, 2,5-dimethoxy-4-iodoamphetamine; M, slope; RMax, asymptotic response; S50%, stimulus intensity eliciting half maximal response; TBS, theta-burst stimulation.
fEPSP S-R curves obtained in slices from DOI-treated animals were markedly different compared with those obtained from saline- or ketamine-treated animals. The slope (M) of fEPSP S-R curves exhibited a significant treatment effect (Fig. 2B, F2,67 = 5.407, p = 0.00665). In post hoc testing, M for DOI was significantly different from saline (p = 0.0182) and ketamine (p = 0.0136), whereas ketamine was not significantly different from saline (p = 0.979). No significant treatment effects were observed for RMax or S50% (RMax: F2,67 = 1.55, p = 0.222; S50%: F2,67 = 2.76, p = 0.0706). Thus, slices from DOI-treated animals exhibited greater excitability compared with ketamine- or saline-treated animals. These data indicate that DOI had acutely induced a form of synaptic plasticity in hippocampus that persists for at least 24 h.
Pretreatment with either DOI or ketamine does not alter LTP
Having observed evidence for an acute, persistent change in excitability in response to DOI, we next asked whether induction and maintenance of LTP itself is different 24 h after administration of DOI or ketamine. S-R curves were obtained, and the stimulus intensity that produced an ∼50% maximal response was selected for the LTP protocol. After a 30-min baseline period, TBS-induced LTP, defined as potentiation that lasted at least 60 min, was readily observed (Fig. 3A, B). However, no difference was observed between treatment groups in the magnitude of LTP at t = 50–60 min (Fig. 3C; F2,56 = 0.67, p = 0.516). Thus, neither DOI nor ketamine affected the magnitude of LTP 24 h after treatment.
FIG. 3.
Summary of LTP across treatment groups. (A) Example pre- and post-TBS fEPSP traces averaged from t = 50–60 min from each treatment group. (B) fEPSP time series data for the three treatment groups from baseline to 60 min post-TBS. Symbols are mean ± standard error of the mean. (C) fEPSP slope averaged between t = 50–60 min post-TBS. Each symbol is one experiment. Box plots show median, upper and lower quartile ranges, and data range excluding outliers. Groups were not significantly different (F2,56 = 0.67, p = 0516).
Pretreatment with DOI enhances short-term plasticity
Although the magnitude of LTP was not different between treatment groups, fEPSP slopes in slices from DOI-treated animals were considerably larger than those from saline- or ketamine-treated animals immediately after TBS (Fig. 3B), indicating that in addition to strengthening synapses, treatment with DOI also increases STP (Fig. 4A, B; F2,56 = 4.41, p = 0.0167). Post hoc tests showed that responses from the DOI group were significantly enhanced compared with saline (p = 0.0352) and ketamine (p = 0.0332), while ketamine was not different from saline (p = 0.999). These data indicate that 24 h after treatment, DOI, but not ketamine, induced changes in plasticity in hippocampus. By contrast, no differences across treatment groups were observed in the time course of decay (τWt) of the fEPSP slope to its steady-state baseline following TBS (Fig. 5C; F2,56 = 0.607, p = 0.549).
FIG. 4.

Summary of STP across treatment groups. (A) Example average pre- and post-TBS fEPSP traces from each treatment group. Five minutes from each treatment group. (B) Average fEPSP slope from t = 0–5 min post-TBS. Groups were significantly different (F2,56 = 4.409, p = 0.0167). Responses from the DOI group were significantly enhanced compared with saline (p = 0.0352) and ketamine (p = 0.0332), while ketamine was not different from saline (p = 0.999). (C) Weighted time constant τWt across treatment groups. No significant differences were observed across groups (F2,56 = 0.607, p = 0.549). In both (A, B), each symbol is one experiment. Box plots show median, upper and lower quartile ranges, and data range excluding outliers. *p < 0.05. STP, short-term potentiation.
FIG. 5.

Summary of FV S-R curves. Averaged FV S-Rs across all slices for each drug condition. DOI was significantly different compared with saline (p = 0.0458) where Ketamine was not (p = 0.868). FV, fiber volley.
The effects of DOI are presynaptic
FV amplitudes represent excitability of the stimulated afferent fibers. FV amplitudes were examined to evaluate presynaptic contributions to the results of Figures 2 and 4. FV S-R curves showed a clear difference between DOI-treated animals compared with ketamine- or saline-treated animals (Fig. 5). Maximal FV amplitudes exhibited a significant treatment effect (F = 3.17, p = 0.0490), and post hoc testing indicated that FV amplitudes were significantly greater in DOI-treated animals compared with the other groups (p = 0.0458), while no difference was observed between saline and ketamine (p = 0.868). This suggests that the increased excitability in slices from DOI-treated animals shown in Figure 2 was at least partly due to presynaptic effects of DOI.
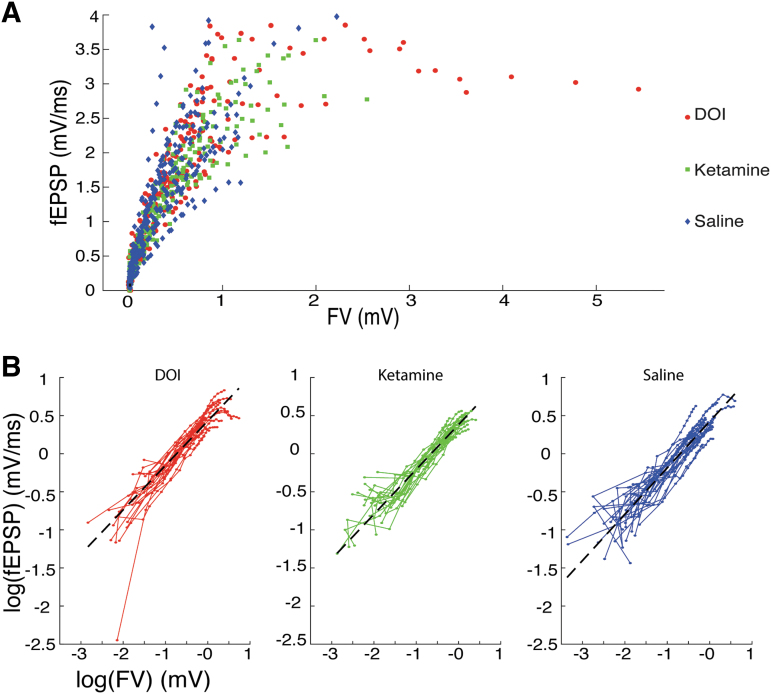
To determine the extent of this presynaptic contribution, we compared presynaptic and postsynaptic responses directly by plotting fEPSP slopes as a function of FV amplitudes (Fig. 6). Here, we reasoned that if the effect of DOI was entirely presynaptic, then there should be no difference between treatment groups when fEPSP slopes are considered directly as a function of FV amplitude, since a given presynaptic input will elicit the same postsynaptic response if there are no postsynaptic changes induced by DOI. Indeed, the data from the three treatment groups largely overlap in Figure 6A, consistent with this hypothesis. To test this quantitatively, we log transformed the data and applied a linear mixed effects model with random slopes and intercepts (Fig. 6B). We found no significant difference in fitted slopes (saline: 0.608; DOI: 0.583; ketamine: 0.588) or intercepts (saline: 0.423; DOI: 0.429; ketamine: 0.382) between treatment groups (all p-values >0.226).
FIG. 6.
No effect of treatment on fEPSPs when plotted versus FV amplitude. (A) Stimulus–response data from all slices and all treatment groups showing overlap of data when plotted as a function of FV amplitude. (B) Data from (A) separated by treatment group and plotted on a log-log scale to demonstrate suitability for linear regression analysis. We found no significant difference in fitted slopes (saline: 0.608; DOI: 0.583; ketamine: 0.588) or intercepts (saline: 0.423; DOI: 0.429; ketamine: 0.382) between treatment groups (all p-values >0.226).
The PPR (i.e., the ratio of the second to the first synaptic response to two stimuli presented at short interstimulus interval) is commonly used to evaluate presynaptic short-term plasticity.74 To further elucidate presynaptic contributions to the observed effects of DOI, we evaluated the PPR for fEPSPs evoked by the first two stimuli (separated by 10 ms) in the LTP-inducing protocol. We found that there was a significant difference between the groups (F2,60 = 3.581, p = 0.0339; Fig. 7), with PPR in DOI-treated animals significantly greater to saline-treated animals (p = 0.0257), but PPR in ketamine-treated animals no different from saline (p = 0.484). These results support a presynaptic component to the mechanism underlying the observed neuroplastic and metaplastic effects of DOI in hippocampus.
FIG. 7.
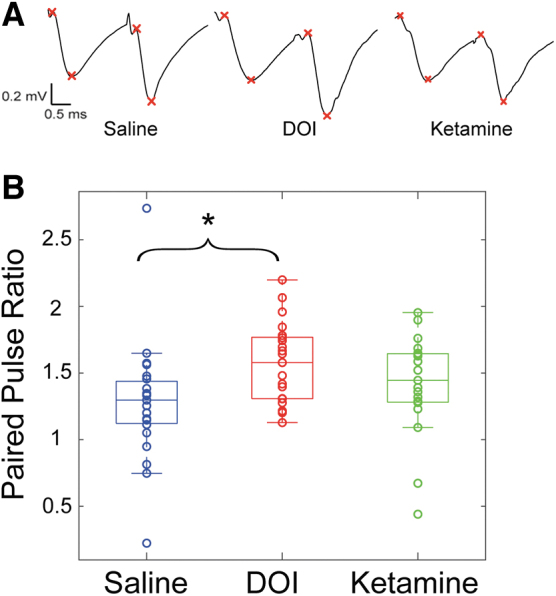
Increased PPR for DOI treated slices. (A) Representative mean paired-pulse responses from single slice experiments. The pairs of x's denote measurements of baseline and peak amplitude values. (B) PPR was measured as the slope of the second fEPSP divided by the first. The groups were significantly different (F2,60 = 3.581, p = 0.0339) with DOI-treated slices having significantly higher ratios compared with saline (p = 0.0257). Ketamine was not significantly different from saline (p = 0.485). *p < 0.05. PPR, paired pulse ratio.
Discussion
The 1- to 2-week period following treatment with ketamine and serotonergic psychedelics is called the “after-glow” period, during which subjects exhibit dramatic changes in mood and perspective, and openness to new experiences, ideas, and narratives.75,76 The pro-neuroplastic properties of ketamine and serotonergic psychedelics, demonstrated most convincingly in preclinical models, likely contribute to these effects.24 The psychedelic experience itself has been described as transformative, transgressing normative boundaries of lived experience so profoundly that many subjects are reminded that there are many possible stories they can construct about themselves and their relationship to the world, and thus change their actions accordingly.63
Neuroscience researchers have taken this a step further; however, proposing that the transformative effects of psychedelics may be mediated by “metaplastic” neural changes, such as altered thresholds for and magnitudes of synaptic plasticity, lasting for days and weeks after the drugs have left the central nervous system (CNS).54–56 This suggestion is based in part on the importance of 5-HT2A receptor signaling during the critical period of neuroplasticity that arises during neurodevelopment.53 Indeed, the integration sessions that follow psychedelic dosing in standard models of psychedelic-assisted psychotherapy rely implicitly on a post-exposure enhancement of capacity for behavioral change.
Here, we evaluated the effects of a serotonergic psychedelic (DOI) and ketamine on plasticity and metaplasticity at Schaffer collateral synapses in CA1 of murine hippocampal brain slices, a widely accepted in vitro molecular model of memory formation.77 We observed an increase in the slope of fEPSP S-R curves at these synapses 24 h after administration of DOI, but not ketamine, suggesting an increase in neuronal excitability. Previous results indicate that a 1 mg/kg IP dose of DOI is fully cleared from the CNS of mice by 24 h, indicating that these changes persist beyond the presence of the drug at these synapses.78 These results for DOI are consistent with previous studies showing direct, rapid, and persistent neuroplastic effects of psychedelics.
Changes in neurite outgrowth and spine and synaptic density have been observed following brief application of lysergic acid diethylamide in cultured rat cortical neurons79 and following a single administration of psilocybin in vivo in murine mPFC.33,38 In addition, enhanced synaptic responses have been observed in hippocampal brain slices 3 days after administration of psilocybin in vivo in a mouse model of stress-induced phenotypes.30
The absence of neuroplastic effects of ketamine at hippocampal synapses in this study is surprising, as previous studies have shown rapid and persistent neuroplastic effects of ketamine in cultured neurons in vitro and in mPFC in vivo.3,32,79,80 Ketamine has also been shown to induce LTP in hippocampal slices when applied directly,80,81 but the post-acute effects reported at the 24-h time point are sex-specific and may depend on pre-existing stress exposure.64 In addition, neuroplastic changes in response to ketamine have been reported to occur after multiple exposures to the drug in vivo followed by exposure ex vitro, and on different time scales in different regions of the brain, which may also contribute to distinct outcomes in hippocampus and mPFC.80,82
Importantly, we observed evidence that DOI induced metaplastic effects, i.e., changes in neuroplasticity, at Schaffer collateral synapses in CA1, consistent with effects observed previously in mPFC 24-h after administration of DOI.38 Although the magnitude of LTP in response to TBS stimulation was indistinguishable between treatment groups, the magnitude of STP was significantly enhanced for animals previously treated with DOI compared with those previously receiving ketamine or saline. STP is defined as the initial transient increase in response amplitude immediately following TBS. This early phase of plasticity is mechanistically distinct from the later phase of LTP at Schaffer collateral synapses in CA183 and likely relies on changes in presynaptic signaling.84
Further support for a presynaptic mechanism underlying the effects of DOI comes from the observations that FV amplitudes were increased following DOI pretreatment (Fig. 5) and from analysis of PPRs (Fig. 7). The observed increases in FV amplitudes may arise due to increased excitability of axonal fibers, or increased numbers of fibers secondary to increased numbers of synaptic release sites. The latter is consistent with previous observations of increased numbers and size of dendritic spines in mPFC following the administration of serotonergic psychedelics in mice.33,38 The observation that PPRs in slices from DOI-treated animals were enhanced compared with saline suggests that these new release sites have smaller release probability compared with pre-treatment release sites. Differences in release probability at newly established release sites following acute neuroplasticity have been shown previously at hippocampal synapses.85 Thus, our observations that STP, FV amplitudes, and PPRs were significantly enhanced in DOI-treated animals are consistent with a presynaptic component to the mechanism for the neuroplastic and metaplastic effects of DOI, but postsynaptic changes may contribute as well.
DOI-induced changes in STP are likely to be functionally significant, even though, compared with LTP, STP occurs on a much shorter timescale, does not involve the transcription of plasticity associated genes, and is less durable.84 STP can have significant effects on neural information transfer86,87 and can modulate neural responses to transient inputs88 and network responses to sustained inputs,89 as well as induce state switching in neurons (up and down states).90
Interestingly, STP has been observed to depend on presynaptic NMDA receptors (NMDARs),83,91 and this may underlie the effects of DOI on both STP and S-R curves (excitability) observed here. DOI binds with high affinity at 5-HT2A receptors, and evidence suggests that 5-HT2A receptors and NMDARs are closely linked. For example, activation of 5-HT2A receptors enhances NMDAR-dependent transmission and plasticity at thalamocortical synapses via a presynaptic mechanism.92 Furthermore, in a study assessing the effects of the agonist 2,5-dimethoxy-4-bromoamphetamine, which, like DOI, has similarly strong affinity for both 5-HT2A and 5-HT2C receptors, activation of 5-HT2A receptors was necessary for the NMDAR-induced changes to excitatory post-synaptic potentials and resting membrane potential through a presynaptic mechanism.93
Serotonergic psychedelics, including DOI, have been evidenced to increase extracellular glutamate in cortical areas through the activation of 5-HT2A receptors on cortical pyramidal cells.94,95 The interplay between these 5-HT2A receptors and glutamate receptors may be due to the close proximity with which they exist on the transmembrane domain and also due to the formation of heterodimeric complex between these receptors.27 Thus, it is possible that the observed effects of DOI on both S-R curves and STP arise due to enhanced glutamatergic neurotransmission through NMDARs downstream of presynaptic 5-HT2A receptor activation. Indeed, literature suggests that DOI-induced metaplasticity may be dependent on NMDA receptor activation, and so this proposed mechanism may also provide context for the absence of an effect on excitability or STP with ketamine, due to its direct antagonism of NMDARs.96
Under the definition first put forward by Abraham and Bear (1996),—“Metaplasticity is manifest as a change in the ability to induce subsequent synaptic plasticity”—DOI's effects on subsequent STP are metaplastic. While effects on subsequent LTP or long-term depression (LTD) have more commonly been provided as examples of such metaplasticity,38,51 the DOI-induced change in STP at 24 h following administration is clearly also reflective of long-lasting changes to functional neuronal dynamics. In all, our work provides evidence for neuroplastic and metaplastic effects at CA1 Schaffer collateral synapses at 24 h following serotonergic psychedelic administration, but not ketamine administration. Our findings provide evidence for a presynaptic mechanism for metaplasticity and a mechanistic basis for change in network level activity in response to psychedelic drug administration.
Future directions
As the data in this study are limited, future experiments should be conducted to elucidate the results here. First, our experiments were focused in dorsal hippocampus, and we did not investigate regional heterogeneity in the observed effects of DOI. For example, dorsal and ventral hippocampus are anatomically distinct, with different patterns of afferent and efferent connections.97 Functionally, the dorsal portion plays a direct role in memory processes where the ventral portion plays a more direct role in stress responses and emotionally motivated behaviors, such as approach and avoidance.97 Future experiments will elucidate whether the effects reported here generalize to ventral hippocampus. The use of additional psychedelics such as psilocybin can be helpful as positive controls along with the addition of an NMDA receptor antagonist pretreatment with DOI and other psychedelics to discern the receptor-level contributions to the observed mechanism. The identity of the subtypes of 5-HT receptors involved also remains an open question. Data suggest that DOI also has high affinity as a partial agonist of the 5-HT2C receptor.98–100
It has also been shown that the 5-HT2C receptor, in part, facilitates 5-HT2A receptor-induced effects on locomotion, suggesting that DOI may exert behavioral effects through coordinated action at both receptor subtypes.101 Future experiments with specific receptor antagonists will help resolve this question. Further clinical and preclinical work is needed to better understand the mechanism and role of plasticity and metaplasticity in the brain following serotonergic psychedelic administration. Considering the enhanced excitability seen here, as well as the proposed evolutionary role of metaplasticity in “tuning” synaptic signaling to prevent saturation that impairs learning, investigations into metaplastic effects of DOI on LTD may be of substantial mechanistic importance. Further assessment of these outcomes across alternative brain regions, time courses, and species will also be key experiments regarding the translational impact of this finding on optimization of psychedelic-assisted psychotherapy protocols.
Authors' Contributions
Z.W.S. contributed to the acquisition of the data, and Z.Z. and Z.W.S. contributed to the analysis, and interpretation of data, and drafting the article. R.A.P. contributed to the acquisition and interpretation of data and drafting the article. B.M.K. contributed to the analysis of data and drafting the article. C.J.W. contributed to the interpretation of data and drafting the article. M.I.B. contributed to the conception of the work, analysis and interpretation of data, and drafting the article.
Author Disclosure Statement
M.I.B. is a paid consultant for VCENNA, Inc., no research support for this work was provided by VCENNA. C.J.W. receives research funding from Psilera, Inc., no research support for this work was provided by Psilera, Inc. Z.Z., Z.W.S., R.A.P., and B.M.K. declare no competing interests.
Funding Information
Funding was provided by the UW Department of Anesthesiology, UW Office of the Vice Chancellor for Research and Graduate Education, the National Institute of Mental Health (R01MH122742 to C.J.W.), the National Institute of General Medicine (R01GM118801 to R.A.P.), and the National Institute of Neurological Disorders and Stroke (T32NS105602 to Z.Z.).
References
- 1. Walsh Z, Mollaahmetoglu OM, Rootman J, et al. Ketamine for the treatment of mental health and substance use disorders: Comprehensive systematic review. BJPsych Open 2021;8(1):e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reiff CM, Richman EE, Nemeroff CB, et al. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry 2020;177(5):391–410. [DOI] [PubMed] [Google Scholar]
- 3. Moda-Sava RN, Murdock MH, Parekh PK, et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science 2019;364(6436):eaat8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fitzgerald PJ, Yen JY, Watson BO. Stress-sensitive antidepressant-like effects of ketamine in the mouse forced swim test. PLoS One 2019;14(4):e0215554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Gregorio D, Popic J, Enns JP, et al. Lysergic acid diethylamide (LSD) promotes social behavior through mTORC1 in the excitatory neurotransmission. Proc Natl Acad Sci U S A 2021;118(5):e2020705118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fadahunsi N, Lund J, Breum AW, et al. Acute and long-term effects of psilocybin on energy balance and feeding behavior in mice. Transl Psychiatry 2022;12(1):330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bouso JC, Dos Santos RG, Alcazar-Corcoles MA, et al. Serotonergic psychedelics and personality: A systematic review of contemporary research. Neurosci Biobehav Rev 2018;87:118–132. [DOI] [PubMed] [Google Scholar]
- 8. Doss MK, Povazan M, Rosenberg MD, et al. Psilocybin therapy increases cognitive and neural flexibility in patients with major depressive disorder. Transl Psychiatry 2021;11(1):574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yaden DB, Griffiths RR. The subjective effects of psychedelics are necessary for their enduring therapeutic effects. ACS Pharmacol Transl Sci 2021;4(2):568–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davis AK, Barrett FS, Griffiths RR. Psychological flexibility mediates the relations between acute psychedelic effects and subjective decreases in depression and anxiety. J Contextual Behav Sci 2020;15:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murphy-Beiner A, Soar K. Ayahuasca's ‘afterglow’: Improved mindfulness and cognitive flexibility in ayahuasca drinkers. Psychopharmacology (Berl) 2020;237(4):1161–1169. [DOI] [PubMed] [Google Scholar]
- 12. Uthaug MV, van Oorsouw K, Kuypers KPC, et al. Sub-acute and long-term effects of ayahuasca on affect and cognitive thinking style and their association with ego dissolution. Psychopharmacology (Berl) 2018;235(10):2979–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gukasyan N, Davis AK, Barrett FS, et al. Efficacy and safety of psilocybin-assisted treatment for major depressive disorder: Prospective 12-month follow-up. J Psychopharmacol 2022;36(2):151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Griffiths RR, Johnson MW, Carducci MA, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J Psychopharmacol 2016;30(12):1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ross S, Bossis A, Guss J, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: A randomized controlled trial. J Psychopharmacol 2016;30(12):1165–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Agin-Liebes GI, Malone T, Yalch MM, et al. Long-term follow-up of psilocybin-assisted psychotherapy for psychiatric and existential distress in patients with life-threatening cancer. J Psychopharmacol 2020;34(2):155–166. [DOI] [PubMed] [Google Scholar]
- 17. Mion G, Villevieille T. Ketamine pharmacology: An update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci Ther 2013;19(6):370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Preller KH, Duerler P, Burt JB, et al. Psilocybin induces time-dependent changes in global functional connectivity. Biol Psychiatry 2020;88(2):197–207. [DOI] [PubMed] [Google Scholar]
- 19. Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, et al. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 1998;9(17):3897–3902. [DOI] [PubMed] [Google Scholar]
- 20. Zhang MW, Ho RC. Controversies of the effect of ketamine on cognition. Front Psychiatry 2016;7:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bălăeţ M. Psychedelic cognition-the unreached frontier of psychedelic science. Front Neurosci 2022;16:832375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mans K, Kettner H, Erritzoe D, et al. Sustained, multifaceted improvements in mental well-being following psychedelic experiences in a prospective opportunity sample. Front Psychiatry 2021;12:647909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCulloch DE, Grzywacz MZ, Madsen MK, et al. Psilocybin-induced mystical-type experiences are related to persisting positive effects: A quantitative and qualitative report. Front Pharmacol 2022;13:841648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aleksandrova LR, Phillips AG. Neuroplasticity as a convergent mechanism of ketamine and classical psychedelics. Trends Pharmacol Sci 2021;42(11):929–942. [DOI] [PubMed] [Google Scholar]
- 25. Kadriu B, Greenwald M, Henter ID, et al. Ketamine and serotonergic psychedelics: Common mechanisms underlying the effects of rapid-acting antidepressants. Int J Neuropsychopharmacol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moliner R, Girych M, Brunello CA, et al. Psychedelics promote plasticity by directly binding to BDNF receptor TrkB. Nat Neurosci 2023;26(6):1032–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Banks MI, Zahid Z, Jones NT, et al. Catalysts for change: The cellular neurobiology of psychedelics. Mol Biol Cell 2021;32(12):1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vargas MV, Meyer R, Avanes AA, et al. Psychedelics and other psychoplastogens for treating mental illness. Front Psychiatry 2021;12:727117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jett JD, Boley AM, Girotti M, et al. Antidepressant-like cognitive and behavioral effects of acute ketamine administration associated with plasticity in the ventral hippocampus to medial prefrontal cortex pathway. Psychopharmacology (Berl) 2015;232(17):3123–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hesselgrave N, Troppoli TA, Wulff AB, et al. Harnessing psilocybin: Antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice. Proc Natl Acad Sci U S A 2021;118(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang J, Qu Y, Chang L, et al. (R)-ketamine rapidly ameliorates the decreased spine density in the medial prefrontal cortex and hippocampus of susceptible mice after chronic social defeat stress. Int J Neuropsychopharmacol 2019;22(10):675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li N, Liu RJ, Dwyer JM, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 2011;69(8):754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shao LX, Liao C, Gregg I, et al. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron 2021;109(16):2535–2544 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ly C, Greb AC, Cameron LP, et al. Psychedelics promote structural and functional neural plasticity. Cell Rep 2018;23(11):3170–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dakic V, Minardi Nascimento J, Costa Sartore R, et al. Short term changes in the proteome of human cerebral organoids induced by 5-MeO-DMT. Sci Rep 2017;7(1):12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu M, Minkowicz S, Dumrongprechachan V, et al. Ketamine rapidly enhances glutamate-evoked dendritic spinogenesis in medial prefrontal cortex through dopaminergic mechanisms. Biol Psychiatry 2021;89(11):1096–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cavalleri L, Merlo Pich E, Millan MJ, et al. Ketamine enhances structural plasticity in mouse mesencephalic and human iPSC-derived dopaminergic neurons via AMPAR-driven BDNF and mTOR signaling. Mol Psychiatry 2018;23(4):812–823. [DOI] [PubMed] [Google Scholar]
- 38. de la Fuente Revenga M, Zhu B, Guevara CA, et al. Prolonged epigenomic and synaptic plasticity alterations following single exposure to a psychedelic in mice. Cell Rep 2021;37(3):109836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Catlow BJ, Song S, Paredes DA, et al. Effects of psilocybin on hippocampal neurogenesis and extinction of trace fear conditioning. Exp Brain Res 2013;228(4):481–491. [DOI] [PubMed] [Google Scholar]
- 40. Lima da Cruz RV, Moulin TC, Petiz LL, et al. A single dose of 5-MeO-DMT stimulates cell proliferation, neuronal survivability, morphological and functional changes in adult mice ventral dentate gyrus. Front Mol Neurosci 2018;11:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rawat R, Tunc-Ozcan E, McGuire TL, et al. Ketamine activates adult-born immature granule neurons to rapidly alleviate depression-like behaviors in mice. Nat Commun 2022;13(1):2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yamada J, Jinno S. Potential link between antidepressant-like effects of ketamine and promotion of adult neurogenesis in the ventral hippocampus of mice. Neuropharmacology 2019;158:107710. [DOI] [PubMed] [Google Scholar]
- 43. Clarke M, Razmjou S, Prowse N, et al. Ketamine modulates hippocampal neurogenesis and pro-inflammatory cytokines but not stressor induced neurochemical changes. Neuropharmacology 2017;112(Pt A):210–220. [DOI] [PubMed] [Google Scholar]
- 44. Jefsen OH, Elfving B, Wegener G, et al. Transcriptional regulation in the rat prefrontal cortex and hippocampus after a single administration of psilocybin. J Psychopharmacol 2020:0269881120959614. [DOI] [PubMed] [Google Scholar]
- 45. González-Maeso J, Yuen T, Ebersole BJ, et al. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J Neurosci 2003;23(26):8836–8843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nichols CD, Sanders-Bush E. A single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain. Neuropsychopharmacology 2002;26(5):634–642. [DOI] [PubMed] [Google Scholar]
- 47. Nichols CD, Garcia EE, Sanders-Bush E. Dynamic changes in prefrontal cortex gene expression following lysergic acid diethylamide administration. Brain Res Mol Brain Res 2003;111(1–2):182–188. [DOI] [PubMed] [Google Scholar]
- 48. Nichols CD, Sanders-Bush E. Molecular genetic responses to lysergic acid diethylamide include transcriptional activation of MAP kinase phosphatase-1, C/EBP-beta and ILAD-1, a novel gene with homology to arrestins. J Neurochem 2004;90(3):576–584. [DOI] [PubMed] [Google Scholar]
- 49. Viana GSB, Vale EMD, Araujo ARA, et al. Rapid and long-lasting antidepressant-like effects of ketamine and their relationship with the expression of brain enzymes, BDNF, and astrocytes. Braz J Med Biol Res 2020;54(2):e10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Davoudian PA, Shao L-X, Kwan AC. Shared and distinct brain regions targeted for immediate early gene expression by ketamine and psilocybin. ACS Chem Neurosci 2023;14(3):468–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nardou R, Sawyer E, Song YJ, et al. Psychedelics reopen the social reward learning critical period. Nature 2023;618(7966):790–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bathje GJ, Majeski E, Kudowor M. Psychedelic integration: An analysis of the concept and its practice. Front Psychol 2022;13:824077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Carhart-Harris RL, Nutt DJ. Serotonin and brain function: A tale of two receptors. J Psychopharmacol 2017;31(9):1091–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lepow L, Morishita H, Yehuda R. Critical period plasticity as a framework for psychedelic-assisted psychotherapy. Front Neurosci 2021;15:710004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Abraham WC, Bear MF. Metaplasticity: The plasticity of synaptic plasticity. Trends Neurosci 1996;19(4):126–130. [DOI] [PubMed] [Google Scholar]
- 56. Abraham WC. Metaplasticity: Tuning synapses and networks for plasticity. Nat Rev Neurosci 2008;9(5):387. [DOI] [PubMed] [Google Scholar]
- 57. Ewald AH, Fritschi G, Bork WR, et al. Designer drugs 2,5-dimethoxy-4-bromo-amphetamine (DOB) and 2,5-dimethoxy-4-bromo-methamphetamine (MDOB): Studies on their metabolism and toxicological detection in rat urine using gas chromatographic/mass spectrometric techniques. J Mass Spectrom 2006;41(4):487–498. [DOI] [PubMed] [Google Scholar]
- 58. Zhai Y, George CA, Zhai J, et al. Group II metabotropic glutamate receptor modulation of DOI-induced c-fos mRNA and excitatory responses in the cerebral cortex. Neuropsychopharmacology 2003;28(1):45–52. [DOI] [PubMed] [Google Scholar]
- 59. Glatfelter GC, Pottie E, Partilla JS, et al. Structure–activity relationships for psilocybin, baeocystin, aeruginascin, and related analogues to produce pharmacological effects in mice. ACS Pharmacol Transl Sci 2022;5(11):1181–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bittar TP, Labonte B. Functional contribution of the medial prefrontal circuitry in major depressive disorder and stress-induced depressive-like behaviors. Front Behav Neurosci 2021;15:699592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 2010;35(1):169–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kessler R, Schmitt S, Sauder T, et al. Long-term neuroanatomical consequences of childhood maltreatment: Reduced amygdala inhibition by medial prefrontal cortex. Front Syst Neurosci 2020;14:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Amada N, Lea T, Letheby C, et al. Psychedelic experience and the narrative self: An exploratory qualitative study. J Conscious Stud 2020;27:6–33. [Google Scholar]
- 64. Logue J, Schoepfer K, Guerrero AB, et al. Sex-specific effects of social isolation stress and ketamine on hippocampal plasticity. Neurosci Lett 2022;766:136301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Torrado Pacheco A, Olson RJ, Garza G, et al. Acute psilocybin enhances cognitive flexibility in rats. Neuropsychopharmacology 2023;48(7):1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Darmani NA, Martin BR, Pandey U, et al. Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol Biochem Behav 1990;36(4):901–906. [DOI] [PubMed] [Google Scholar]
- 67. Jiang K, Liu X, Su R. Contrasting effects of DOI and lisuride on impulsive decision-making in delay discounting task. Psychopharmacology 2022;239(11):3551–3565. [DOI] [PubMed] [Google Scholar]
- 68. Berquist MD, Fantegrossi WE. Effects of 5-HT2A receptor agonist 2,5-dimethoxy-4-iodoamphetamine on alcohol consumption in Long-Evans rats. Behav Pharmacol 2021;32(5):382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Choi KH, Berman RY, Zhang M, et al. Effects of ketamine on rodent fear memory. Int J Mol Sci 2020;21(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Morena M, Colucci P, Mancini GF, et al. Ketamine anesthesia enhances fear memory consolidation via noradrenergic activation in the basolateral amygdala. Neurobiol Learn Mem 2021;178:107362. [DOI] [PubMed] [Google Scholar]
- 71. Zanos P, Moaddel R, Morris PJ, et al. Ketamine and ketamine metabolite pharmacology: Insights into therapeutic mechanisms. Pharmacol Rev 2018;70(3):621–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Figueroa AG, Benkwitz C, Surges G, et al. Hippocampal β2-GABA(A) receptors mediate LTP suppression by etomidate and contribute to long-lasting feedback but not feedforward inhibition of pyramidal neurons. J Neurophysiol 2021;126(4):1090–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol 2002;64:355–405. [DOI] [PubMed] [Google Scholar]
- 74. Singh N, Bartol T, Levine H, et al. Presynaptic endoplasmic reticulum regulates short-term plasticity in hippocampal synapses. Commun Biol 2021;4(1):241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Majic T, Schmidt TT, Gallinat J. Peak experiences and the afterglow phenomenon: When and how do therapeutic effects of hallucinogens depend on psychedelic experiences? J Psychopharmacol 2015;29(3):241–253. [DOI] [PubMed] [Google Scholar]
- 76. Sumner RL, Chacko E, McMillan R, et al. A qualitative and quantitative account of patient's experiences of ketamine and its antidepressant properties. J Psychopharmacol 2021;35(8):946–961. [DOI] [PubMed] [Google Scholar]
- 77. Bear MF. A synaptic basis for memory storage in the cerebral cortex. Proc Natl Acad Sci U S A 1996;93(24):13453–13459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. de la Fuente Revenga M, Shin JM, Vohra HZ, et al. Fully automated head-twitch detection system for the study of 5-HT2A receptor pharmacology in vivo. Sci Rep 2019;9(1):14247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ly C, Greb AC, Vargas MV, et al. Transient stimulation with psychoplastogens is sufficient to initiate neuronal growth. ACS Pharmacol Transl Sci 2021;4(2):452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kim JW, Autry AE, Na ES, et al. Sustained effects of rapidly acting antidepressants require BDNF-dependent MeCP2 phosphorylation. Nat Neurosci 2021;24(8):1100–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jang G, MacIver MB. Ketamine produces a long-lasting enhancement of CA1 neuron excitability. Int J Mol Sci 2021;22(15):8091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gass N, Becker R, Reinwald J, et al. Differences between ketamine's short-term and long-term effects on brain circuitry in depression. Transl Psychiatry 2019;9(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Park P, Volianskis A, Sanderson TM, et al. NMDA receptor-dependent long-term potentiation comprises a family of temporally overlapping forms of synaptic plasticity that are induced by different patterns of stimulation. Philos Trans R Soc Lond B Biol Sci 2014;369(1633):20130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Volianskis A, Collingridge GL, Jensen MS. The roles of STP and LTP in synaptic encoding. PeerJ 2013;1:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Schulz PE, Cook EP, Johnston D. Using paired-pulse facilitation to probe the mechanisms for long-term potentiation (LTP). J Physiol Paris 1995;89(1):3–9. [DOI] [PubMed] [Google Scholar]
- 86. Fuhrmann G, Segev I, Markram H, et al. Coding of temporal information by activity-dependent synapses. J Neurophysiol 2002;87(1):140–148. [DOI] [PubMed] [Google Scholar]
- 87. Fung CCA, Wong KYM, Wang H, et al. Dynamical synapses enhance neural information processing: Gracefulness, accuracy, and mobility. Neural Comput 2012;24(5):1147–1185. [DOI] [PubMed] [Google Scholar]
- 88. Tsodyks M, Wu S. Short-term synaptic plasticity. Scholarpedia 2013;8. [Google Scholar]
- 89. Barak O, Tsodyks M. Persistent activity in neural networks with dynamic synapses. PLoS Comput Biol 2007;3(2):e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Melamed O, Barak O, Silberberg G, et al. Slow oscillations in neural networks with facilitating synapses. J Comput Neurosci 2008;25(2):308–316. [DOI] [PubMed] [Google Scholar]
- 91. France G, Fernández-Fernández D, Burnell ES, et al. Multiple roles of GluN2B-containing NMDA receptors in synaptic plasticity in juvenile hippocampus. Neuropharmacology 2017;112:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Barre A, Berthoux C, De Bundel D, et al. Presynaptic serotonin 2A receptors modulate thalamocortical plasticity and associative learning. Proc Natl Acad Sci U S A 2016;113(10):E1382–E1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Arvanov VL, Liang X, Magro P, et al. A pre- and postsynaptic modulatory action of 5-HT and the 5-HT2A, 2C receptor agonist DOB on NMDA-evoked responses in the rat medial prefrontal cortex. Eur J Neurosci 1999;11(8):2917–2934. [DOI] [PubMed] [Google Scholar]
- 94. Scruggs JL, Schmidt D, Deutch AY. The hallucinogen 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) increases cortical extracellular glutamate levels in rats. Neurosci Lett 2003;346(3):137–140. [DOI] [PubMed] [Google Scholar]
- 95. Wojtas A, Bysiek A, Wawrzczak-Bargiela A, et al. Effect of psilocybin and ketamine on brain neurotransmitters, glutamate receptors, DNA and rat behavior. Int J Mol Sci 2022;23(12):6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chen A, Hough CJ, Li H. Serotonin type II receptor activation facilitates synaptic plasticity via N-methyl-D-aspartate-mediated mechanism in the rat basolateral amygdala. Neuroscience 2003;119(1):53–63. [DOI] [PubMed] [Google Scholar]
- 97. Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 2010;65(1):7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Marek GJ, Aghajanian GK. LSD and the phenethylamine hallucinogen DOI are potent partial agonists at 5-HT2A receptors on interneurons in rat piriform cortex. J Pharmacol Exp Ther 1996;278(3):1373–1382. [PubMed] [Google Scholar]
- 99. Porter RH, Benwell KR, Lamb H, et al. Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br J Pharmacol 1999;128(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Canal CE, Booth RG, Morgan D. Support for 5-HT2C receptor functional selectivity in vivo utilizing structurally diverse, selective 5-HT2C receptor ligands and the 2,5-dimethoxy-4-iodoamphetamine elicited head-twitch response model. Neuropharmacology 2013;70:112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Halberstadt AL, van der Heijden I, Ruderman MA, et al. 5-HT(2A) and 5-HT(2C) receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology 2009;34(8):1958–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]