Abstract
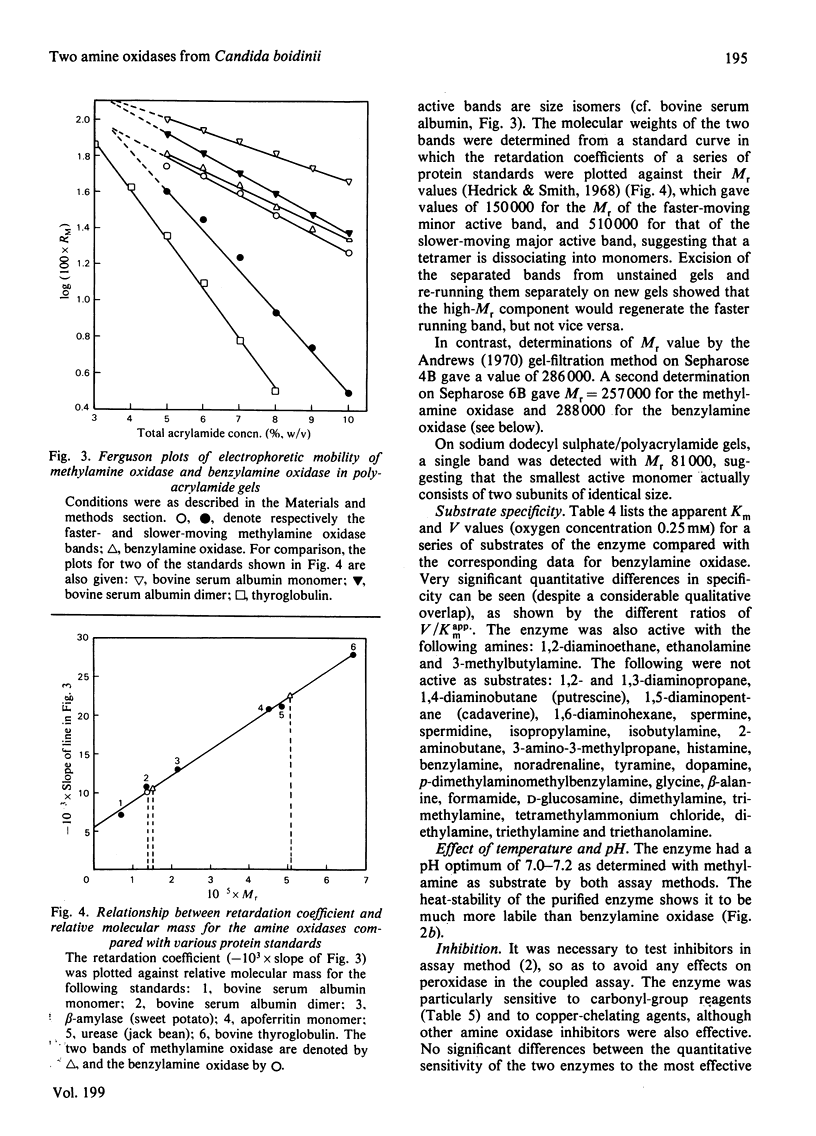
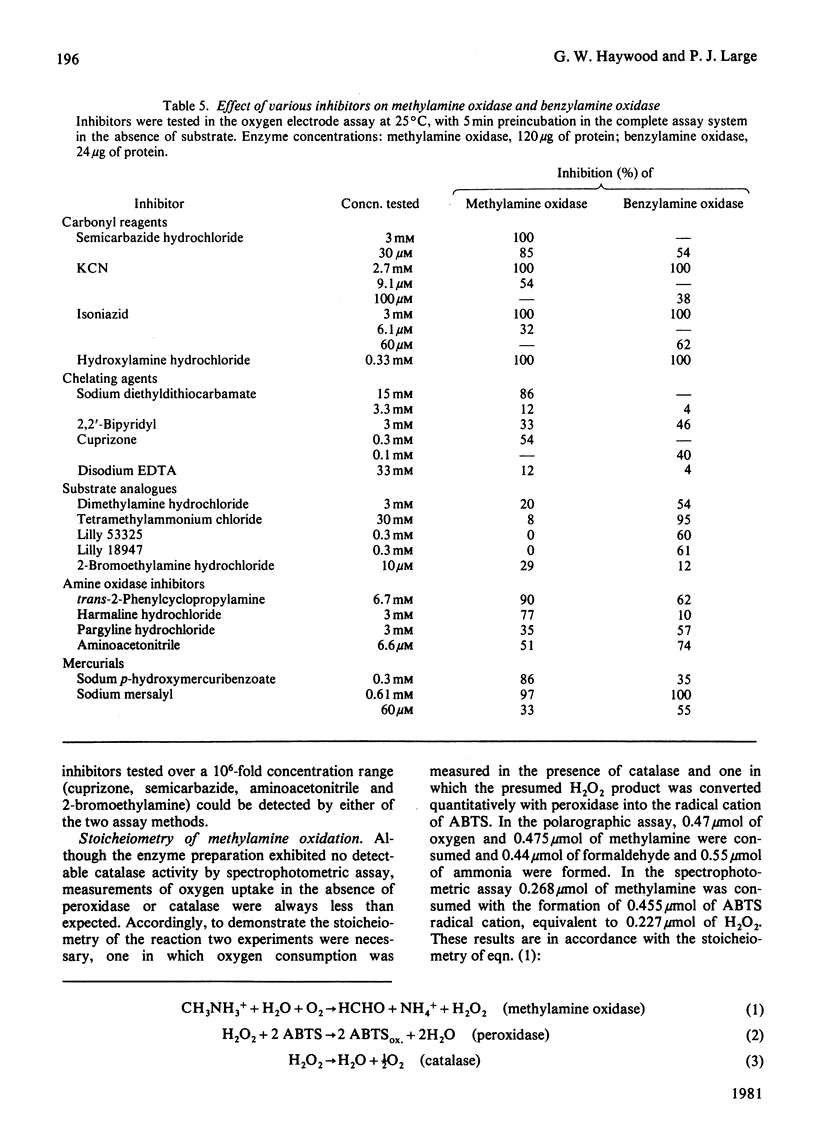
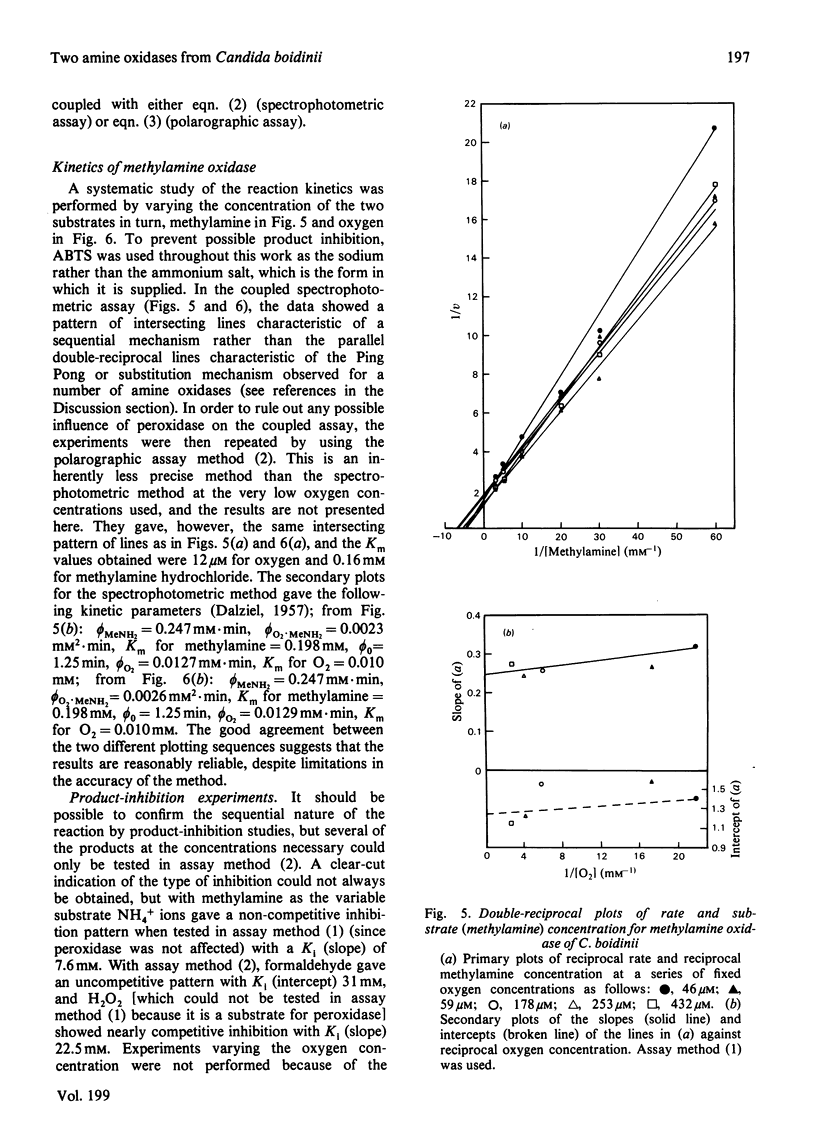
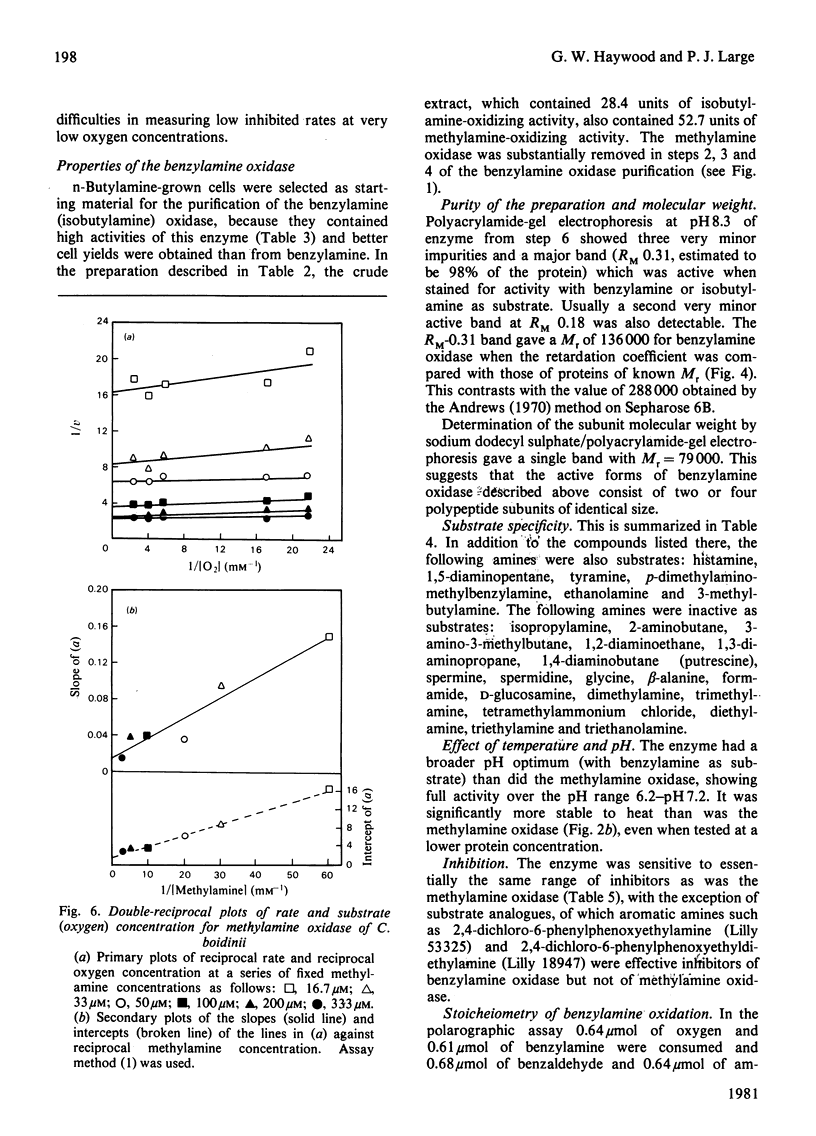
1. The yeast Candida boidinii was grown on glucose as carbon source with a range of amines and amino acids as nitrogen sources. Cells grown on amines contained elevated activities of catalase. If the amines contained N-methyl groups, formaldehyde dehydrogenase, formate dehydrogenase and S-formylglutathione hydrolase were also elevated in activity compared with cells grown on (NH4)2SO4. 2. Cells grown on all the amines tested, but not those grown on urea or amino acids, contained an oxidase attacking primary amines, which is referred to as methylamine oxidase. In addition, cells grown on some amines contained a second amine oxidase, which is referred to as benzylamine oxidase. 3. Both amine oxidases were purified to near homogeneity. 4. Benzylamine oxidase was considerably more stable at 45 and 50°C than was methylamine oxidase. 5. Both enzymes had a pH optimum in the region of 7.0, and had a considerable number of substrates in common. There were, however, significant differences in the substrate specificity of the two enzymes. The ratio V/Kapp.m increased with increasing n-alkyl carbon chain length for benzylamine oxidase, but decreased for methylamine oxidase. 6. Both enzymes showed similar sensitivity to carbonyl-group reagents, copper-chelating agents and other typical `diamine oxidase inhibitors'. 7. The stoicheiometry for the reaction catalysed by each enzyme was established. 8. The kinetics of methylamine oxidase were examined by varying the methylamine and oxygen concentrations in turn. A non-Ping Pong kinetic pattern with intersecting double-reciprocal plots was obtained, giving Km values of 10μm for O2 and 198μm for methylamine. The significance of this unusual kinetic behaviour is discussed. Similar experiments were not possible with the benzylamine oxidase, because it seemed to have an even lower Km for O2. 9. Both enzymes had similar subunit Mr values of about 80000, but the benzylamine oxidase behaved as if it were usually a dimer, Mr 136000, which under certain conditions aggregated to a tetramer, Mr 288000. Methylamine oxidase was mainly in the form of an octamer, Mr 510000, which gave rise quite readily to dimers of Mr 150000, and on gel filtration behaved as if the Mr was 286000.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achee F. M., Chervenka C. H., Smith R. A., Yasunobu K. T. Amine oxidase. XII. The association and dissociation, and number of subunits of beef plasma amine oxidase. Biochemistry. 1968 Dec;7(12):4329–4336. doi: 10.1021/bi00852a027. [DOI] [PubMed] [Google Scholar]
- Agro A. F., Rotilio G., Costa M. T., Mondovi B. Evidence for a ping-pong mechanism in the diamine oxidase reaction. FEBS Lett. 1969 Jul;4(1):31–32. doi: 10.1016/0014-5793(69)80188-2. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of molecular size and molecular weights of biological compounds by gel filtration. Methods Biochem Anal. 1970;18:1–53. [PubMed] [Google Scholar]
- Bardsley W. G., Crabbe M. J., Shindler J. S. Kinetics of the diamine oxidase reaction. Biochem J. 1973 Mar;131(3):459–469. doi: 10.1042/bj1310459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge I., Green P. E. Species of Trichuris in domestic ruminants in Australia. Aust Vet J. 1981 Mar;57(3):141–142. doi: 10.1111/j.1751-0813.1981.tb00489.x. [DOI] [PubMed] [Google Scholar]
- Boulton C. A., Large P. J. Properties of Pseudomonas AM1 primary-amine dehydrogenase immobilized on agarose. Biochim Biophys Acta. 1979 Sep 12;570(1):22–30. doi: 10.1016/0005-2744(79)90197-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bradsley W. G., Crabbe M. J., Scott I. V. The amine oxidases of human placenta and pregnancy plasma. Biochem J. 1974 Apr;139(1):169–181. doi: 10.1042/bj1390169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook D. F., Large P. J. A steady-state kinetic study of the reaction catalysed by the secondary-amine mono-oxygenase of Pseudomonas aminovorans. Biochem J. 1976 Jul 1;157(1):197–205. doi: 10.1042/bj1570197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANEY A. L., MARBACH E. P. Modified reagents for determination of urea and ammonia. Clin Chem. 1962 Apr;8:130–132. [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Crabbe M. J., Waight R. D., Bardsley W. G., Barker R. W., Kelly I. D., Knowles P. F. Human placental diamine oxidase. Improved purification and characterization of a copper- and manganese-containing amine oxidase with novel substrate specificity. Biochem J. 1976 Jun 1;155(3):679–687. doi: 10.1042/bj1550679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Duine J. A., Frank J., Westerling J. Purification and properties of methanol dehydrogenase from Hyphomicrobium x. Biochim Biophys Acta. 1978 Jun 9;524(2):277–287. doi: 10.1016/0005-2744(78)90164-x. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Easterby J. S. Coupled enzyme assays: a general expression for the transient. Biochim Biophys Acta. 1973 Feb 15;293(2):552–558. doi: 10.1016/0005-2744(73)90362-8. [DOI] [PubMed] [Google Scholar]
- FERGUSON K. A. STARCH-GEL ELECTROPHORESIS--APPLICATION TO THE CLASSIFICATION OF PITUITARY PROTEINS AND POLYPEPTIDES. Metabolism. 1964 Oct;13:SUPPL–SUPPL1002. doi: 10.1016/s0026-0495(64)80018-4. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Tipton K. F. The reaction pathway of membrane-bound rat liver mitochondrial monoamine oxidase. Biochem J. 1973 Dec;135(4):735–750. doi: 10.1042/bj1350735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large P. J., Eady R. R., Murden D. J. An enzymic method for the micro estimation of methylamine, ethylamine, and n-propylamine. Anal Biochem. 1969 Dec;32(3):402–407. doi: 10.1016/s0003-2697(69)80007-2. [DOI] [PubMed] [Google Scholar]
- NASH T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J. 1953 Oct;55(3):416–421. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata F., Ushiwata A., Nakamura Y. Spectorphotometric assay of monoamine oxidase using 2,4,6-trinitrobenzene-1-sulfonic acid. J Biochem. 1971 Feb;69(2):349–354. doi: 10.1093/oxfordjournals.jbchem.a129473. [DOI] [PubMed] [Google Scholar]
- Oi S., Inamasu M., Yasunobu K. T. Mechanistic studies of beef plasma amine oxidase. Biochemistry. 1970 Aug 18;9(17):3378–3383. doi: 10.1021/bi00819a013. [DOI] [PubMed] [Google Scholar]
- Pionetti J. M. Analytical-band centrifugation of the active form of pig kidney diamine oxidase. Biochem Biophys Res Commun. 1974 May 20;58(2):495–498. doi: 10.1016/0006-291x(74)90392-1. [DOI] [PubMed] [Google Scholar]
- Robinson J., Cooper J. M. Method of determining oxygen concentrations in biological media, suitable for calibration of the oxygen electrode. Anal Biochem. 1970 Feb;33(2):390–399. doi: 10.1016/0003-2697(70)90310-6. [DOI] [PubMed] [Google Scholar]
- TABOR C. W., TABOR H., ROSENTHAL S. M. Purification of amine oxidase from beef plasma. J Biol Chem. 1954 Jun;208(2):645–661. [PubMed] [Google Scholar]
- Tipton K. F. Kinetic mechanism and enzyme function. Biochem Soc Trans. 1980 Jun;8(3):242–245. doi: 10.1042/bst0080242. [DOI] [PubMed] [Google Scholar]
- Tipton K. F. The reaction pathway of pig brain mitochondrial monoamine oxidase. Eur J Biochem. 1968 Aug;5(3):316–320. doi: 10.1111/j.1432-1033.1968.tb00372.x. [DOI] [PubMed] [Google Scholar]
- Uotila L. Preparation and assay of glutathione thiol esters. Survey of human liver glutathione thiol esterases. Biochemistry. 1973 Sep 25;12(20):3938–3943. doi: 10.1021/bi00744a024. [DOI] [PubMed] [Google Scholar]
- VISHNIAC W., SANTER M. The thiobacilli. Bacteriol Rev. 1957 Sep;21(3):195–213. doi: 10.1128/br.21.3.195-213.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki E. F., Swindell R., Reed D. J. Some aspects of catalysis by the amine oxidase of pea seedlings. Biochemistry. 1970 Mar 3;9(5):1206–1210. doi: 10.1021/bi00807a022. [DOI] [PubMed] [Google Scholar]
- Zwart K., Veenhuis M., van Dijken J. P., Harder W. Development of amine oxidase-containing peroxisomes in yeasts during growth on glucose in the presence of methylamine as the sole source of nitrogen. Arch Microbiol. 1980 Jun;126(2):117–126. doi: 10.1007/BF00511216. [DOI] [PubMed] [Google Scholar]
- van Dijken J. P., Bos P. Utilization of amines by yeasts. Arch Microbiol. 1981 Jan;128(3):320–324. doi: 10.1007/BF00422538. [DOI] [PubMed] [Google Scholar]
- van Dijken J. P., Harder W. Optimal conditions for the enrichment and isolation of methanol-assimilating yeasts. J Gen Microbiol. 1974 Oct;84(2):409–411. doi: 10.1099/00221287-84-2-409. [DOI] [PubMed] [Google Scholar]
- van Dijken J. P., Otto R., Harder W. Growth of Hansenula polymorpha in a methanol-limited chemostat. Physiological responses due to the involvement of methanol oxidase as a key enzyme in methanol metabolism. Arch Microbiol. 1976 Dec 1;111(1-2):137–144. doi: 10.1007/BF00446560. [DOI] [PubMed] [Google Scholar]


