ABSTRACT
Enterococci are natural inhabitants of the intestinal tract in humans and many animals, including food-producing and companion animals. They can easily contaminate the food and the environment, entering the food chain. Moreover, Enterococcus is an important opportunistic pathogen, especially the species E. faecalis and E. faecium, causing a wide variety of infections. This microorganism not only contains intrinsic resistance mechanisms to several antimicrobial agents, but also has the capacity to acquire new mechanisms of antimicrobial resistance. In this review we analyze the diversity of enterococcal species and their distribution in the intestinal tract of animals. Moreover, resistance mechanisms for different classes of antimicrobials of clinical relevance are reviewed, as well as the epidemiology of multidrug-resistant enterococci of animal origin, with special attention given to beta-lactams, glycopeptides, and linezolid. The emergence of new antimicrobial resistance genes in enterococci of animal origin, such as optrA and cfr, is highlighted. The molecular epidemiology and the population structure of E. faecalis and E. faecium isolates in farm and companion animals is presented. Moreover, the types of plasmids that carry the antimicrobial resistance genes in enterococci of animal origin are reviewed.
INTRODUCTION
Enterococcus species are natural inhabitants of the intestinal tract in humans and animals, and due to their ubiquity in human and animal feces and their persistence in the environment, enterococci are considered indicators of fecal contamination in water (1). Moreover, enterococci serve as important key indicator bacteria for several human and veterinary resistance surveillance systems.
During the evisceration process at slaughterhouses, fecal enterococci can contaminate food products of animal origin. Some studies reported that over 90% of food samples of animal origin are contaminated with enterococci at the slaughterhouse, mostly with Enterococcus faecalis, followed by Enterococcus faecium (1, 2). In addition, enterococci are opportunistic pathogens which have become one of the main causes of nosocomial and community-acquired human infections, including septicemia, endocarditis, and urinary tract infections, among others (3).
The genus Enterococcus presently contains over 50 species, and E. faecalis and E. faecium are the predominant isolated species, accounting for more than 80% of isolates. In addition, these two species are considered the third- and fourth-most prevalent nosocomial pathogens worldwide (4). Other Enterococcus species, such as E. hirae, E. avium, E. durans, E. gallinarum, E. casseliflavus, and E. raffinosus, are rare causes of human clinical infections and are thought to be more opportunistic in nature than E. faecium and E. faecalis (5–10). E. faecalis and E. faecium are also the most representative enterococcal species detected in the human intestine, whereas other species, such as E. durans and E. avium, are occasionally detected (11). The most commonly encountered enterococcal species in the guts of animals are E. faecalis, E. faecium, E. hirae, and E. durans; other species are also detected sporadically or in particular age groups (such as E. cecorum in older poultry) (11, 12). Several members of the genus Enterococcus can cause bovine mastitis, endocarditis, septicemia and amyloid encephalopathy with sudden death in chickens (13), and diarrhea in dogs, cats, pigs, and rats (12). In the past decade, E. cecorum has emerged as an important poultry pathogen, associated with arthritis and osteomyelitis (14–15).
The intrinsic resistance of these bacteria to several antimicrobial agents has compromised the choice of therapeutic options to treat enterococcal infections. Those intrinsic resistances confer resistance to semisynthetic penicillins (low-level resistance), aminoglycosides (low-level resistance), vancomycin (E. gallinarum, E. casseliflavus, and E. flavescens), and polymyxins and streptogramins (E. faecalis) (11). Moreover, enterococci frequently acquire antimicrobial resistance genes through plasmids and/or transposons. The antibiotic resistances in Enterococcus species have been reviewed previously (3, 16–18), with focuses on specific agents (such as vancomycin [19–22] or aminoglycosides [23]) or sources (livestock/food [24–26]). The zoonotic transmission potential of antimicrobial-resistant enterococci has also been reviewed (27). In this review, we update the available knowledge on the prevalence and molecular mechanisms of antimicrobial resistance in enterococcal isolates from a wide range of animals (livestock, pets, and wildlife) and animal-derived food, with particular emphasis on beta-lactams, vancomycin, and linezolid. Furthermore, we outline the major clonal lineages and plasmids responsible for antimicrobial resistance in Enterococcus from farm and companion animals.
DIVERSITY OF ENTEROCOCCAL SPECIES IN THE ANIMAL INTESTINAL TRACT
Enterococci are ubiquitous bacteria in the gastrointestinal tract of humans and a wide range of animals (mammals, reptiles, birds, and some invertebrates). In addition, they are commonly found in vegetables, water, soil, and food derived from animals (including fermented and dairy products) (11). Enterococci are classified as lactic acid bacteria and are highly adaptable to different environmental conditions. They survive over a wide range of temperature (10 to 45°C), and pH (4.8 to 9.6) and are able to grow at high salt concentrations (up 6.5% NaCl). Most of them can hydrolyze esculin in the presence of 40% bile salts, a characteristic used for phenotypic identification processes (11). These and other properties explain the utilization of enterococci in diverse roles; for instance, they have been used as probiotics, starter cultures, bio-preservatives, and indicators of fecal contamination of water and sanitary quality of food (28–30).
Genomic analysis revealed that members of the genus Enterococcus have a low G+C content, ranging from 34.29 to 44.75% (31). For a long time, Enterococcus species were considered streptococci of Lancefield group D. In 1984, application of nucleic hybridization and 16S rRNA sequencing led to a reclassification of Streptococcus faecium and Streptococcus faecalis in the genus Enterococcus (32). Currently, this genus includes around 50 species (33). Many of them were discovered in this century, mostly recovered from nonhuman sources, such as plants (E. plantarum, E. ureilyticus), water (E. quebecensis, E. rivorum, E. ureasiticus), animals (E. canis, E. phoeniculicola, E. devriesei), and food products (E. thailandicus, E. italicus) (34–42).
A recent genomic study which compared the concatenated nucleotide sequences of the core genes of 37 enterococci belonging to a variety of species divided these strains into 6 branches: (i) the E. faecium branch (containing E. faecium, E. mundtii, E. durans, E. hirae, E. ratti, E. villorum, E. thailandicus, E. phoeniculicola), (ii) the E. faecalis branch (E. faecalis, E. termitis, E. quebecensis, E. moraviensis, E. caccae, E. haemoperoxidus, E. silesiacus), (iii) the E. dispar branch (E. dispar, E. canintestini, E. asini), (iv) the E. casseliflavus branch (E. casseliflavus, E. gallinarum, E. aquimarinus, E. saccharolyticus, E. italicus, E. sulfureus, E. cecorum, E. columbae), (v) the E. pallens branch (E. pallens, E. hermanniensis, E. devriesei, E. gilvus, E. malodoratus, E. avium, E. raffinosus), and (vi) the E. canis branch, which contained only one strain (31). Results showed that most strains from human and other mammals were clustered into the E. faecium, E. faecalis, E. dispar, and E. pallens branches, whereas the majority of the bird isolates belonged to the E. casseliflavus branch.
In 1963, Mundt and colleagues carried out a survey of the occurrence of enterococci among animals living in the wild environment (43). They obtained enterococci from the feces of 71% of the studied mammals, 86% of the reptiles, and 32% of the birds. In addition, patterns of food and animal species dependence were observed. In general, enterococci were only isolated sporadically in samples recovered from herbivorous mammals. However, they were abundant in rodents, bats, and larger animals with omnivorous or carnivorous diets (43), but as demonstrated in several other reports, the differences in the proportions of enterococci in each niche, as well as the species distributions, varied not only according to the diet, but also according to seasonal changes, individual characteristics (gender, age), and geographic location (11, 44).
In general, E. faecium, E. faecalis, E. hirae, and E. durans are the most prevalent enterococcal species in the gastrointestinal tract of humans and other mammals (11). E. cecorum is also a relevant member of the normal enterococcal microbiota in the gut of farm and pet animals (cattle, pigs, dogs, cats) and birds (poultry and pigeons) (45–47). However, in chickens, a significant age-dependent increase in gut colonization has been reported for this species. E. cecorum has been found to be a dominant part of the enterococcal gastrointestinal microbiota in mature chickens (48). Some other species, such as E. gallinarum and E. avium, which were first described in chickens, have not been frequently detected among enterococcal gut populations in poultry (49, 50).
In cattle and swine, the proportions of the enterococcal species vary across studies. E. faecium, E. durans, E. hirae, and E. faecalis were unanimously found in different surveys (46, 50–52). In some works, E. faecalis was the predominant enterococcal species in the gut of bovines and swines (46, 53). In others, E. hirae and E. faecium were described as the more abundant bacteria in both livestock species (44, 51, 52). As observed, variations between geographical regions might explain these differences in the composition of the enterococcal populations (44). E. casseliflavus, E. gallinarum, E. avium, and E. cecorum have also been reported as part of the bovine and swine microbiota, but they were present in lower proportions (46, 50, 51). Additionally, some minoritary species, such as E. villorum and E. thailandicus, have been sporadically detected in feces from cattle and pigs (52, 54, 55).
The enterococcal microbiota of the intestinal tract of dogs and cats showed a predominance of E. faecalis and E. faecium, followed by E. hirae (56–59). E. avium has been commonly isolated in canines and also, although in smaller proportions, in feline feces (56, 57). Other species, such as E. durans, E. gallinarum, E. casseliflavus, E. cecorum, and E. raffinosus, have been occasionally reported (56, 58, 59). In addition, some newly characterized species were isolated from anal swabs and chronic otitis externa (E. canis) and fecal samples (E. canintestini) of dogs (34, 60).
Enterococci are also normal residents of the gut of a wide range of free-living animals. In pigeons, the predominant species is E. columbae and, to a lesser extent, E. cecorum. However, E. faecium and E. faecalis are rare in these birds (61). Another study reported a high prevalence of enterococci among three species of coraciiform birds (74%), with a dominance of E. faecalis, followed by E. casseliflavus (62). In Portugal, E. faecium was the most frequently encountered species in buzzard fecal samples (63), and E. faecium, E. durans, and E. gallinarum were found in the feces of a variety of wild birds (64). The enterococcal gut microbiota has also been analyzed in wild marine species. E. faecium was identified as the most abundant species in echinoderms collected from Azorean waters. Minor species, such as E. hirae, E. faecalis, and E. gallinarum, were also detected (65). In a recent study in southern Brazil, different wild marine animals were analyzed using real-time quantitative PCR to identify and quantify enterococci in feces. These bacteria were found in all the studied animal species, with a dominance of E. faecalis and E. mundtii in most of the marine mammals; E. faecalis in green turtles, Magellanic penguins, and albatross; and E. hirae and E. gallinarum in white-backed stilts (66). Enterococci are also a relevant part of the facultative anaerobic microbiota of the gastrointestinal tract of large wild mammals (wolf, wild-boar, deer, etc.) and rodents (67–69).
Administration of antibiotics in both human and animal medicine may shift the gut microbial community, allowing drug-resistant strains (e.g., vancomycin-resistant enterococci) to proliferate dramatically. Because many enterococcal infections are caused by normal inhabitants of the gastrointestinal tract that become opportunistic pathogens, the selection of antibiotic-resistant strains raises the risk of developing difficult-to-treat infections. The following sections give an overview of the mechanisms and prevalence of antimicrobial resistance in enterococci in the animal setting.
ANTIMICROBIAL RESISTANCE IN ENTEROCOCCI OF ANIMALS AND FOODS OF ANIMAL ORIGIN
Beta-Lactam Resistance
Enterococci are intrinsically resistant to cephalosporins and present a natural reduced susceptibility to penicillins, due to the expression of low-affinity penicillin binding proteins (PBPs) that bind weakly to beta-lactam antibiotics. For this reason, the MICs for penicillins are higher in enterococci than in streptococci or other Gram-positive organisms, which do not produce chromosomally encoded low-affinity PBPs (17). E. faecalis isolates normally exhibit lower MIC values for penicillins than E. faecium (18).
All enterococci have at least five PBPs, and six putative PBP genes have been detected by genomic analysis in E. faecalis and E. faecium (class A: ponA, pbpF, pbpZ; class B: pbp5, pbpA, pbpB) (18). The expression of the species-specific chromosomally located pbp5 gene, which encodes PBP5, with low affinity binding for penicillins and cephalosporins, is associated with intrinsic resistance to beta-lactams. In E. faecium, the pbp5 gene is included within an operon, together with two other genes that are also implicated in cell wall synthesis (psr and ftsW) (18).
Acquired (enhanced) resistance for penicillins (penicillin or ampicillin) has been frequently detected among clinical E. faecium isolates, being rare in E. faecalis. High-level ampicillin resistance in E. faecium (MIC, ≥128 μg/ml) has been associated with increased production of PBP5 (requiring a higher concentration of the agent to saturate the active site) or with specific amino acid changes in its sequence, which make the low-affinity PBP5 even less susceptible to inhibition by penicillins (70, 71). The amino acid substitutions near the Ser-Thr-Phe-Lys, Ser-Asp-Ala, and Lys-Thr-Gly motifs, which are part of the active-site cavity, seem to be the most significant ones (16).
Combinations of specific amino acid changes in the C-terminal transpeptidase domain of PBP5 (especially the substitution Met-485-Ala/Thr, but also the changes Ala-499-Ile/Thr, Glu-629-Val, and Pro-667-Ser), and the insertion of serine or aspartic acid after position 466, have been associated with ampicillin resistance in E. faecium isolates (72–76). It has been found that single substitutions at positions 485, 499, 629, and 466-insertion have only slight influence on ampicillin MIC, but when combined, the effect increases. Mutations in genes encoding other species-specific proteins that participate in cell wall synthesis may also slightly increase the MIC value (76).
Two distinct allelic forms have been identified when the whole sequence of the pbp5 gene is considered, which differ in 5% of the sequence, yielding two types of PBP5 (PBP5-S and PBP5-R) with changes in 21 amino acid residues. PBP5-S is usually detected in community-associated ampicillin-susceptible E. faecium isolates (MIC of usually ≤2 μg/ml), and PBP5-R is usually detected in hospital-associated ampicillin-resistant isolates (MIC of usually ≥16 μg/ml) (77, 78). A hybrid-like type of PBP5 (PBP5-S/R), with a sequence between the other two types, has been observed in some isolates, with a MIC for ampicillin of around 4 μg/ml (77, 78).
Considering the population structure of E. faecium, two main lineages have been postulated in humans: (i) subclade A1, hospital-associated, enriched in mobile genetic elements, usually implicated in human infections, and in most cases, ampicillin-resistant (MIC, ≥16 μg/ml) with the consensus allele pbp5-R, and (ii) clade B: community-associated, detected in isolates from healthy humans (not implicated in infections), generally ampicillin-susceptible (MIC, ≤2 μg/ml), and harboring the consensus allele pbp5-S. The subclade A2 includes E. faecium isolates mostly from animal settings, exhibits a wide range of ampicillin MIC values (0.5 to 128 μg/ml), and generally carries the hybrid-like pbp5 allele (pbp5-S/R) (72, 78, 79). In addition to amino acid sequence alteration in PBP5, elevated levels of this protein are also observed in highly ampicillin-resistant isolates of clade A (subclade A1 and part of A2), but not in the ampicillin-susceptible isolates of subclade A2 and clade B, suggesting a differential regulation process in each clade. The upstream region of pbp5 seems to have a role in the level of expression of the gene (72).
In E. faecalis, acquired ampicillin resistance is unusual but is generally mediated by mutations in pbp4 (27, 80). Selected strains of E. faecalis produce a plasmid-mediated beta-lactamase that is similar to the enzyme produced by Staphylococcus aureus (17, 81), encoded by the blaZ gene, although some polymorphisms in this gene have also been detected in some isolates. This beta-lactamase is expressed in a constitutive way in E. faecalis, in contrast to the inducible production in S. aureus. The enzyme is produced in low amounts in E. faecalis, and for this reason, the strain can appear as ampicillin susceptible when the MIC is tested in vitro. In any case, this mechanism of resistance is very infrequently seen in E. faecalis. Very unusual beta-lactamase-producing E. faecium strains have also been reported (82). Chromosomal beta-lactamase-encoding genes conferring ampicillin resistance have also been detected in E. faecium isolates (83).
The in vitro transferability of pbp5 in E. faecium isolates (84), which suggests a mechanism by which high-level ampicillin resistance conferred by mutated pbp5 alleles could be disseminated among clinical isolates, has been reported. Moreover, Novais et al. (85) demonstrated in vitro ampicillin-resistance transference by conjugation in 28% of the E. faecium isolates from a pig farm environment, although the genetic basis of this transference was not determined. Codiversification of the E. faecium core genome and pbp5 has been recently analyzed, showing evidence of pbp5 horizontal transfer (86).
Various studies have evaluated the prevalence of penicillin or ampicillin resistance in enterococci from food-producing animals, pets, or wild animals, as well as in those from food of animal origin. For E. faecium, the prevalence of resistance is variable depending on the country and the type of animal. Reflecting this, no resistant E. faecium isolates were detected in a surveillance study performed in a cattle population at slaughter in Australia (87), but a rate of 30% resistance was detected in isolates of poultry in Portugal (88). For pets, the following ampicillin resistance rates were reported among E. faecium isolates: 63% and 37% in dogs and cats, respectively, in the United States and 3% in pets in Portugal (58, 88). Moreover, ampicillin-resistant E. faecium isolates were detected in 23% of the dogs screened in a cross-sectional study in the United Kingdom and in 76% of the dogs analyzed in a longitudinal study in Denmark (89). Most of these resistant isolates belonged to the hospital-adapted clonal complex CC17. Frequencies of ampicillin resistance in the range of 4.5 to 7.7% have been detected in E. faecium isolates recovered from wild animals (wild boar, Iberian wolf, and gilt-head seabream) (74, 90, 91), but no resistant isolates were detected in Iberian lynx (92).
A surveillance study was performed analyzing the prevalence of antimicrobial resistance in 21,077 Enterococcus isolates obtained from retail meat samples in the United States between 2002 and 2014, through the National Antimicrobial Resistance Monitoring System (NARMS) (2). A low frequency of ampicillin resistance was detected among E. faecium isolates from ground beef and pork chops (4% and 2.7%, respectively), but higher percentages were detected in retail chicken (26%), and even higher in ground turkey (62.6%). Bortolaia et al. (25) reviewed ampicillin resistance data reported in European countries (Denmark, Sweden, The Netherlands, Slovenia) and the United States for E. faecium isolates recovered from poultry meat, comparing them to human isolates in the same countries (93–95). Human isolates showed very high rates of ampicillin resistance in all countries (>80%), but resistance in food isolates was significantly lower than in those of humans. Of note is the detection of 10% ampicillin resistance in E. faecium of (imported) broiler meat in Denmark and >50% resistance in isolates of turkey meat in the United States. Almost no ampicillin-resistant E. faecalis isolates (with very few exceptions) have been reported in animals or food of animal origin.
Glycopeptide Resistance
Mechanism of resistance
Vancomycin and teicoplanin are important members of the glycopeptide family and are used for the treatment of severe human infections. Avoparcin, another member of this family, has been extensively used in the past as a growth-promoter in food-producing animals in many countries.
The mechanism of action of glycopeptides is the inhibition of the synthesis of the bacterial cell wall, by the link to the d-Ala-d-Ala terminus of the pentapeptide precursor of the peptidoglycan, preventing cross-linking of the peptidoglycan chain and inhibiting cell wall synthesis. The main mechanism of glycopeptide resistance in enterococci implicates the alteration of the peptidoglycan synthesis pathway. In this sense, the terminus d-Ala-d-Ala of the pentapeptide to which vancomycin binds is modified to d-Ala-d-Lac (causing high-level vancomycin resistance; MIC, >64 μg/ml) or to d-Ala-d-Ser (low-level vancomycin resistance; MIC, 4 to 32 μg/ml). These modified cell-wall precursors bind glycopeptides with reduced affinity (about 1,000-fold and 7-fold for d-Lac and d-Ser substitutions, respectively) (18, 22).
The first vancomycin-resistant enterococci (VRE) with an acquired mechanism of resistance were detected three decades ago in clinical E. faecium isolates in France and the United Kingdom (96, 97). Since then, VRE have been extensively described in hospitals worldwide; they have been seen especially frequently in the United States since the 1990, mostly in patients in intensive care units, and at lower levels in Europe since the 2000s (21). According to surveillance data from the European Centre for Disease Prevention and Control (EARS-Net), the European Union/European Economic Area population-weighted mean percentage of vancomycin resistance in E. faecium was 11.8% in 2016, and national percentages ranged from 0 to 46.3%; the prevalence of vancomycin resistance for E. faecalis was lower (98).
Vancomycin resistance is mediated by van operons, which encode the modified peptidoglycan precursors. To date, eight van operons have been identified in enterococci mediating acquired vancomycin resistance (vanA, vanB, vanD, vanE, vanG, vanL, vanM, and vanN), and one additional operon in intrinsic vancomycin resistance (vanC) (18, 19, 99–102). Three variants of the gene vanC have been described (vanC1, vanC2, and vanC3), intrinsic to E. gallinarum, E. casseliflavus, and E. flavescens, respectively. Moreover, different subtypes have been identified for vanB (vanB1, vanB2, and vanB3), vanD (vanD1 to vanD5) and vanG (vanG1, vanG2) (100, 103, 104). An additional variant, vanF, has also been described, but until now only in the environmental microorganism Paenibacillus popilliae (105).
vanA and vanB are the most frequent genotypes among VRE with acquired resistance mechanisms of humans and animals, mostly among E. faecalis and E. faecium. The genotypes vanD, vanE, vanG, vanL, vanM, and vanN are very unusual in VRE isolates, and E. faecalis (vanE/G/L) and E. faecium (vanD/M/N) are the most common carriers (22).
The vanA operon is associated with the transposon Tn1546 and includes seven open reading frames transcribed under two different promoters (106). Regulation is mediated by a vanS-vanR (sensor-kinase-response regulator) two-component system, transcribed with a common promoter (107). The remaining genes are transcribed from a second promoter (22). The proteins encoded by vanH (dehydrogenase that converts pyruvate into lactate) and vanA (ligase that forms a d-Ala-d-Lac dipeptide) modify the synthesis of peptidoglycan precursors; moreover, the proteins encoded by both vanX (dipeptidase that cleaves d-Ala-d-Ala) and vanY (d,d-carboxipeptidase), interrupt the formation of the d-Ala-d-Ala end of the pentapeptide, and the vanZ gene is related to teicoplanin resistance (22, 108). Different insertion elements (ISs) can be included in the vanA operon, rendering different variants (109).
The vanB operon has been associated with different transposons (Tn1547, Tn1549, and Tn5382). Tn1549 is widely prevalent among vanB-type enterococci, usually located in the chromosome and less frequently on plasmids (22). The structure of the vanB operon is similar to that of vanA, with two promoters and seven open reading frames, but with important differences, mostly in the two-component signaling regulatory system (encoded by vanRB and vanSB) and in the absence of a homolog of vanZ (substituted by vanW, of unknown function); consequently, vanB enterococci show vancomycin resistance (high or low level) but teicoplanin susceptibility (22, 108). The structure of the different van operons and their mechanisms of action have been extensively reviewed in previous studies (17–19, 21, 22, 108, 110).
Origin of vancomycin resistance
Partially preassembled glycopeptide resistance-associated gene clusters present in environmental organisms are suggested as the source of the vancomycin resistance genes in VRE (105, 111). The environmental organism P. popilliae, carrier of a vanF variant with high similarity at the amino acid level to vanA, has been suggested as the potential origin of vancomycin resistance in enterococci. To a lesser extent, this role could also be attributed to glycopeptide-producing organisms (e.g., the vancomycin-producing organism Amycolatopsis orientalis), which require these genes to inhibit the action of produced glycopeptides (111). Nevertheless, the genes in these organisms are probably not the direct source of the enterococcal vancomycin resistance genes since they are similar but not identical; in this sense, transference could have occurred from a common ancestral bacterium or via one or more bacterial intermediaries. In addition, considering the differences in G+C content, as well as the sequence homology among different organisms, it is possible that the genes of the van cluster could have more than one origin (111).
Historical aspects related to glycopeptide resistance
During the 1990s, VRE with the vanA genotype emerged in food-producing animals, healthy humans, food products, and environmental samples throughout Europe and other countries; this emergence was linked to the use of the glycopeptide avoparcin since the mid-1970s, in subtherapeutic concentrations, as an animal growth promoter (22, 26, 112, 113). This hypothesis was tested in poultry flocks and pig herds receiving or not receiving avoparcin, confirming the significant role of avoparcin in VRE selection in the animals (112, 113). This association was also corroborated in an animal model with young chickens receiving avoparcin supplementation (114). Avoparcin was banned as a growth promoter in the European Union in 1997, and a clear decrease in VRE fecal carriage in food-producing animals and healthy humans was observed (115), as well as in food-derived products. Nevertheless, VRE persisted in the animal setting many years after the avoparcin ban (116, 117). A similar situation happened in Taiwan after the ban of avoparcin in 2000 that resulted in a clear decrease of VRE prevalence in chickens, although it still persisted in this animal population (118). In dogs, high rates of fecal VRE carriage were reported before the avoparcin ban in the European Union (119), although no VRE was detected in dogs in Spain a decade after the ban (120). The frequency of human infection by VRE in the European Union was low during the period of high prevalence in animals, but an increase in the frequency of VRE-related human infections was evidenced since 1999 (22).
The situation in the United States and Canada was completely different than that in the European Union. Avoparcin use has never been approved in animal production in those countries, and VRE was not reported in animals until the end of the 2000s (20, 76, 121, 122). Nevertheless, in North America, VRE was a frequent cause of human infections, especially in patients in intensive care units, which was attributed to the high use of vancomycin in humans (22, 123). The differences in VRE prevalence in humans and animals in the European Union and the United States before and after the avoparcin ban in the European Union introduce some doubts about the possible routes of transmission of VRE determinants between animals and humans (22, 124).
Different theories have been postulated to explain the persistence of VRE in food-producing animals after the avoparcin ban in the European Union and in other countries, such as coselection by the use of other antimicrobials (e.g., erythromycin and tetracycline). It has been shown that vanA and erm(B) genes (the latter implicated in erythromycin resistance) are frequently located in the same transferable plasmids (113). Moreover, the gene tcrB, implicated in copper resistance, has been detected in pig E. faecium isolates in the same plasmid as vanA and erm(B) (125). However, the presence of plasmid addition systems in the same plasmid that carries the vanA gene could force bacteria to retain the resistance (125).
VRE in food-producing animals and food of animal origin
Tables 1 and 2 summarize the papers that have been published related to the prevalence and mechanisms of vancomycin resistance in enterococci isolated from food-producing animals and food of animal origin, respectively, as well as the genetic lineages of the isolates (when available). The data are organized by animal species (poultry, pigs, and cattle, among others) and by the year the isolates were recovered. Many of the studies were performed in European countries, but studies in the American, African and Asian countries, as well as Australia and New Zealand are also included.
TABLE 1.
Summary of reports about detection of VRE with acquired mechanisms of resistance in healthy food-producing animals
| Animal species | Year of recovery of tested isolates | Country | % Prevalencea | Species ST (CC)b (genotype) | Vancomycin selection method | Reference |
|---|---|---|---|---|---|---|
| Poultry | 1996 | Spain | 11/15 | E. hirae/E. faecium (vanA) | + | 114 |
| Poultry | 1997 | Netherlands | 29 (poultry and farmers) | E. faecium/E. durans/E. hirae (vanA) | + | 126 |
| E. faecalis (vanA) | ||||||
| Poultry | 1997 | Norway | 0.8–4.6 | E. faecium ST26/ST146/ST195/ST242/ST248/ST9/ST241/ST244/ST245 (vanA) | + | 127 |
| Poultry | 1997–1998 | Spain | 16 | E. durans (vanA) | + | 128 |
| Poultry | 1998–1999 | Norway | 34 | E. faecium (vanA) | + | 125 |
| Poultry | 2000–2007 | Sweden | <1 (2000), 40 (2005), 30 (2006–2007) | E. faecium ST13/ST370/ST310 (vanA) | + | 129 |
| Poultry | 2001–2004 | Hungary | 8.6 (2001), 23 (2002), 0 (2003–2007) | E. faecium/E. durans/E. mundtii (vanA) | + | 130 |
| Poultry | 2002–2003 | New Zealand | 5.8 | E. faecium/E. faecalis/E. durans (vanA) | + | 131 |
| Poultry | 2002–2004 | Czech Republic | 2.1 | E. faecium (vanA), E. faecium/E. faecalis (vanB) | – | 132 |
| Poultry | 2003 | Korea | 1.4 | E. faecium (vanA) | + | 133 |
| Poultry | 2003–2004 | Canada | 0 | – | 134 | |
| Poultry | 2003–2004 | Canada | 0 | – | 135 | |
| Poultry | 2004 | Portugal | 9.2 | E. faecium/E. durans/E. hirae (vanA) | + | 136 |
| Poultry | 2005 | Hungary | 1.1 | E. faecium/E. faecalis (vanA) | + | 137 |
| Poultry | 2005–2008 | Greece | 14.4 | E. faecium (vanA) | + | 138 |
| Poultry | 2009 | Germany | 17.6 | Enterococcus spp. (vanA) | + | 139 |
| Poultry | 2000 | Australia | 8.6 | E. faecium/E. mundtii/E. faecalis (vanC1) | + | 140 |
| 2008–2009 | 0 | - | + | |||
| Poultry | 2010 | Denmark | 47 | E. faecium ST10/ST12/ST22/ST26/ST38/ST157/ST417/ST520/ST587/ST784/ST785/ST839/ST840/ST841/ST842 (vanA) | + | 27 |
| Poultry | 2010–2011 | Germany | 0 | – | 141 | |
| Poultry | 2013–2014 | Poland | 0.11 | NSc | – | 142 |
| Poultry | NS | Italy | 8.7 | NS | – | 143 |
| Poultry | NS | Sweden | 55–70 (few samples) | E. faecium- ST310- (vanA) | + | 144 |
| Pigs | 1998 | Spain | 6.1 | E. faecium (vanA), E. hirae (vanB2) | + | 145 |
| Pigs | 1998–1999 | Spain | 8.0 | E. faecium (NS), E. hirae (NS), E. durans (NS) | + | 146 |
| Pigs and farm facilities | 2006–2007 | Portugal | 5 | E. faecium ST132/ST185 (CC5) (vanA), E. faecium ST443 (vanA), E. faecalis ST6 (CC2) (vanA) | + | 85 |
| Pigs | 2009 | USA | 10.9 | E. faecium ST5/ST6/ST185 (CC5) (vanA) | + | 121 |
| Pigs | 2009–2010 | USA | 8.2 | E. faecium ST5/ST6/ST185 (CC5) (vanA) | + | 122 |
| Pigs | 2014 | South Africa | NS | Enterococcus spp. (vanB) | – | 147 |
| Pigs | NS | Australia | 0 | NS | 148 | |
| Poultry and pigs | 1998–1999 | Costa Rica | 14.8 (poultry), 10.9 (pigs) | E. faecium/E. faecalis/E. durans/E. hirae (vanA) | + | 149 |
| Poultry and pigs | 1998–2000 | European countries | 8.2 | E. faecium/E. faecalis/E. hirae (vanA) | + | 150 |
| Poultry and pigs | 2002 | UK and Wales | 24 (poultry), 5 (pigs) | E. faecium (vanA) | + | 151 |
| Poultry and pigs | 2005 | France | 1.6 (poultry), 6.2 (pigs) | E. faecium/E. faecalis/Enterococcus spp. (vanA) | + | 152 |
| Poultry and pigs | 2009 | China | 0 | – | 153 | |
| Poultry and cattle | 2014 | Nigeria | 0 | – | 154 | |
| Poultry and cattle | NS | Ethiopia | 30–54 | NS | – | 155 |
| Poultry, pigs, cattle | 2008–2013 | China | 0.2 (pigs), 0 (poultry), 0 (cattle) | E. faecium ST6 (CC5) (vanA) | + | 156 |
| Poultry, pigs, cattle | NSa | Austria | 47.8 (poultry), 0.5 (cattle), 0 (pigs) | E. faecium/E. durans (vanA) | + | 157 |
| Cattle | 2003–2004 (healthy cattle)–2006 (sick cattle) | France | 0.1 (healthy cattle), 0.4 (sick cattle) | E. faecium/E. faecalis/E. casseliflavus (vanA) | + | 158 |
| Cattle | NSa | Australia | 0 | – | 87 | |
| Equines and swine | 2005 | Italy | 6.7 (equine), 16.1 (swine), | E. faecium/E. faecalis/E. casseliflavus (vanA) | + | 159 |
| Sheep, pigs, cattle | 2008–2009 | Portugal | 25.3 (pigs), 2.7 (sheep), 0 (cattle) | E. faecium/E. hirae (vanA) | + | 160 |
| Farm animals | 1998–2003 | USA | 0 | + | 161 | |
| Farm animals | 2000–2001 | Korea | 0.67 | E. faecium (vanA) | + | 162 |
| Farm animals | 2001 | Korea | 16.7 (chicken), 1.9 (pigs), 0 (cattle) | E. faecium (vanA) | + | 163 |
| Ostriches | 2009–2010 | Portugal | 7.4 | E. durans (vanA) | + | 164 |
| Mullet fish | 2006–2007 | Portugal | 3.9 | E. faecium (vanA) | + | 165 |
Some characteristics (year of isolation, or type of samples) are included in parenthesis
ST (CC), sequence type (clonal complex), if data are available
NS, not specified.
TABLE 2.
Summary of reports about detection of VRE with acquired mechanisms of resistance in food samples of animal origin
| Origin of the food sample | Year of recovery of tested isolates | Country | % Prevalencea | Species-ST (CC)b (genotype) | Vancomycin selection method | Reference |
|---|---|---|---|---|---|---|
| Chicken | 1995–1996 | Japan | 3.0 | E. faecium/E. faecalis (vanA) | – | 166 |
| Chicken | 1997–1998 | Spain | 27.2 | E. faecium/E. faecalis/E. durans/E. hirae (vanA) | + | 128 |
| Chicken | 1997–1998 | Belgium | 1.3 | E. faecium (NSc) | – | 167 |
| Chicken | 2000–2001 | Korea | 60 (15 samples) | E. faecium (vanA) | + | 162 |
| Chicken | 2000–2003 | Taiwan | 13.7 (2000), 3.7 (2003) (E. faecalis), 3.4 (2000)–0 (2003) (E. faecium) | E. faecium/E. faecalis (vanA) | + | 118 |
| Chicken | 2009 | Japan | 4.5 (22 samples) | E. cecorum (vanA) | + | 168 |
| Chicken | 2009 | Colombia | 3.7 | NSc | – | 169 |
| Chicken | 2009–2010 | Turkey | 0 | – | – | 170 |
| Chicken | NS | USA | 0 | – | – | 171 |
| Chicken | NS | UK | 18.5 | NS | + | 172 |
| Food and poultry | 1999–2001 | Portugal | 34.0 | E. faecium/E. faecalis/Enterococcus spp. (vanA) | + | 173 |
| Chicken and pork | 1996–1997 | Germany | 12.3 | E. faecium/E. durans/E. faecalis/E. hirae (vanA) | + | 174 |
| Chicken and pork | 1997–1998 | Italy | 15.9 (1997), 8.1 (1998) (poultry product)4.2 (1997), 6.9 (1998) (pork products) | E. faecium/E. faecalis/E. durans/E. hirae (vanA) | + | 175 |
| Chicken and pork | 1999 | France | 10.2 | E. faecium/E. durans (vanA) | + | 176 |
| Chicken and pork | 2011 | Japan | 0.6 (chicken meat) | E. faecium (vanN) | + | 177 |
| Various types of food | 2000–2002 | Germany | 0 | – | 178 | |
| Various types of food | 2010 | Argentina | 7.5 | E. faecium (vanA) | – | 179 |
| Various types of food | 2010 | Iran | NS | E. faecium ST669 (vanA) | + | 180 |
| Various types of food | 2010–2012 | Greece | 21.9 | E. faecium (vanA), E. faecium (vanA + vanB2/3), E. faecium (vanB2/3) | + | 181 |
| Various types of food | 2012 | Italy | 3.53 | NS | – | 182 |
| Various types of food | NS | Spain | 5.4 | NS | – | 183 |
| Various meats | 1997–1998 | Italy | 14.6 (1997), 8.0 (1998) | E. faecium/E. faecalis/E. durans/E. hirae (vanA) | + | 184 |
| Various meats | 2001–2002 | USA | 0 | – | 185 | |
| Various meats | 2003 | Korea | 44 | E. faecium (vanA) | + | 133 |
| Various meats and feces | 2004–2006 | Japan | 0 | + | 186 | |
| Various meats | 2007–2008 | Canada | 0 | – | 187 | |
| Various meats | 2007–2009 | Spain | 3.9 | E. faecium ST78 (CC17)/ST425 (vanA), E. durans/E. hirae (vanA), E. faecium ST17 (CC17) (vanB2) | + | 188 |
| Various meats | NS | Canada | 0 | – | 189 | |
| Various meats | NS | Spain | 19.4 | E. faecium/E. durans/E. hirae (vanA), E. faecium (vanB) | + | 190 |
| Retail meats | 2002–2014 | USA | 0 | – | 2 | |
| Cheese | 2005 | France | 0 | + | 191 | |
| Milk (sheep) | NS | Spain | 1.7 | E. faecalis ST168 (vanC1) | – | 192 |
| Shellfish | NS | UK | 2.7 | NS | – | 172 |
| Fish (Tilapia) | NS | Egypt | 37.5 (8 samples) | E. faecalis/E. gallinarum (vanA) | – | 193 |
| Frozen food | NS | Thailand | 9.7 | NS | – | 194 |
Some characteristics (year of isolation or type of samples) are included in parenthesis.
ST (CC), Sequence type (clonal complex), if data are available.
NS, not specified.
Most of the surveys of food-producing animals reported E. faecium as the major species of the genus Enterococcus exhibiting acquired resistance to vancomycin, in most cases with the vanA genotype. However, vanA-containing E. faecalis isolates, and to a lesser extent E. durans and E. hirae isolates, have also been frequently detected in food-producing animals (Table 1) (27, 85, 87, 114, 121, 122, 125–165). Other enterococcal species have occasionally been reported as vanA carriers, such as E. mundtii in poultry in Hungary (130) and E. casseliflavus in cattle in France (158) and in horses and swine in Italy (159). Available data indicate that vanA was, by far, the main gene responsible for acquired VRE in food-producing animals worldwide, regardless of the species. Nevertheless, vanB (and especially the vanB2 variant) was occasionally detected. The first detection of vanB2 in animals was in a vancomycin-resistant E. hirae isolate recovered from a pig in Spain in 2008 (145); later, vanB-positive E. faecium and E. faecalis isolates were detected in poultry in Czech Republic (132) and in Enterococcus species in pigs in South Africa (147). Moreover, vanC1 was detected as an acquired gene in isolates of E. faecium, E. faecalis, and E. mundtii in poultry in Australia (140). In most of the studies, VRE were detected when a selective protocol with media supplemented with vancomycin was used (Table 1). Resistance frequencies varied depending on the type of animals tested (poultry, 0 to 77%; pigs, 0 to 25.3%; cattle, 0 to 0.5%), the year the study was performed, the country, and the protocol used for VRE recovery (see Table 1). vanA-containing enterococci have also been detected in ostriches and mullet fish in Portugal (prevalence of resistance of 7.4% and 3.9%, respectively) (164). In eight of the reviewed papers in which VRE were detected in food-producing animals, the multilocus sequence typing (MLST) data were provided for vanA-positive E. faecium (most isolates) or E. faecalis isolates. A wide variety of sequence types were identified among the E. faecium isolates from poultry and pigs (>30 sequence types) (27, 85, 121, 122, 127, 129, 144, 156). Also, the lineage sequence type 6 (ST6) (CC2) was identified in E. faecalis of pig origin (85).
The E. faecium species carrier of the vanA gene was the most frequent VRE detected in food of animal origin. Nevertheless, vanA-containing E. faecalis, E. durans, and E. hirae isolates were also frequently detected in these types of samples (Table 2) (2, 118, 128, 133, 162, 166–194). VRE with the vanB gene was found in E. faecium isolates from veal and chicken in Spain (ST17-vanB2) (188) and in different types of food in Greece (vanB2/3) and Spain (vanB) (181, 190). The identification of the unusual vanN gene in five E. faecium isolates from chicken meat in Japan is interesting, showing a low level of vancomycin resistance (MIC, 12 μg/ml) (177). Also notable is the unusual detection of vanA-containing E. cecorum isolates in chicken samples in Japan (168), vanA-positive E. gallinarum in fish in Egypt (193), and vanC1-positive E. faecalis isolates from sheep milk samples in Spain (192). The frequencies of detection of VRE with acquired resistance in food samples were variable (Table 1). In chicken and pork food samples analyzed from 1996 to 1999, the prevalence was in the range of 4.2 to 34% (Table 2), with a few exceptions (1.3%) (167). Very high frequencies were detected in different types of food in Korea (44%) (133), but no VRE were found in the studies performed in the United States (2, 171, 185). In some cases, isolates showing a phenotype usually associated with the vanB genotype (high-level resistance to vancomycin, susceptibility to teicoplanin) were detected in Enterococcus strains harboring the vanA gene (118, 168, 173).
VRE in companion animals
Table 3 shows the detection of VRE with acquired mechanisms of resistance in companion animals. vanA-containing E. faecium is a unique type of VRE with acquired resistance reported in dogs and cats (136, 145, 195–202). These isolates, recovered from fecal samples from 1996 to 2003, were found in the United States, Spain, and Portugal, with variable frequencies of detection (ranging from 2.8 to 22.7%) (136, 145, 195, 196). No VRE were detected in studies performed in the following years (Table 3), not even in sick dogs (197, 200). Vancomycin-resistant E. faecium and E. durans isolates were detected in fecal samples of equids obtained in 2007 to 2008 (prevalence 4.4%) in a study performed in Portugal (202).
TABLE 3.
Summary of reports about detection of VRE with acquired mechanisms of resistance in companion and free-living animals
| Animal species | Isolation year | Country | % Prevalencea | Species-ST (CC)b (genotype) | Vancomycin selection method | Reference |
|---|---|---|---|---|---|---|
| Companion animals | ||||||
| Dogs (sick) | 1996–1998 | USA | 2.8 | E. faecium (vanA) | – | 195 |
| Dogs/cats | 1998 | Spain | 22.7 (22 samples) | E. faecium (vanA) | + | 145 |
| Dogs | 1998–2003 | Spain | 12.6 | E. faecium (vanA) | + | 196 |
| Dogs/cats | 2003 | Portugal | 2.6 (dogs)0 (cats) | E. faecium (vanA) | + | 136 |
| Dogs (sick) | 2008–2009 | USA | 0 | – | 197 | |
| Dogs | 2009 | Spain | 0 | + | 120 | |
| Dogs/cats (antibiotic-treated) | 2011–2012 | Japan | 0 | – | 198 | |
| Dogs/cats | 2014 | Egypt | 0 | – | 199 | |
| Dogs (sick) | NSc | Portugal | 0 | – | 200 | |
| Dogs/cats | NS | Turkey | 3.8 (dogs)0 (cats) | NSc | – | 201 |
| Equids | 2007–2008 | Portugal | 4.4 | E. faecium/E. durans (vanA) | + | 202 |
| Free-living animals | ||||||
| Wild mammals | 1997–2000 | England | 1.2 (badgers)4.6 (woodmice) | E. faecium (vanA) | – | 203 |
| Wild animals | 2004 | Portugal | 0 | + | 136 | |
| Wild animals | 2008–2013 | Spain | 2.0 | E. faecium-ST915 (vanA), E. faecalis ST6 (CC2) (vanB2) | + | 204 |
| Wild animals | 2012 | Poland | 14.9 | NS | – | 205 |
| Wild animals | 2014–2015 | Spain | 0.3 | E. faecium ST993 (vanA) | + | 206 |
| Wild birds | 2006–2010 | Portugal | 2.7 | E. faecium/E. durans (vanA) | + | 207 |
| Wild birds | 2009–2010 | Portugal | 1.3 | E. faecium/E. durans/E. hirae (vanA) | + | 208 |
| Wild birds | 2010 | Portugal | 0.7 | E. faecalis (vanA) | 209 | |
| Wild birds | 2012 | Tunisia | 3.6 | E. faecium (vanA) | + | 210 |
| Corvids(American crows) | 2012 | USA | 2.5 | E. faecium ST18 (CC17)/ST555 (CC17)/ST749/ST750/ST751/ST752–(vanA), E. faecalis ST179 (CC16)/ST16 (CC16)/ST6 (CC2) (vanA) | + | 211 |
| Corvids(American crows) | 2012–2015 | USA | 2.7 | E. faecium-ST362/ST412 (CC17)-(vanA) | + | 212 |
| Corvids | 2012–2013 | Canada | 0.7 | E. faecium ST448 (vanA) | + | 213 |
| Corvids | 2013 | Slovakia | 1.3 | E. faecium ST917/ST6 (CC5) (vanA) | + | 214 |
| Wild boars | 2005–2006 | Portugal | 3 | E. faecium (vanA) | + | 215 |
| Mullets fish | 2006–2007 | Portugal | 3.8 | E. faecium ST273 (CC17)/ST280 (CC17) (vanA) | + | 165 |
| Seagulls | 2007 | Portugal | 10.5 | E. faecium-ST5 (CC17)-(vanA), E. durans-(vanA) | + | 216 |
| Gilt-head seabream | 2007 | Portugal | 5.9 | E. faecium ST273 (CC17)/ST313 (CC17)/ST76 (vanA), E. faecalis ST6 (CC2) (vanA), E. durans (vanA) | + | 217 |
| Wild rabbits | 2007–2008 | Portugal | 3.9 | E. faecium-(vanA) | + | 218 |
| Pigeons | 2007–2008 | Brazil | 0 | – | 219 | |
| Buzzards | 2007–2009 | Portugal | 9 | E. faecium ST273 (CC17)/ST5 (vanA), E. durans (vanA) | + | 220 |
| Partridges | 2015–2016 | Portugal | 2 | E. faecium-ST18(CC17)/ST448(CC17)/ST139(CC5)-(vanA) | + | 221 |
| Red foxes | 2008–2009 | Portugal | 13.5 | E. faecium ST262 (CC17)/ST273 (CC17) (vanA), E. durans (vanA) | + | 222 |
| Iberian wolf, Iberian lynx | 2008–2010 | Portugal | 0.5 (Iberian wolf)0 (Iberian lynx) | E. faecium ST18 (CC17)/ST573 (vanA) | + | 223 |
| Buffalo, wildebeest, zebra | 2010–2011 | Tanzania | 2.8 (buffalo)20 (zebra)2.5 (wildebeest) | NS | + | 224 |
| Camels | NS | Spain | 0 | – | 225 | |
| Wild game meat | NS | Spain | 30.9 | E. faecium/E. faecalis/E. durans (vanA), E. faecium (vanB) | – | 226 |
Some characteristics (year of isolation, type of samples) are included in parenthesis.
ST (CC), sequence type (clonal complex), if data are available.
NS, not specified.
VRE in free-living animals
Table 3 also shows the detection of VRE with acquired mechanisms of resistance in free-living animals, including different species of mammals and birds (136, 165, 203–226). Many studies have been performed with this type of animal, including in various countries in Europe, the Americas (United States, Canada, and Brazil), and Africa (Tunisia and Tanzania). The most frequently detected mechanism of resistance was vanA, mainly among E. faecium isolates, followed by E. faecalis (E. durans and E. hirae were infrequently detected). Occasionally, enterococci were found to be vanB carriers: two small mammals (Rattus rattus) harbored vanB2-containing E. faecalis ST6 isolates in Spain (204), and E. faecium vanB was detected in wild game meat, also in Spain (226). The frequencies of detection of vanA-containing enterococci in wild animals ranged from 0 to 13.5%, with the highest values detected in red foxes, seagulls, and buzzards in Portugal (9 to 13.5%) (216, 220, 222). Interestingly, vanA-containing E. faecium isolates detected were ascribed to different sequence types included in the high-risk clonal complex CC17 (ST18, ST262, ST273, ST280, ST313, ST362, ST412, ST448, and ST555). These isolates were detected in corvids in the United States and in mullet fish, gilt-head seabream, seagulls, buzzards, partridges, red foxes, and Iberian wolves in Portugal (Table 3).
Resistance to Linezolid
The widespread occurrence of VRE in many countries makes it necessary to look for other therapeutic options, and linezolid is an important one. This oxazolidinone, introduced in 2000 in the United States and in 2001 in the United Kingdom, is an important agent for the treatment not only of VRE, but also of other Gram-positive bacteria, such as methicillin-resistant S. aureus.
Linezolid resistance is still unusual among enterococci but has emerged in recent years in human and animal isolates (227). Mutations in the central loop of domain V of the 23S rDNA is the most common mechanism of resistance in enterococci, the amino acid change G2576T being the predominant one, although other changes have also been described (G2505A, U2500A, G2447U, C2534U, and G2603U) (18). E. faecalis and E. faecium possess four and six 23S rDNA alleles per genome, respectively, and depending on the number of mutated versus wild-type alleles per genome, these correlate with the level of resistance of the isolates (227). In some cases, this mechanism appears during the course of treatment with oxazolidinones, and nosocomial transmission of linezolid-resistant enterococci has been reported (228). Linezolid-resistant E. faecalis and E. gallinarum isolates of swine origin were detected in China (MIC, 8 to 16 μg/ml), and the nucleic acid change G2576T was identified in the 23S rDNA of these isolates (229). Mutations in the ribosomal proteins L3, L4, and L22 can confer decreased susceptibility to linezolid in enterococci and staphylococci (230).
In recent years, there has been concern about the emergence of transferable resistance to linezolid, associated with the acquisition of the cfr gene or with the recently described optrA gene. The cfr gene has been detected in enterococci of both human and animal origin (231) and encodes an rRNA methyltransferase that modifies the adenine residue at position 2503 in domain V of the 23S rRNA; it confers resistance to oxazolidinones, phenicols, lincosamides, pleuromutilins, and streptogramin A (the phenotype named PhLOPSA) (18). Among oxazolidinones, linezolid is mostly affected by cfr; tedizolid, a new compound of this family, showed increased activity in cfr-positive enterococci, so these isolates are susceptible to this agent. Table 4 summarizes the data published until now in relation to linezolid resistance mechanisms in enterococci of animal and food origin, as well as in enterococci of environmental origin (229, 232–241).
TABLE 4.
Mechanisms implicated in linezolid resistance in enterococci of animals, food of animal origin, and the environment
| Origin | Species | Sample | Year of isolation | Country | Number of linezolid resistant isolates (%) | Linezolid MIC (μg/ml) | Mechanism of linezolid resistance (genetic location, number of isolates)a | ST (number of isolates)b | Characteristics (number of isolates) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Food-producing animals | ||||||||||
| Cattle | E. faecalis | Feces | 2009 | China | 1 | 4 | cfr (P) | fexB-linked | 232 | |
| Pigs | E. faecalis | Feces | 2009 | China | 1 (0.3% of E. faecalis) | 4 | cfr (P) | ST21 | fexA (in a different plasmid) | 233 |
| Pigs | E. faecalis | Rectal swabs | 2013 | Brazil | 5 (2% of E. faecalis) | 8–>8 | cfr (5)Mutation in ribosomal L3 protein (V149I) (1) | ST591 (2); ST29, ST590, ST592 (1 each) | 234 | |
| Pigs | E. gallinarum (n = 1), E. casseliflavus (n = 24) | Rectal swabs | 2012 | China | 25 (31.5% of florfenicol-resistant enterococci) | 8–16 | cfr (25) (C,1; P, 8) | fexA-linked (5), fexA-fexB-linked (1) | 235 | |
| Poultry and pigs | E. faecalis | Feces | 2012–2013 | China | 20 (10% of E. faecalis of animal origin) | 2c–8 | optrA (C,8; P, 9; P/C, 3) | ST21 (2); ST27, ST59, ST74, ST93, ST116, ST256, ST330, ST403, ST475, ST476, ST480, ST593, ST618, ST619, ST620, ST621, ST622, ST623 (1 each) | fexA-linked (14), fexB-linked (2), fexA-fexB-linked (1)optrA amino acid changes: Y176D, T481P (2); K3E, Y176D, G393D (1); T112K, Y176D (2); Y176D, G393D (1) | 236, 237 |
| Poultry and pigs | E. faecium | Feces | 2005, 2009, 2012, 2014 | China | 5 (5.7% of E. faecium of animal origin) | 4d–8 | optrA (C, 1; P/C, 4) | ST957 (2); ST32, ST184, ST29 (1 each) | fexB-linked (4) | 236, 237 |
| Poultry and pigs | E. faecalis | Feces and carcass | 2008, 2012, 2014 | Korea | 12 (0.2% of E. faecalis) | 8–>16 | optrA (12) | ST21 (4); ST49 (2); ST16, ST32, ST256, ST403, ST728, ST729 (1 each) | fexA-linked (11) | 238 |
| Poultry and cattle | E. faecium | Feces and carcass | 2008, 2010, 2012–2014 | Korea | 27 (0.7% of E. faecium ) | 8–>16 | optrA (23)Mutation in ribosomal L4 protein (N130K) (7) | ST195 (6); ST32 (2); ST157 (2); ST1171 (2); ST1168 (2); ND (4); ST8, ST120, ST121, ST236, ST241, ST1166, ST1167, ST1169, ST1170 (1 each) | fexA-linked (19) | 238 |
| Pigs | E. faecalis (n = 5), E. gallinarum (n = 1) | China | 6 (17.14% of enterococci) | 8–16 | 23S rRNA mutation: G2576T | ST29, ST146, ST220, ST283, ST535 | 229 | |||
| Meat | ||||||||||
| Cattle | E. faecalis | Danish veal | 2015 | Denmark | 1 (<0.1% of E. faecalis) | 8 | optrA (P) | ST22 | fexA-linked | 239 |
| Poultry | E. faecium | Imported turkey and broiler meat | 2012, 2013 | Denmark | 2 (<0.1% of E. faecium) | 8 | optrA (P, 1) | ST22, ST873 | 239 | |
| Poultry | E. faecalis | Meat | 2010–2011 | Colombia | 3 (0.5% of enterococci) | 8 | optrA (P, 3) | ST59 (2), ST489 | fexA-linked | 240 |
| Environment | ||||||||||
| Wastewater | E. faecalis | Urban wastewater treatment plant | 2014 | Tunisia | 2 (1% of chloramphenicol-resistant enterococci) | 4 | optrA (P) | ST86 (2) | fexA-linked (2), optrA amino acid changes:M1L (2), K3E (1) and I622M (1) | 241 |
P, plasmid; C, chromosome.
ND, not determined.
Five optrA-positive E. faecalis isolates showed a linezolid MIC of 2 μg/ml, and five isolates showed an MIC of 4 μg/ml.
Two optrA-positive E. faecium isolates showed a linezolid MIC of 4 mcg/ml.
The cfr gene was identified for the first time in enterococci in 2011, specifically in an E. faecalis isolate recovered on a dairy farm in China (232). Since then, cfr has been detected in human clinical E. faecalis isolates (242), as well as in swine E. casseliflavus, E. gallinarum, and E. faecalis isolates in China and Brazil (233–235) and in a cattle E. faecalis isolate in China (234). A second variant of the cfr gene, named cfr(B), has been described in E. faecium isolates of human origin. This new plasmid-located variant is more similar to a cfr-like gene of Clostridium difficile than to the cfr genes of staphylococci or other enterococcal species (243, 244), and it has so far not been detected in enterococci of animal origin.
The novel optrA gene confers transferable resistance to oxazolidinones (both linezolid and telizolid) and phenicoles (chloramphenicol and florfenicol) and has been detected in E. faecalis and E. faecium isolates of both human and animal origin (236). This gene encodes an ABC transporter and has been detected more frequently in E. faecalis than in E. faecium isolates and more frequently in isolates from food-producing animals (pigs and chickens) than in those of human origin (236). The optrA gene has been detected both in chromosomal and in plasmidic locations in animal and human E. faecalis and E. faecium isolates. As shown in Table 4, optrA-positive enterococci have been detected in food-producing animals (poultry, pigs, and occasionally, cattle) in Asiatic countries, mostly in E. faecalis and E. faecium belonging to many different sequence types, and sporadically in E. gallinarum. The prevalence of optrA-positive enterococci represents 10% and 5.7% of total E. faecalis and E. faecium isolates, respectively, obtained from fecal samples of poultry and pigs in a study performed in China (236). In a recent study carried out in Korea, 11,659 E. faecalis and E. faecium isolates obtained from fecal and carcass samples of healthy cattle, pigs, and chickens from farms and slaughter houses from 2003 to 2014 were tested for linezolid resistance, detecting a rate of resistance of 0.33%, mainly attributed to optrA carriage (238). The optrA gene has also been detected in sporadic isolates of E. faecalis and E. faecium (n = 3) obtained in meat samples in Denmark (imported poultry and veal), which represented <0.1% of total enterococci recovered from these samples (239). In Colombia, optrA has been detected in three E. faecalis isolates from poultry meat, coharboring the fexA, tet(L), and Isa(A) resistance genes (240). Both cfr and optrA have been detected in VRE isolates of human origin (245), but not in animal isolates so far.
The optrA gene has also been detected in two E. faecalis isolates of the lineage ST86 recovered from urban wastewater in Tunisia, accounting for 1% of all chloramphenicol-resistant enterococci tested (241); optrA was located within a transferable mosaic plasmid, which also contained the fexA and erm(A) genes.
At least 12 and 5 polymorphic variants of the optrA gene have been detected among human and animal enterococci, respectively (237, 246–248). The wild OptrA type (OptrAE349) and the variants Tyr176Asp + Lys3Glu-Gly393Asp or Thr481Pro or Thr112Lys or Gly393Asp have been found in animal isolates (237, 246). Functional cfr and optrA genes have been identified in both enterococci and S. aureus. In most of the animal isolates, optrA is located close to other genes, as is the case of fexA (implicated in phenicol resistance) and a novel erm(A)-like gene. This erm(A)-like gene encodes an rRNA methylase, which shows 85.2% amino acid identity to the Erm(A) protein of transposon Tn554 of S. aureus (237).
Most of the cfr-positive enterococci of food-producing animals (>90%) showed a MIC for linezolid of ≥8 μg/ml, but two E. faecalis isolates presented a MIC of 4 μg/ml. optrA-positive isolates of food-producing animal and food origin showed a linezolid MIC in the range of 2 to >8 μg/ml, presenting 19% of the isolates’ MICs in the range of 2 to 4 μg/ml (categorized as susceptible according to EUCAST breakpoints and susceptible-intermediate according to CLSI) (Table 4). It is interesting to note that cfr- and optrA-positive enterococci could appear as linezolid-susceptible, probably leading to an underestimation of their actual incidence.
Oxazolidinones are not used in food-producing animals. Nevertheless, the emergent detection in these animals of linezolid-resistant enterococci carrying the optrA gene in transferable plasmids, linked to resistance genes for antibiotics commonly used in animals (phenicols, tetracyclines, lincosamides, and aminoglycosides), suggests its role in the coselection of multiresistant bacteria, which poses a risk for public health.
To summarize, transferable linezolid resistance genes, mostly optrA, have been detected in enterococci of food-producing animals and food of animal origin in various European, South American, and Asian countries, but so far not in Africa. These mechanisms of resistance have not been detected so far, to our knowledge, in pets or in wild animals.
Resistance to Aminoglycosides
Enterococci are intrinsically resistant to clinically achievable concentrations of aminoglycosides due to their low cell wall permeability. In addition, some species, such as E. faecium [aac(6′)-Ii], E. durans [aac(6′)-Id], and E. hirae [aac(6′)-Ih], intrinsically express a chromosomal-encoded acetyltransferase that confers resistance to tobramycin, kanamycin, and amikacin (249). The chromosomally encoded methyltransferase EfmM has been exceptionally described in an E. faecium isolate (250) codifying resistance to kanamycin and tobramycin. Acquired resistances to aminoglycosides are detected in strains from both animals and humans and usually confer a high level of resistance to gentamicin, kanamycin, and streptomycin.
High-level resistance to gentamicin in enterococcal isolates of animal origin was first described in 1998 in Denmark (251) and in 2001 in the United States (252). The acquired genetic mechanisms identified in animal isolates are identical to those described in human isolates. The most frequent ones are the bifunctional enzyme encoded by aac(6′)-Ie-aph(2″)-Ia (conferring resistance to gentamicin, kanamycin, amikacin, netilmicin, and tobramycin) and aph(3)-IIIa (conferring resistance to kanamycin and amikacin) (23, 253). High-level gentamicin resistance can also be due to the expression of the unusual aph(2″)-Ic, aph(2″)-Id, aph(2″)-Ie, and aph(2″)-Ib genes (17, 23); aph(2″”)-Ic seems to be more frequent in enterococci of animal origin, and some farm animals could be a reservoir of this gene (252). High-level resistance to streptomycin is commonly caused by punctual ribosomal mutations, although acquisition of some modifying enzymes has been also described [ant(3″)-Ia and ant(6′)-Ia]. Table 5 summarizes papers (from 2013 to 2017) that analyzed the rates of antimicrobial resistance (high level to gentamicin and others such as tetracycline, erythromycin, and ciprofloxacin) in enterococcal isolates from animals (65, 15, 87, 90, 92, 135, 141, 143, 147, 153, 154, 198, 205, 209, 254–276).
TABLE 5.
Summary of articles on the antimicrobial resistance in Enterococcus isolated from animals from 2013 to 2017
| Animal | Country | Enterococcal specie | Percentage of resistance toa | Reference | |||
|---|---|---|---|---|---|---|---|
| TET | ERY | HLR-GEN | CIP | ||||
| Food-producing and farm animals | |||||||
| Camels | Tunisia | Enterococcus spp. | 4 | 11 | 0 | ND | 254 |
| Chickens and pigs | China | Enterococcus spp. | 92.5 | 72.8 | 30 | ND | 153 |
| Aquaculture | Spain | E. faecalis | 0 | 5 | ND | 62 | 255 |
| E. faecium | 5 | 37 | ND | 26 | |||
| Rainbow trout | Spain | Enterococcus spp. | 27.1 | 41 | 7 | 0 | 256 |
| Marine aquaculture | Italy | Enterococcus spp. | 66 | 25 | 0 | ND | 257 |
| Tibetan pigs | Tibet | E. faecalis | 93.5 | 93.5 | 0 | 0 | 258 |
| E. faecium | 44.4 | 20 | 0 | 0 | |||
| Farm animals | Argentina | E. faecalis | 100 | 50 | 0 | ND | 259 |
| Chickens | Southeast Asian countries | E. faecalis | 69.2 | 70.9 | 11.1 | 17.9 | 260 |
| E. faecium | 92.2 | 79.4 | 13.9 | 82.8 | |||
| Poultry | Italy | Enterococcus spp. | 65.2 | 87.8 | 60 | 70.4 | 143 |
| Beef and sheep | Tunisia | E. faecalis | 15 | 30 | 2.5 | 22.5 | 261 |
| Pigs | South Africa | E. faecalis | ND | 100 | ND | 45 | 147 |
| E. faecium | ND | 98.3 | ND | 94.1 | |||
| Poultry | Germany | E. faecalis | 82 | 70 | 98 | 14 | 141 |
| E. faecium | 67 | 89 | 89 | 72 | |||
| Poultry | Nigeria | Enterococcus spp. | 81.6 | 100 | 20 | ND | 262 |
| Chicken and turkey | Canada | Enterococcus spp. | 94 | 64–74 | 8–15 | 2–8 | 135 |
| Fish and seafood | Switzerland | E. faecalis | 16 | ND | 4 | 0 | 263 |
| Infection in poultry | Poland | E. cecorum | 29 | 51 | 1 | ND | 15 |
| Poultry and cattle | Nigeria | Enterococcus spp. | 61 | 61 | 32.7 | 9.7 | 154 |
| Mastitis in cows | China | Enterococcus spp. | 6.7 | ND | 50 | 25 | 264 |
| Cattle | Australia | E. faecalis | 7.3 | 10.4 | 0 | ND | 87 |
| E. faecium | 11.7 | 8.3 | 0 | ND | |||
| Pigs | Nigeria | E. faecalis | 60 | 68.7 | 85 | 0.5 | 265 |
| E. faecium | 20 | 31.5 | 40 | 7.4 | |||
| Donkey | Spain | E. faecium | 68 | 44 | 0 | 60 | 266 |
| Pets and companion animals | |||||||
| Healthy dogs and cats | Japan | Enterococcus spp. | ND | 40 | 44 | 25 | 267 |
| Hospitalized dogs | Korea | E. faecalis | 64.3 | 50 | 7.1 | 0 | 268 |
| E. faecium | 66.7 | 44.4 | 33.3 | 55.6 | |||
| Antibiotic-treated dogs and cats | Japan | E. faecalis | 58 | 52 | 38 | 30 | 198 |
| E. faecium | 15 | 28 | 21 | 8 | |||
| Various animals | Poland | E. faecalis | 36 | 48 | ND | 38 | 205 |
| E. faecium | 7 | 60 | ND | 46 | |||
| Healthy cats and dogs | Italy | Enterococcus spp. | 97.4 | 81.7 | 40.8 | 7.8 | 269 |
| Wild animals | |||||||
| Iberian lynx | Spain | Enterococcus spp. | 33 | 30 | 4 | 7 | 92 |
| Iberian wolf | Portugal | Enterococcus spp. | 55 | 22 | 1 | 15 | 90 |
| Echinoderms | Portugal | E. faecium | 31.7 | 33 | 0.8 | 30.8 | 65 |
| Red foxes | Portugal | E. faecalis | 81 | 36 | 18 | ND | 270 |
| E. faecium | 96 | 56 | 12 | ND | |||
| Wild birds | Portugal | E. faecalis | 32.3 | 15.3 | 0 | 15.3 | 209 |
| E. faecium | 45 | 15 | 0 | 32.5 | |||
| Eurasian otter | Portugal | E. faecalis | 32 | ND | 52 | ND | 271 |
| E. faecium | 78 | ND | 11 | ND | |||
| House flies | USA | E. faecalis | 60 | 18 | 5 | 0 | 272 |
| Wild birds | Tunisia | E. faecalis | 33 | 33 | 50 | 66 | 261 |
| E. faecium | 16 | 53 | 11 | 38 | |||
| Wild fur seals | Brazil | E. faecalis | 0 | 25 | 0 | 10 | 273 |
| Wild marine animals | Brazil | Enterococcus spp. | 14.5 | 32.2 | 0 | 20.9 | 274 |
| Seafood | Tunisia | E. faecalis | 42.9 | 38.1 | 14.3 | 52.4 | 275 |
| E. faecium | 18.2 | 27.3 | 0 | 63.6 | |||
| House flies | Thailand | Enterococcus spp. | 75 | 42.5 | ND | ND | 276 |
TET, tetracycline; ERY, erythromycin; HLR-GEN, high-level resistance to gentamicin; CIP, ciprofloxacin.
Resistance to Tetracycline
This family of antimicrobials integrates several antibacterial active compounds (277), although tetracycline, chlortetracycline, oxytetracycline, and doxycycline are the most used in veterinary. Despite Roberts’ extensive 1996 review about tetracycline resistance mechanisms (278), a more recent update was published in 2005 (279). Almost 60 tetracycline resistance genes have been described, although the most frequent ones in Enterococcus are those implicated in ribosomal protection [tet(M), tet(O), tet(S)], efflux, or enzymatic inactivation [tet(K), tet(L)]. In Enterococcus, as occurs in other Gram-positive microorganisms, the ribosomal protection protein mechanism encoded by the tet(M) gene is the most frequent, independent of the origin of the strains. The transferability of the tetracycline resistance determinants in the absence of plasmids has been described (280); the Tn916/Tn1545 conjugative transposon family carrying the tet(M) gene is responsible, usually in combination with erm(B).
Resistance to Macrolides/Lincosamines/Streptogramins
Numerous chemically diverse compounds are integrated into the macrolide family, with erythromycin being the most representative. Resistance to this antibiotic was immediately reported after its introduction in human clinical use in 1952; moreover, enterococci are intrinsically resistant to clindamycin and lincomycin. Tylosin, spiramycin, and virginiamycin were widely used in pigs and other animals before the European Union limited their use. After the ban, erythromycin resistance in Enterococcus strains from animals decreased spectacularly (281), demonstrating the link between consumption of the antibiotic and the increase in resistance rates, even in different environments.
Chromosomal intrinsic resistance to macrolides by msr(A) and to lincosamides by linB in E. faecium has been described (282, 283). Acquired resistance to macrolides can be codified by various genetic determinants (up to 92 have been described) (284), although the most common worldwide is erm(B), usually carried by Tn917, which is widespread in human and animal isolates. Other relevant genes in the genus Enterococcus are the efflux genes mef(A), conferring resistance to macrolides, vgb(A), conferring resistance to virginiamycin, lnu(B), conferring resistance to lincosamide, and vat(D) and vat(E), conferring resistance to streptogramins.
Resistance to Quinolones
Fluoroquinolones have reduced antimicrobial activity against enterococci, with levofloxacin and moxifloxacin being the most active compounds. Acquired resistance is the consequence of mutations in the gyrA and parC genes (285, 286, 287) or the acquisition of the qnr genes (287). Efflux pumps such as EmeA for E. faecalis (288) and NorA-like for E. faecium (289) have also been described, although their frequency is low. Resistance to ciprofloxacin is a conserved feature among the high-risk E. faecium CC17 clone linked to nosocomial outbreaks (290) and among almost all isolates with resistance to glycopeptides. Fluoroquinolones have never been used as growth promoters, although their use for veterinary therapy is common.
MOLECULAR EPIDEMIOLOGY AND POPULATION STRUCTURE OF ENTEROCOCCI IN FARM AND COMPANION ANIMALS
Epidemiological studies of farm and companion animals were originally driven by the interest in establishing a relationship between antibiotic-resistant isolates from human and nonhuman hosts. At present, the resistance phenotypes of clinical relevance that may be linked to animals mainly comprise resistance to ampicillin, gentamicin, quinupristin-dalfopristin, vancomycin, and linezolid.
Molecular typing of enterococci strains has been performed by different methods, including pulsed field gel electrophoresis (PFGE), amplified fragment length polymorphism (AFLP), MLST, cgMLST, Bayesian analysis of population structure (BAPS) and whole-genome sequencing (WGS) (revised in 291).
The emergence of VRE in European food-producing animals and food of animal origin in the early 1990s (128, 291, 292–296), as well as in the feces of healthy volunteers and food handlers (297–299), led to surveillance studies in the community setting that suggested a relationship between the extensive use of animal growth promoters in veterinary medicine (e.g., avoparcin and tylosin), the colonization pressure in animals, and the subsequent transmission to human hosts throughout the food chain (300, 301).
The first report of VRE in nonhuman hosts occurred in 1993 in the United Kingdom and documented the similarity between isolates of different origins (300). This study was followed by others, which confirmed the similarity of VRE strains from humans and farm animals exposed to avoparcin in different European countries (26, 292, 302–305). The potential selection of antibiotic-resistant enterococci by antibiotics led to the ban of avoparcin as an animal growth promoter in Sweden in 1986, Denmark and Switzerland in 1995, and in the rest of the European countries 2 years later (Commission Directive 97/6/EC). By 1999, other antibiotics (such as bacitracin, virginiamycin, and tylosin) were also banned as growth promoters for healthy animals in Europe, and this was followed in 2006 by a ban on all antibiotics as growth promoters. In this way, Europe led the first intervention against VRE at a global level. In contrast to western countries, the use of antimicrobials in livestock and poultry, as well as the standard policies on antimicrobial use, varies significantly among Asian countries (reviewed in 306). In Korea, avoparcin was used in the management of poultry and swine from 1983 to 1997 but was banned thereafter to reduce exposure of humans to VRE (133). After several years of avoparcin discontinuance in Korea, the prevalence of VRE in Korean livestock was investigated, and some studies reported that the VRE incidence rate in chicken samples was higher than that in pig samples (163, 307).
The ban led to a significant reduction of VRE colonization in animals, foods, and fecal samples of community-based people in different countries. However, VRE was recovered in feces of animals and humans years later, reflecting the important effects of previous livestock practices in the population structure of enterococci in animals.
Most information came from the species E. faecium and E. faecalis, the predominant species in the gastrointestinal tract of mammals, along with E. hirae, E. durans, and E. cecorum (11, 45, 46).
E. faecium
PFGE remained the “gold standard” for molecular typing of E. faecium until the recent introduction of WGS-based epidemiology (291, 308). By using PFGE, clonal dissemination of E. faecium strains with clinically relevant phenotypes (ampicillin, gentamicin, quinupristin-dalfopristin, and vancomycin) has been extensively documented between animals from the same or different farms and has also been suggested between animals and humans (309, 310). The data vary greatly among geographic areas and are normally associated with the use of antibiotics.
Ecological differentiation of E. faecium has been documented in epidemiological studies using AFLP, MLST, and/or BAPS (311–314). AFLP analysis originally revealed different subpopulations (or ecotypes) corresponding to hospitalized patients, community-based people, and farm animals, including veal calves, poultry, and swine (311, 315). Later, MLST results using eBURST confirmed the split of E. faecium into host-specific subgroups, one from hospitalized patients (originally termed clonal complex 17 [CC17]) and others from domesticated animals (291, 316). More recently, BAPS analysis allowed the partitioning of 519 sequence types of 1,720 E. faecium isolates into 13 nonoverlapping groups. Again, BAPS groups were significantly associated with isolates from hospitalized patients (BAPS 3-3) and farm animals (BAPS 2-1 and 2-4) (313). More recently, single-nucleotide polymorphism-based phylogenetic analysis of WGS data split E. faecium into isolates causing infections (clade A1), isolates from healthy humans (clade B), and isolates from healthy humans and animals (clade A2) (79). Clade A1 mostly comprises isolates from hospitalized humans associated with lineages 17 (including ST16 and ST17), 18 (ST18), and 78 (ST78 and ST192), although isolates from animals have been extensively reported (89, 304, 313). The ST78 isolates show putative evolutionary hallmarks with respect to pets (dogs and cats) and poultry isolates and diversified mainly through recombination and acquisition or loss of mobile genetic elements, which eventually led to adaptation to different ecological niches. Thus, ecological distinction is not absolute, and the main zoonotic risk linked to E. faecium isolates is represented by transfer of mobile genetic elements harboring antimicrobial resistance genes.
Poultry
E. faecium isolates resistant to macrolides, quinupristin-dalfoprisitin, or other streptogramins were extensively reported in poultry farms, revealing high heterogeneity of PFGE types and sequence types, although some similar patterns were eventually detected on farms in Europe, the United States, and Asia (317–319). Clonal dissemination of VRE of the E. faecium species (VREfm) in poultry farms exposed to antibiotics before and after the avoparcin ban (109, 302) were documented in European and Asian countries, with sequence types belonging to CC9 or CC96 being predominant in Europe and Malaysia, respectively (320). A dramatic increase of VREfm in Sweden from 2000 to 2009 was due to the clonal expansion of the clone ST310, despite the absence of selection by antibiotics in this country, where the use of antibiotics as animal growth promoters has been forbidden since 1986 (129). A Danish study showed a high rate of VREfm in Danish farms after the 15-year ban of avoparcin, with different sequence types and the presence of an ST842 clone in 36 flocks analyzed corresponding to eight farms broadly distributed across the country (85). Recently, clonally unrelated E. faecium isolates resistant to linezolid emerged on farms in China (236, 237). Common PFGE profiles or sequence types between humans and broilers have also been documented (321–323), but the human health risk associated with the presence of E. faecium in poultry meat is under debate (25).
Swine
VREfm has been extensively reported in pig farms in European countries before and after the avoparcin ban (113, 324, 325). Clonal spread of VREfm was documented in Denmark, Norway, Finland (113), Switzerland (326), Portugal (304), and Spain (327), with predominance of sequence types belonging to the CC5 lineage (ST5, ST6, ST185). The persistence of VREfm in pig farms after the avoparcin ban was associated later with the use of tylosin, which facilitated the coselection of strains resistant to both glycopeptides and macrolides due to the presence of both vanA and erm(B) genes in the same plasmid (113). VREfm was also detected in county fairs in Michigan from 2008 to 2010, which represents the first and only report of VREfm in livestock in the United States to date (121, 122). In Asia, the occurrence varies among countries and is sporadic in China (156). In all these studies, CC5 strains were predominantly identified. A particular ST6 (CC5) clone was identified on farms in different European Union countries and the United States, as well as in healthy volunteers and hospitalized patients, all carrying a Tn1546 in orf1 and a G-T point mutation in position 8234 at vanX (304, 328). In addition to tylosin, copper is frequently added to pig and cattle feeds, so colocation of heavy metal resistance determinants has also been demonstrated in Europe and the United States (329, 330). Copper resistance is often associated with resistance to macrolides [erm(B)], tetracyclines [tet(M)], and glycopeptides (vanA). Although clonal dissemination has been reported (330), a great diversity has been observed on farms (331). Major human clones CC9 and CC22 (previously classified as CC17) have also been documented in some studies (85, 332, 333).
Companion animals
A few studies have analyzed the fecal carriage of ampicillin-resistant E. faecium (AREfm) and VREfm in companion animals. High rates of AREfm were observed among fecal samples of dogs collected in the United Kingdom and Denmark in 2006 and 2008 (23% and 76%, respectively) (89, 334). Most of these isolates belonged to the major human clonal lineage originally called CC17, which suggested a possible transmission between hosts. Later, de Regt et al. demonstrated some unique metabolic features in these CC17 canine isolates that could have facilitated niche adaptation (335). A recent large Dutch country-wide population-based study reported a higher prevalence of fecal carriers of AREfm in dogs and cats than in a healthy human population (25.6%, 5.1%, and 1.5%, respectively). This study concluded that isolates from pets were genetically distinct from those of humans based on the lack of co-occurrence and the cgMLST results (336). Prior antibiotic use and eating raw meat were considered a risk factor for acquiring AREfm in all the available studies (197, 336). Clinical isolates from dogs and cats treated with amoxicillin belong to high clonal complex risks and were similar to those from humans (197, 337).
E. faecalis
A plethora of molecular methods have been used to type this species, including PFGE, AFLP, and MLST. In contrast to E. faecium, E. faecalis isolated from different sources/hosts cannot be grouped using MLST or AFLP. Studies using MLST data revealed the presence of many sequence types in different hosts, including farm animals, companion animals, and hospitalized patients (338, 339). Moreover, some sequence types are associated with a higher prevalence of antibiotic resistance, represented by ST2, ST8, ST9, ST16, ST40, and ST87 (303, 339, 340), all of them being overrepresented in humans. To date, ST16 has been recovered from humans and farm animals and is considered a zoonotic lineage (25), involved in the spread of resistance to all antibiotics used in animals, including bacitracin, phenicols, oxazolidinones (341). Clonal outbreaks of E. faecalis ST82, a common cause of amyloid arthropathy in poultry, have been reported on farms in Denmark, the United States, France, and Germany (342).
Although the detection of more-prevalent E. faecalis sequence types in distant geographical locations and different hosts suggests frequent horizontal gene transfer between different host populations (69, 211, 241, 339, 340, 343), some studies using comparative genomics discarded the idea of global transmission (344).
The incongruence in the topologies of the seven MLST gene trees revealed that this species is highly recombinogenic (291, 343). Subsequent analysis of the E. faecalis population structure based on MLST data using BAPS also yielded incongruent results and confirmed the lack of host-specific groups or ecotypes (313, 314). This issue was also demonstrated by studies that characterized the phylogenetic diversity of E. faecalis using whole genomes (phylogenomics and cgMLST) of clinical, human commensal, and animal isolates and that observed a lack of distinct clustering of isolates according to the source (291, 345).
Additional WGS studies are necessary to characterize and describe the role of animals in the evolution, genetic diversity, and population structure of E. faecalis.
PLASMIDS IN ENTEROCOCCI FROM FOODBORNE AND COMPANION ANIMALS
Horizontal gene transfer plays a relevant role in the dissemination of antibiotic resistance in nonhuman hosts, and plasmids play a central role in this dissemination. Classically, plasmid categorization is based on the presence and diversity of their replication machinery (346), which were established by replication-initiator protein (rep) schemes (347, 348) identified in Gram-positive species to date. In Fig. 1 we show the plasmid content (percentage and diversity of rep sequences) of the 67 E. faecium and 47 E. faecalis genomes of animal origin obtained from the WGS database of the NCBI. The enterococcal genomes from public databases were classified according to their origin (Table 6), information obtained from the Pathosystems Resource Integration Center (PATRIC) database (349). The rep genes obtained by the PlasmidFinder bioinformatics tool (350) belong to plasmid families with theta (RepaA_N, Inc18, Rep3_small tetha) or rolling-circle replication mechanisms (Fig. 1).
FIGURE 1.
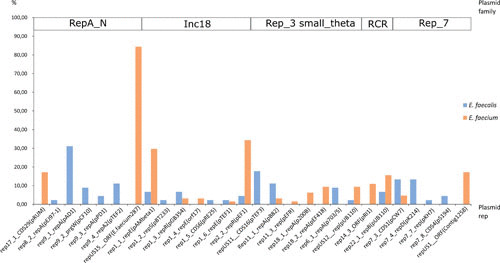
Plasmid gene content of 67 E. faecium and 47 E. faecalis genomes of animal origin from the NCBI whole-genome database. Plasmid data were obtained by the PlasmidFinder bioinformatics tool. The genomes from the database were classified by source, extracting the isolate information from the Pathosystems Resource Integration Center (PATRIC) database (344). Reps, replicases.
TABLE 6.
Enterococcus isolates with animal source from the Genbank database included in the plasmid rep genes in silico screening
| Species | Isolate | MLST | Plasmid rep | Isolation source | Year | Isolation country | Assembly accession |
|---|---|---|---|---|---|---|---|
| Enterococcus faecalis | PF3 | 0 | Adélie penguin (Pygoscelis adeliae) | 2010 | Antarctica | GCA_000505585.1 | |
| DBH18 | 1 | Mallard duck (Anas platyrhynchos) | 2005 | Spain | GCF_001563075.1 | ||
| ATCC 27959 | 40 | 0 | Cattle | 1975 | United States | GCA_000395265.1 | |
| ATCC 35038 | 59 | 2 | Chicken (Gallus gallus) | 1980 | GCA_000392955.1 | ||
| ATCC 29212 | 0 | Chicken (Gallus gallus) | GCA_900117325.1 | ||||
| ATCC 29212 | 2 | Chicken (Gallus gallus) | GCA_900117335.1 | ||||
| F1 | 72 | 2 | Cow | 1900 | GCA_000393095.1 | ||
| ATCC 6055 | 113 | 2 | Cow | 1937 | GCA_000393375.1 | ||
| ARO1/DG | 108 | 3 | Dog (Canis lupus familiaris) | GCA_000157275.1 | |||
| UCD-PD3 | 1 | Felis catus | 2015 | United States | GCF_001692955.1 | ||
| Fly1 | 101 | 0 | Fruit fly (Drosophila) | United States | GCA_000157415.1 | ||
| Fly 2 | 102 | 1 | Fruit fly (Drosophila) | 2005 | GCA_000393215.1 | ||
| P9-1 | 2 | Magellanic penguin (Spheniscus magellanicus) | 2013 | Brazil | GCF_001400065.1 | ||
| P8-1 | 3 | Magellanic penguin, (Spheniscus magellanicus) | 2013 | Brazil | GCF_001400055.1 | ||
| KB1 | 1 | Mus musculus | 2011 | Germany | GCA_002221625.2 | ||
| KB1 | 0 | Mus musculus | 2015 | Switzerland | GCF_001689055.1 | ||
| 7330257-1 | 16 | 1 | Pig (Sus scrofa) | 2001 | Denmark | GCA_000390805.1 | |
| 7330259-5 | 16 | 1 | Pig (Sus scrofa) | 2001 | Denmark | GCA_000390825.1 | |
| 7330948-5 | 16 | 1 | Pig (Sus scrofa) | 2001 | Denmark | GCA_000390845.1 | |
| 7330112-3 | 16 | 6 | Pig (Sus scrofa) | 2001 | Denmark | GCA_000390765.1 | |
| 7430416-3 | 16 | 0 | Pig (Sus scrofa) | 2002 | Denmark | GCA_000390905.1 | |
| 19116 | 16 | 1 | Pig (Sus scrofa) | 2002 | Denmark | GCA_000390685.1 | |
| 7430275-3 | 16 | 1 | Pig (Sus scrofa) | 2002 | Denmark | GCA_000390865.1 | |
| 7430821-4 | 16 | 1 | Pig (Sus scrofa) | 2002 | Denmark | GCA_000390925.1 | |
| D6 | 16 | 0 | Pig (Sus scrofa) | Denmark | GCA_000157455.1 | ||
| 7430315-3 | 35 | 3 | Pig (Sus scrofa) | 2002 | Denmark | GCA_000390885.1 | |
| D1 | 40 | 0 | Pig (Sus scrofa) | GCA_000393575.1 | |||
| 7330082-2 | 40 | 0 | Pig (Sus scrofa) | 2001 | Denmark | GCA_000390745.1 | |
| D32 | 40 | 1 | Pig (Sus scrofa) | 2001 | Denmark | GCA_000281195.1 | |
| D3 | 47 | 2 | Pig (Sus scrofa) | GCA_000392915.1 | |||
| 7330245-2 | 47 | 2 | Pig (Sus scrofa) | 2001 | Denmark | GCA_000390785.1 | |
| L9 | 0 | Pig (Sus scrofa) | 2013 | Brazil | GCF_001878735.1 | ||
| L12 | 0 | Pig (Sus scrofa) | 2013 | Brazil | GCF_001886675.1 | ||
| IBUN9046YE | 5 | Pig (Sus scrofa) | 2014 | Colombia | GCA_002250145.1 | ||
| 19 | 1 | Pig (Sus scrofa) | 2011 | Denmark | GCA_000788165.1 | ||
| 17 | 1 | Pig (Sus scrofa) | 2011 | Denmark | GCA_000788185.1 | ||
| 1 | 2 | Pig (Sus scrofa) | 2011 | Denmark | GCA_000788175.1 | ||
| 32 | 2 | Pig (Sus scrofa) | 2011 | Denmark | GCA_000788255.1 | ||
| 12 | 3 | Pig (Sus scrofa) | 2011 | Denmark | GCA_000788155.1 | ||
| 18 | 3 | Pig (Sus scrofa) | 2011 | Denmark | GCA_000788235.1 | ||
| Enfs51 | 2 | Pig (Sus scrofa) | 2012 | Malaysia | GCF_001806515.1 | ||
| P.En250 | 3 | Pig (Sus scrofa) | 2012 | Malaysia | GCF_002009545.1 | ||
| P. En090 | 3 | Pig (Sus scrofa) | 2012 | Malaysia | GCF_002009565.1 | ||
| Enfs85 | 5 | Pig (Sus scrofa) | 2012 | Malaysia | GCF_002009465.1 | ||
| XJ05 | 1 | Sheep (Ovis aries) | 2002 | China | GCF_001263775.1 | ||
| OG1X | 92 | 1 | Tunicate (Lissoclinum patella) | 2006 | Solomon Islands | GCA_000320305.1 | |
| 2924 | 116 | 1 | Turkey meat | 2005 | Denmark | GCA_000390725.1 | |
| Enterococcus faecium | E1573 | 21 | 1 | Bison (Bison sp.) | 1994 | Belgium | GCA_000321765.1 |
| 825 | 4 | Cattle | 2004 | United States | GCA_002360185.1 | ||
| E0045 | 9 | 3 | Chicken (Gallus gallus) | 1992 | United Kingdom | GCA_000321465.1 | |
| E2134 | 12 | 6 | Chicken (Gallus gallus) | 2004 | Netherlands | GCA_000322185.1 | |
| VAN 342 | 22 | 6 | Chicken (Gallus gallus) | 2010 | Denmark | GCA_000395785.1 | |
| VAN 332 | 38 | 4 | Chicken (Gallus gallus) | 2010 | Denmark | GCA_000395745.1 | |
| VAN 345 | 38 | 5 | Chicken (Gallus gallus) | 2010 | Denmark | GCA_000395805.1 | |
| E1575 | 158 | 4 | Chicken (Gallus gallus) | 1995 | Belgium | GCA_000321805.1 | |
| F9730129-1 | 245 | 4 | Chicken (Gallus gallus) | 1997 | Denmark | GCA_000395965.1 | |
| VAN 335 | 417 | 4 | Chicken (Gallus gallus) | 2010 | Denmark | GCA_000395765.1 | |
| VAN 219 | 784 | 3 | Chicken (Gallus gallus) | 2010 | Denmark | GCA_000395705.1 | |
| VAN 222 | 784 | 3 | Chicken (Gallus gallus) | 2010 | Denmark | GCA_000395725.1 | |
| 7527 | 3 | Chicken (Gallus gallus) | 2003 | United States | GCA_002360135.1 | ||
| 6605 | 4 | Chicken (Gallus gallus) | 2003 | United States | GCA_002360225.1 | ||
| 5209 | 6 | Chicken (Gallus gallus) | 2003 | United States | GCA_002360255.1 | ||
| E1574 | 27 | 3 | Dog (Canis lupus familiaris) | 1995 | Belgium | GCA_000321785.1 | |
| E4389 | 78 | 5 | Dog (Canis lupus familiaris) | Denmark | GCA_000322425.1 | ||
| E4453 | 192 | 5 | Dog (Canis lupus familiaris) | GCA_000239095.2 | |||
| E4452 | 266 | 5 | Dog (Canis lupus familiaris) | 2008 | Netherlands | GCA_000239115.2 | |
| GL3 | 0 | Dromedary | 2013 | Algeria | GCF_001277795.1 | ||
| ICIS 96 | 5 | Equus caballus | 2014 | Russia | GCA_002265255.1 | ||
| LS170308 | 2 | Moschus berezovskii | 2017 | China | GCA_002831505.1 | ||
| E1622 | 104 | 0 | Mouse | 1959 | Netherlands | GCA_000321945.1 | |
| E1576 | 159 | 3 | Ostrich (Struthio camelus) | 2001 | South Africa | GCA_000321825.1 | |
| E0688 | 5 | 4 | Pig (Sus scrofa) | Spain | GCA_000321605.1 | ||
| HF50104 | 5 | 2 | Pig (Sus scrofa) | 2008 | United States | GCA_000396685.1 | |
| HF50215 | 5 | 4 | Pig (Sus scrofa) | 2008 | United States | GCA_000396785.1 | |
| A17 Sv1 | 6 | 1 | Pig (Sus scrofa) | 1995 | Denmark | GCA_000392125.1 | |
| E8sv3 | 6 | 1 | Pig (Sus scrofa) | 1995 | Denmark | GCA_000392145.1 | |
| 9730219-1 | 6 | 1 | Pig (Sus scrofa) | 1997 | Denmark | GCA_000391945.1 | |
| 9730357-1 | 6 | 1 | Pig (Sus scrofa) | 1997 | Denmark | GCA_000391965.1 | |
| 9731352-4 | 6 | 2 | Pig (Sus scrofa) | 1997 | Denmark | GCA_000392005.1 | |
| 9830091-5 | 6 | 1 | Pig (Sus scrofa) | 1998 | Denmark | GCA_000392025.1 | |
| 9830512-2 | 6 | 1 | Pig (Sus scrofa) | 1998 | Denmark | GCA_000392045.1 | |
| 9931110-4 | 6 | 3 | Pig (Sus scrofa) | 1999 | Denmark | GCA_000392105.1 | |
| 7330446-2 | 6 | 1 | Pig (Sus scrofa) | 2001 | Denmark | GCA_000391825.1 | |
| 7330519-3 | 6 | 1 | Pig (Sus scrofa) | 2001 | Denmark | GCA_000391845.1 | |
| 7330884-2 | 6 | 1 | Pig (Sus scrofa) | 2001 | Denmark | GCA_000391885.1 | |
| 7430166-3 | 6 | 1 | Pig (Sus scrofa) | 2002 | Denmark | GCA_000391905.1 | |
| S658-3 | 6 | 1 | Pig (Sus scrofa) | 2002 | Denmark | GCA_000395425.1 | |
| HF50204 | 6 | 1 | Pig (Sus scrofa) | 2008 | United States | GCA_000396765.1 | |
| HF50105 | 6 | 3 | Pig (Sus scrofa) | 2008 | United States | GCA_000396705.1 | |
| HF50106 | 6 | 3 | Pig (Sus scrofa) | 2008 | United States | GCA_000396725.1 | |
| 9731349-1 | 82 | 4 | Pig (Sus scrofa) | 1997 | Denmark | GCA_000391985.1 | |
| 9930238-2 | 133 | 1 | Pig (Sus scrofa) | 1999 | Denmark | GCA_000392085.1 | |
| 7330381-1 | 133 | 1 | Pig (Sus scrofa) | 2001 | Denmark | GCA_000391805.1 | |
| E0679 | 150 | 2 | Pig (Sus scrofa) | Belgium | GCA_000321565.1 | ||
| E0680 | 151 | 3 | Pig (Sus scrofa) | Germany | GCA_000321585.1 | ||
| E1578 | 160 | 1 | Pig (Sus scrofa) | 2001 | Germany | GCA_000321845.1 | |
| 9830565-4 | 185 | 1 | Pig (Sus scrofa) | 1998 | Denmark | GCA_000392065.1 | |
| 7330614-1 | 185 | 1 | Pig (Sus scrofa) | 2001 | Denmark | GCA_000391865.1 | |
| HF50203 | 185 | 1 | Pig (Sus scrofa) | 2008 | United States | GCA_000396745.1 | |
| CICYT-205 | 437 | 3 | Pig (Sus scrofa) | Spain | GCF_001622975.1 | ||
| 7230532-1 | 1 | Pig (Sus scrofa) | 2000 | Denmark | GCA_000391785.1 | ||
| 2006-70-121 | 0 | Pig (Sus scrofa) | 2006 | Denmark | GCA_000391765.1 | ||
| 1970-07-08 | 0 | Pig (Sus scrofa) | 2011 | Denmark | GCA_000767345.1 | ||
| 70-40-11 | 1 | Pig (Sus scrofa) | 2011 | Denmark | GCA_000804405.1 | ||
| 70-61-3 | 1 | Pig (Sus scrofa) | 2011 | Denmark | GCA_000767355.1 | ||
| 1970-08-02 | 2 | Pig (Sus scrofa) | 2011 | Denmark | GCA_000804415.1 | ||
| 70-36-8 | 2 | Pig (Sus scrofa) | 2011 | Denmark | GCA_000767365.1 | ||
| 70-61-7 | 2 | Pig (Sus scrofa) | 2011 | Denmark | GCA_000804385.1 | ||
| E2071 | 27 | 1 | Poultry | 2001 | Denmark | GCA_000322165.1 | |
| FDAARGOS_397 | 2 | Tonsil crypt | GCA_002554355.1 | ||||
| E0269 | 9 | 3 | Turkey (Meleagris gallopavo) | 1996 | Netherlands | GCA_000321525.1 | |
| E0164 | 26 | 3 | Turkey (Meleagris gallopavo) | 1996 | Netherlands | GCA_000321505.1 | |
| M3K31 | 3 | Vulture | 2010 | Spain | GCF_001039515.1 | ||
| 58M | 1 | Wooly mammoth (Mammuthus primigenius) | 2014 | Russia | GCF_001280775.1 |
Plasmids conferring resistance in enterococci to vancomycin, macrolides, tetracycline, aminoglycosides, and heavy metals (copper, cadmium, bacitracin zinc) have been detected on farms that were exposed to antimicrobials used as growth promoters (avoparcin, virginiamycin, tylosin, or bacitracin zinc), therapeutically (tetracyclines, gentamicin, penicillins), or as dietary supplements (e.g., copper). Antibiotic-resistant plasmids have also been recovered from areas where selection was not apparent. Some emblematic examples are transferable vanA in commercial animal husbandry on farms in Michigan, where avoparcin has never been licensed for use in growth promotion (121, 122), and persistent vanA-Inc18 plasmids in Norwegian broiler flocks after the ban of some antibiotics. These studies suggest alternative routes of selection, introduction, and spread of vanA-type vancomycin resistance, plasmid fitness, and other phenomena (351).
Plasmids Conferring Resistance to Glycopeptides
Tn1546 (vanA), the predominant mechanism of glycopeptide resistance in enterococci, has been successfully disseminated among poultry and swine through plasmids of the Inc18 and RepA_N families, respectively (352, 353). In poultry, an 18- to 25-kb fragment that includes the 10.85 kb of Tn1546 (vanA), is conserved in Inc18 plasmids detected in Norwegian broiler flocks for more than 1 decade (from 1999 to years after the avoparcin ban) and in pIP186, the first Inc18 (vanA) plasmid described, in 1986, in an E. faecium clinical isolate (354, 355). The persistence of vanA plasmids on Norwegian poultry farms is attributed to the toxin-antitoxin system ω-ε-ζ originally described in pRE25, a plasmid of E. faecalis that carries resistance to different antibiotic families and is prevalent in animals and foods (127, 354). Analyses of the Tn1546 insertion sites and plasmid backbones suggest spread of the vanA transposon across clonal lines in the broiler industry (125, 354–356). Bortolaia and Guardabassi recently associated the persistence of glycopeptide resistance in Danish poultry flocks after 15 years of the avoparcin ban with a nontransferable 54-kb plasmid in isolates that only confer resistance to glycopeptides (27). It is notable that broiler flocks raised in Denmark come from parent birds imported from Sweden, and the high occurrence of VREfm was also observed in Swedish broiler flocks until 2011 (129).
In swine, large plasmids belonging to the RepA_N family (150 to 190 kb, reppLG1), which carry a truncated variant of Tn1546 and tcrB (coding for resistance to copper), have been detected in a pandemic CC5 E. faecium clone that has been circulating in swine farms in Spain, Portugal, Denmark, Switzerland, and the United States for decades and in other E. faecium lineages of pigs and humans, which suggests transmission (304). These plasmids used to carry the erm(B) gene (macrolide resistance) and, eventually, trcB (copper resistance) (see below).
Also, sporadic reports have documented the occurrence of strains carrying other vanB or vanN operons on plasmids in poultry meat (178, 188), farmed game meat and wild game meat (226). Finally, vancomycin-susceptible E. faecalis strains carrying vanC1 on transferable elements (plasmids, transposons, and integrons) have also been reported in cloacal swabs of broilers (357) and feces of diseased pigs from different farms (358). Transmission of species-specific vanC1 and vanC2/C3 genes could be currently underestimated given the high prevalence of E. gallinarum and E. casseliflavus, respectively, in food-producing animals (159, 359–360) and the scarcity of studies that screen vanC genes in other species.
Plasmids Conferring Resistance to Macrolides, Streptogramins, and Lincosamides
These plasmids have been extensively recovered in enterococci from poultry and pig farms where macrolides (spiramycin and tylosin) and streptogramins (virginiamycin) were used as growth promoters and pleuromutilins (tiamulin and valnemulin) were used to treat infections. Lincomycin, alone or in combination with spectinomycin, has been widely used to control respiratory and gastrointestinal bacterial pathogens in cattle, swine, poultry, dogs, and cats, and pirlimycin has been only used to treat bovine mastitis cases. Clindamycin is a common therapeutic option for topical infections in dogs and cats.
Macrolides
The most widespread gene that confers resistance to macrolides in enterococci is erm(B), which is located in different transposons and plasmids in species of the Enterococcus, Streptococcus, Staphylococcus, and Clostridium genera (346, 361). pRE25, a multidrug-resistant plasmid originally recovered from an E. faecalis isolate from a sausage sample, is the paradigm of the Inc18 family and has greatly contributed to the spread of erm(B) among animals and humans (346, 353, 362). This plasmid encodes resistance to 12 antimicrobials from 5 structural classes (macrolides, lincosamides, streptothricin, chloramphenicol, aminoglycosides) due to the presence of erm(B) (macrolide-lincosamide-streptogramin B), catpIP501 (chloramphenicol), and Tn5405, which comprises the genes aadE-sat4-aphA3 (aminoglycoside-streptothricin) (363, 364). The genes carried by pRE25 are present in several animal pathogens, namely, Streptococcus pyogenes, Streptococcus agalactiae, S. aureus, Bacillus subtilis, Campylobacter coli, Clostridium perfringens, and C. difficile. The erm(B) gene has also been found in small plasmids in poultry samples (365) and in large plasmids in food samples in addition to other genes such as msr(C) and lnu(B), tet(L), and tet(W) (366). Its presence in chromosomes is also frequent.
The gene erm(A), associated with Tn554 and commonly found in staphylococci from swine, has also been found in streptococci and sporadic isolates of E. faecalis and E. faecium from pigs, suggesting transfer events (282, 367). More recently, a novel erm(A)-like gene that confers high-level resistance to erythromycin (MIC, >128 μg/ml) has been detected in Inc18 plasmids with genes encoding resistance to phenicols and oxazolidinones (see below). This gene differs from the widespread erm(A) gene on Tn554 and the erm(A) gene formerly called ermTR, predominant in staphylococci and streptococci, respectively (82 to 85% homology at the amino acid level). This erm(A) enterococcal variant has a 116-bp deletion in the translational attenuator (237).
Streptogramins
Genes conferring resistance to streptogramins (acetyltransferases encoded by satG/vatE and satA/vatA genes and ABC transporters encoded by vgb/vgbB) and macrolides [23S rRNA methylases encoded by erm(B), erm(A), erm(C) genes] are observed in a diversity of plasmids and clonal backgrounds. In addition, vat genes are often cotranscribed and cotransferred along with vga, vgaB, vgb, vgbB, or erm(B) genes through transposable elements, some of them previously observed in staphylococci (364, 368–373). Transferability of vat genes and streptogramin resistance in E. faecium strains through contaminated pork and chicken meat, raw manure, and surface/ground water has been extensively documented (374, 375).
Lincosamides
Resistance to this antibiotic family can be due to the presence of genes coding for ABC transporters or modifying enzymes, most of them located on plasmids and/or transposable elements. These elements have been extensively documented in staphylococci and to a lesser extent in streptococci, Clostridium, and other species of Gram-positive bacteria in animals.
ABC transporters that confer resistance to pleuromutilins, lincosamides, and streptogramin A antibiotics (PLSA) include the genes vga and vga(A)v, vga(C), vga(E), vga(E)v, eat(A)v, sal(A), lsa(A), lsa(C), and lsa(E). They frequently appear within clusters in plasmids or transferable chromosomal regions previously reported in S. aureus (230). A 8,705-bp region flanked by ISEfa8 and IS1216, and comprising genes coding for one or more antibiotics, namely lnu(B) (lincosamide), lsa(E) (PLSA), spw (spectinomycin), aadE (streptomycin), and erm(B) (macrolide-lincosamide-streptogramin B), is common for plasmids of S. aureus (pV7037) and E. faecium (pY13, pXD4, pXD5) strains recovered from pigs (230, 376, 377). The pY13 plasmid also contains a copy of the genes lnu(B) (lincosamide) and aphA3 (kanamycin/neomycin) and a second copy of erm(B), highlighting the redundancy of determinants in settings under high selective pressure.
Two genes coding for nucleotidyl transferases (lnu), which only confer resistance to lincosamides, have been described in Enterococcus from swine recovered on Chinese farms (229, 378). lnu(G) is part of a 4,738-bp functionally active transposon designated Tn6260, which was first detected in an E. faecalis isolate of swine origin; this element is similar to others of the Tn554 family, which includes different antibiotic resistance genes (378). lnu(B) has been detected in porcine E. faecium isolates, and it has been found in a nonconjugative plasmid linked to the erm(B), lsa(E), spw, aadE, and aphA3 genes, which account for resistance to macrolides, lincosamides, streptogramins, pleuromutilins, streptomycin, spectinomycin, and kanamycin/neomycin (229).
Plasmids Conferring Resistance to Phenicols and Oxazolidinones
Genes coding for resistance to nonfluorinated phenicols (cat), nonfluorinated and fluorinated phenicols (fexA, fexB), and to both phenicols and oxazolidinones (cfr, optrA) have been detected in enterococcal species from animals, foods, and humans.
The production of chloramphenicol acetyltransferase (CAT) enzymes seems to be the main mechanism of resistance to chloramphenicol, although the number of studies addressing the diversity and the genetic context of cat genes in Enterococcus is still scarce. The predominant cat variants are cat(A-7), associated with pRE25-like plasmids of the Inc18 family, which are widely disseminated in food and farm animals, predominantly poultry (241), and cat(A-8), also known as catpC223, associated with pC223 plasmids originally detected in S. aureus that are now predominant in E. faecalis from swine. This gene eventually appears in tandem with tet(M) and tet(L) genes within the transposon Tn6245, and relics of this transposon have been observed in plasmids that also carry fexA and optrA (237). Although isolates positive for the cat(A-9) gene have been recently identified in E. faecalis from swine, their genetic context has not been characterized (A. Freitas, personal communication).
The florfenicol exporter gene fexB was initially detected in nonconjugative plasmids of E. faecium, E. faecalis, and E. hirae isolates collected on Chinese swine farms heavily exposed to florfenicol (379). These plasmids share common regions with the backbone of Inc18 plasmid derivatives (e.g., pVEF4), widely disseminated in Norwegian poultry farms (355). The fexB gene is bracketed by IS1216 and would have been acquired by widespread pRE25-like plasmids, as occurred for other antimicrobial resistance genes flanked by this IS. The fexB gene has also been identified in enterococci from other farm animals (bovine) and aquaculture, although the plasmids have not been characterized (241, 380). A different epidemiological landscape occurs for the fexA gene, which is located on plasmids (241) and chromosomes (236) of enterococcal animal isolates, often in tandem with the optrA gene (237, 241) or the cfr gene (235). The fexA gene is inserted in the emblematic Tn554 of staphylococci, although in enterococci traces of this transposon might be absent as a consequence of different events of horizontal gene transfer (237).
Enterococcal plasmids carrying optrA have been detected in poultry, swine, and humans. Despite differences in size (30 to 80 kb) and the backbone, all share similar regions upstream and downstream of the optrA gene (236, 237, 241). The presence of a novel erm(A)-like gene that confers a high level of resistance to erythromycin is notable (237). The genetic context of optrA is flanked by copies of IS1216 in the same or opposite direction, which determine the mobility.
Conjugative and nonconjugative plasmids carrying the cfr gene flanked by different ISs (IS1216, ISEnfa4, ISEnfa5, IS256) have been described in animal isolates of various Gram-positive species, including enterococci. The nonconjugative pEF-01 (32.2 kb) plasmid represents the first description of a cfr-plasmid in this bacterial genus and was identified in a fecal E. faecalis isolate of bovine origin collected in 2009 on a Chinese farm (232). This plasmid has three Rep proteins of the Inc18 and Rep3 plasmid families and 9-kb and 6-kb regions which exhibit high similarity with the backbone of vanA Inc18 plasmids (pVEF1-2-3), which have been widely isolated on poultry farms (232). Moreover, the cfr gene was flanked by IS1216, which would facilitate recombination processes, and the plasmid also contains the fexA gene, which provides resistance to phenicols. Conjugative plasmids carrying the cfr gene bracketed by ISEnfa4 copies were isolated from E. thailandicus and E. faecalis from Chinese swine farms. These are closely related to another emblematic Inc18 plasmid, pAMb1, and contained erm(B) and erm(A) genes, conferring the MLSB phenotype and the ω-ε-ζ toxin-antitoxin module, which may promote the persistence of plasmids by encoding a system that kills or prevents the growth of plasmid-free cells (55). This genetic context has also been detected in streptococci and staphylococci and points to independent acquisition events for the cfr gene. The cfr gene bracketed by two copies of ISEnfa5 has been documented in E. gallinarum and E. casseliflavus of swine origin (235).
Plasmids Conferring Resistance to Bacitracin
Bacitracin has been used as an animal growth promoter in China, and recent reports documented E. faecalis isolates with high-level resistance to this antibiotic (MIC, ≥256 μg/ml), due to the presence of the bcrABDR cluster, which is composed by the bcrABD operon and its regulatory gene, bcrR. The cluster bracketed by either two, one, or no ISEnfa1 copies is located on transferable plasmids (341) or chromosomes. The structure ISEnfa1-bcrABDR-ISEnfa1 may be circulating and may have been transferred to other species by IS-mediated recombination. A multiresistant 79-kb pheromone-responsive plasmid carrying this ISEnfa1-bcrABDR-ISEnfa1 platform as well as optrA, fexA, Tn6425 (catpC223-tetM-tetL), Tn5405 (aph-sat-str), and genes for resistance to copper and cadmium seems to be disseminated in Chinese farms (341), frequently associated with E. faecalis ST16. This bcrABDR cluster is also common in E. cecorum, a chicken commensal species (341).
Plasmids Conferring Resistance to Copper
Transferable resistance to copper (tcrB) in enterococci has been detected in piglets, calves, poultry, and humans in Europe, Asia, Australia, and North America (148, 331, 368, 381–383). Plasmids carrying tcrB are identified in intensively copper-supplemented livestock species, but plasmids with additional linkage with erythromycin [erm(B)] and/or vancomycin resistance (vanA) genes have only been observed in heavily copper-exposed swine (often with different copper compounds) in European countries where avoparcin was used as growth enhancer in the 1990s (148, 329, 381, 383, 384). The plasmids were detected in several enterococcal species (E. faecium, E. faecalis, E. gallinarum, E. casseliflavus, E. mundtii, E. hirae), and conjugation has been experimentally demonstrated from E. faecalis to E. faecium (381). Copper fed to feedlot cattle at a growth-promotion concentration (10 × basal requirement) was associated with increased frequencies of tcrB-positive, macrolide-resistant erm(B) and, eventually, tetracycline-resistant tet(M) enterococci; however, copper susceptibilities were not increased in piglets in which the effect of in-feed tylosin or chlortetracycline was evaluated (382, 385). Cotransmission of tcrB and erm(B) genes between E. hirae from a sediment-derived livestock isolate and E. faecalis has been experimentally demonstrated (386). A recent analysis of WGS of E. faecalis from copper-supplemented Danish pigs also documented the presence of a chromosomal cluster of genes involved in susceptibility to copper, including the tcrYAZB operon, in three of six isolates analyzed, all containing plasmids (387). A detailed characterization of this chromosomal region was not provided, although other authors, who also identified redundancy of copper genes in chromosomes, demonstrated its cotransferability with ampicillin resistance (331).
CONCLUDING REMARKS
This review summarizes the current knowledge concerning the epidemiology and population structure of antibiotic-resistant Enterococcus species from food-producing, wild, and companion animals. Members of this genus are normal components of the intestinal microbiota of animals, and some species may also be etiological agents of a wide variety of infections with E. faecalis ST16 (considered a zoonotic pathogen) or ST82 (an etiological agent of the amyloid encephalopathy in chickens).
Enterococcus species are frequent contaminants on foods (especially poultry meat), although the risk of transmission from animals to humans through the food chain is based on indirect evidence, and thus, the bacterial load necessary to colonize the human gut remains greatly unknown. The food and animal industries seem to have contributed to the spread of certain pathogenic lineages (E. faecalis ST82 and ST16 lineages) and multidrug-resistant strains. Other species adapted to animals seem to act as important reservoirs of adaptive traits (E. cecorum). However, transmission of antimicrobial resistance by horizontal gene transfer events represents the main risk of foods contaminated by enterococci. Genes encoding resistance to vancomycin, macrolides, phenicols, and linezolid have been extensively documented in animals, frequently in response to heavy selection by antimicrobials (antibiotics and heavy metals) used in prophylaxis or as growth promoters. Although the same genes and plasmids may be present in humans and animals, particular plasmid variants are often documented on farms, suggesting certain host specificity and transmission at a local level. Deep analysis of antimicrobial-resistant genes reveals a wide diversity of alleles [e.g., erm(A), optrA, cfr] and also the frequent presence of ISs (e.g., IS1216) that highlight the risk of frequent and independent acquisition and selection events of antimicrobial resistance on farms. More studies are necessary to establish the risks of the emergence and transmission of antibiotic-resistant enterococci from animals to humans.
ACKNOWLEDGMENTS
Work in University of La Rioja is financed by project SAF2016-76571-R of the Agencia Estatal de Investigación (AEI) of Spain and the Fondo Europeo de Desarrollo Regional (FEDER). Work in TMC lab is supported by grants funded by the Joint Programming Initiative in Antimicrobial Resistance (JPIAMR Third call, STARCS, JPIAMR2016 AC16/00039 and the European Development Regional Fund “A way to achieve Europe” (ERDF) that co-funds the Spanish R&D National Plan 2012-2019 (PI15-01307), CIBER (CIBER in Epidemiology and Public Health, CIBERESP; CB06/02/0053), and the Regional Government of Madrid (InGeMICS- B2017/BMD-3691). L. Ruiz-Ripa has a predoctoral FPI fellowship of the University of La Rioja, Spain.
Contributor Information
Carmen Torres, Biochemistry and Molecular Biology Unit, University of La Rioja, 26006 Logroño, Spain.
Carla Andrea Alonso, Biochemistry and Molecular Biology Unit, University of La Rioja, 26006 Logroño, Spain.
Laura Ruiz-Ripa, Biochemistry and Molecular Biology Unit, University of La Rioja, 26006 Logroño, Spain.
Ricardo León-Sampedro, Department of Microbiology, Ramón y Cajal University Hospital, Ramón y Cajal Health Research Institute (IRYCIS), Madrid, Spain; Centro de Investigación Biomédica en Red de Epidemiología y Salud Pública (CIBER-ESP), Madrid, Spain.
Rosa Del Campo, Department of Microbiology, Ramón y Cajal University Hospital, Ramón y Cajal Health Research Institute (IRYCIS), Madrid, Spain; Red Española de Investigación en Patología Infecciosa (REIPI), Instituto de Salud Carlos III, Madrid, Spain.
Teresa M. Coque, Department of Microbiology, Ramón y Cajal University Hospital, Ramón y Cajal Health Research Institute (IRYCIS), Madrid, Spain Centro de Investigación Biomédica en Red de Epidemiología y Salud Pública (CIBER-ESP), Madrid, Spain.
Frank Møller Aarestrup, Technical University of Denmark, Lyngby, Denmark.
Stefan Schwarz, Freie Universität Berlin, Berlin, Germany.
Jianzhong Shen, China Agricultural University, Beijing, China.
Lina Cavaco, Statens Serum Institute, Copenhagen, Denmark.
REFERENCES
- 1.Boehm AB, Sassoubre LM. 2014. Enterococci as indicators of environmental fecal contamination. In Gilmore MS, Clewell DB, Ike Y, Shankar N (ed), Enterococci: from Commensals to Leading Causes of Drug Resistant Infection. Eye and Ear Infirmary, Boston, MA. [PubMed] [PubMed] [Google Scholar]
- 2.Tyson GH, Nyirabahizi E, Crarey E, Kabera C, Lam C, Rice-Trujillo C, McDermott PF, Tate H. 2017. Prevalence and antimicrobial resistance of enterococci isolated from retail meats in the United States, 2002–2014. Appl Environ Microbiol 84:e01902–e01917. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arias CA, Murray BE. 2012. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 10:266–278 10.1038/nrmicro2761. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Centre for Disease Prevention and Control (ECDC). 2011. Annual epidemiological report on communicable diseases in Europe. Euro Surveill 16:20012. [PubMed] [Google Scholar]
- 5.Iaria C, Stassi G, Costa GB, Di Leo R, Toscano A, Cascio A. 2005. Enterococcal meningitis caused by Enterococcus casseliflavus. First case report. BMC Infect Dis 5:3 10.1186/1471-2334-5-3. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canalejo E, Ballesteros R, Cabezudo J, García-Arata MI, Moreno J. 2008. Bacteraemic spondylodiscitis caused by Enterococcus hirae. Eur J Clin Microbiol Infect Dis 27:613–615 10.1007/s10096-008-0476-8. [PubMed] [DOI] [PubMed] [Google Scholar]
- 7.Mastroianni A. 2009. Enterococcus raffinosus endocarditis. First case and literature review. Infez Med 17:14–20. [PubMed] [PubMed] [Google Scholar]
- 8.Antonello VS, Zenkner FM, França J, Santos BR. 2010. Enterococcus gallinarum meningitis in an immunocompetent host: a case report. Rev Inst Med Trop São Paulo 52:111–112 10.1590/S0036-46652010000200009. [PubMed] [DOI] [PubMed] [Google Scholar]
- 9.Escribano JA, Solivera J, Vidal E, Rivin E, Lozano J. 2013. Otogenic cerebellar abscess by Enterococcus avium, a very rare infectious agent. J Neurol Surg A Cent Eur Neurosurg 74(Suppl 1):e155–e158 10.1055/s-0032-1333418. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Kenzaka T, Takamura N, Kumabe A, Takeda K. 2013. A case of subacute infective endocarditis and blood access infection caused by Enterococcus durans. BMC Infect Dis 13:594 10.1186/1471-2334-13-594. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lebreton F, Willems RJL, Gilmore MS. 2014. Enterococcus diversity, origins in nature, and gut colonization. In Gilmore MS, Clewell DB, Ike Y, Shankar N (ed), Enterococci: from Commensals to Leading Causes of Drug Resistant Infection. Eye and Ear Infirmary, Boston, MA. [PubMed] [Google Scholar]
- 12.Aarestrup FM, Butaye P, Witte W. 2002. Nonhuman reservoirs of enterococci, p 1281–1289. In Gilmore MS, Clewell DB, Courvalin P, Dunny GM, Murray BE, Rice LB (ed), The Enterococci: Pathogenesis, Molecular Biology, and Antibiotic Resistance. ASM Press, Washington, DC. [Google Scholar]
- 13.Aarestrup FM (ed). 2006. Antimicrobial Resistance in Bacteria of Animal Origin. ASM Press, Washington, DC. [Google Scholar]
- 14.Stalker MJ, Brash ML, Weisz A, Ouckama RM, Slavic D. 2010. Arthritis and osteomyelitis associated with Enterococcus cecorum infection in broiler and broiler breeder chickens in Ontario, Canada. J Vet Diagn Invest 22:643–645 10.1177/104063871002200426. [DOI] [PubMed] [Google Scholar]
- 15.Dolka B, Chrobak-Chmiel D, Makrai L, Szeleszczuk P. 2016. Phenotypic and genotypic characterization of Enterococcus cecorum strains associated with infections in poultry. BMC Vet Res 12:129 10.1186/s12917-016-0761-1. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kak V, Chow JM. 2002. Acquired antibiotic resistances in enterococci, p 355–383. In Gilmore MS, Clewell B, Courvalin P, Dunny GM, Murray BM, Rice LB (ed), The Enterococci: Pathogenesis, Molecular Biology, and Antibiotic Resistance. ASM Press, Washington, DC. [Google Scholar]
- 17.Hollenbeck BL, Rice LB. 2012. Intrinsic and acquired resistance mechanisms in enterococcus. Virulence 3:421–433 10.4161/viru.21282. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller WR, Munita JM, Arias CA. 2014. Mechanisms of antibiotic resistance in enterococci. Expert Rev Anti Infect Ther 12:1221–1236 10.1586/14787210.2014.956092. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Courvalin P. 2006. Vancomycin resistance in Gram-positive cocci. Clin Infect Dis 42(Suppl 1):S25–S34 10.1086/491711. [PubMed] [DOI] [PubMed] [Google Scholar]
- 20.Nilsson O. 2012. Vancomycin resistant enterococci in farm animals: occurrence and importance. Infect Ecol Epidemiol 2:16959 10.3402/iee.v2i0.16959. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cattoir V, Leclercq R. 2013. Twenty-five years of shared life with vancomycin-resistant enterococci: is it time to divorce? J Antimicrob Chemother 68:731–742 10.1093/jac/dks469. [PubMed] [DOI] [PubMed] [Google Scholar]
- 22.Ahmed MO, Baptiste KE. 2017. Vancomycin-resistant enterococci: a review of antimicrobial resistant mechanisms and perspectives of human and animal health. Microb Drug Resist. Epub ahead of print. 10.1089/mdr.2017.0147. [DOI] [PubMed] [Google Scholar]
- 23.Chow JW. 2000. Aminoglycoside resistance in enterococci. Clin Infect Dis 31:586–589 10.1086/313949. [PubMed] [DOI] [PubMed] [Google Scholar]
- 24.Hammerum AM, Lester CH, Heuer OE. 2010. Antimicrobial-resistant enterococci in animals and meat: a human health hazard? Foodborne Pathog Dis 7:1137–1146 10.1089/fpd.2010.0552. [PubMed] [DOI] [PubMed] [Google Scholar]
- 25.Bortolaia V, Espinosa-Gongora C, Guardabassi L. 2016. Human health risks associated with antimicrobial-resistant enterococci and Staphylococcus aureus on poultry meat. Clin Microbiol Infect 22:130–140 10.1016/j.cmi.2015.12.003. [PubMed] [DOI] [PubMed] [Google Scholar]
- 26.Hammerum AM. 2012. Enterococci of animal origin and their significance for public health. Clin Microbiol Infect 18:619–625 10.1111/j.1469-0691.2012.03829.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 27.Bortolaia V, Guardabassi L. 2015. Zoonotic transmission of antimicrobial resistant enterococci: a threat to public health or an overemphasized risk? p 407–431. In Sing A (ed), Zoonoses: Infections Affecting Humans and Animals. Focus on Public Health Aspects. Springer, New York, NY. 10.1007/978-94-017-9457-2_16 [DOI] [Google Scholar]
- 28.Stiles ME, Holzapfel WH. 1997. Lactic acid bacteria of foods and their current taxonomy. Int J Food Microbiol 36:1–29 10.1016/S0168-1605(96)01233-0. [DOI] [PubMed] [Google Scholar]
- 29.Lund B, Adamsson I, Edlund C. 2002. Gastrointestinal transit survival of an Enterococcus faecium probiotic strain administered with or without vancomycin. Int J Food Microbiol 77:109–115 10.1016/S0168-1605(02)00047-8. [DOI] [PubMed] [Google Scholar]
- 30.Foulquié Moreno MR, Sarantinopoulos P, Tsakalidou E, De Vuyst L. 2006. The role and application of enterococci in food and health. Int J Food Microbiol 106:1–24 10.1016/j.ijfoodmicro.2005.06.026. [PubMed] [DOI] [PubMed] [Google Scholar]
- 31.Zhong Z, Zhang W, Song Y, Liu W, Xu H, Xi X, Menghe B, Zhang H, Sun Z. 2017. Comparative genomic analysis of the genus Enterococcus. Microbiol Res 196:95–105 10.1016/j.micres.2016.12.009. [PubMed] [DOI] [PubMed] [Google Scholar]
- 32.Schleifer KH, Kilpper-Balz R. 1984. Transfer of Streptococcus faecalis and Streptococcus faecium to the genus Enterococcus nom. rev. as Enterococcus faecalis comb. nov. and Enterococcus faecium comb. nov. Int J Syst Bacteriol 34:31–34 10.1099/00207713-34-1-31. [DOI] [Google Scholar]
- 33.Parte AC. 2014. LPSN: list of prokaryotic names with standing in nomenclature. Nucleic Acids Res 42(D1):D613–D616 10.1093/nar/gkt1111. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Graef EM, Devriese LA, Vancanneyt M, Baele M, Collins MD, Lefebvre K, Swings J, Haesebrouck F. 2003. Description of Enterococcus canis sp. nov. from dogs and reclassification of Enterococcus porcinus Teixeira et al. 2001 as a junior synonym of Enterococcus villorum Vancanneyt et al. 2001. Int J Syst Evol Microbiol 53:1069–1074 10.1099/ijs.0.02549-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 35.Law-Brown J, Meyers PR. 2003. Enterococcus phoeniculicola sp. nov., a novel member of the enterococci isolated from the uropygial gland of the red-billed woodhoopoe, Phoeniculus purpureus. Int J Syst Evol Microbiol 53:683–685 10.1099/ijs.0.02334-0. [DOI] [PubMed] [Google Scholar]
- 36.Fortina MG, Ricci G, Mora D, Manachini PL. 2004. Molecular analysis of artisanal Italian cheeses reveals Enterococcus italicus sp. nov. Int J Syst Evol Microbiol 54:1717–1721 10.1099/ijs.0.63190-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 37.Švec P, Vancanneyt M, Koort J, Naser SM, Hoste B, Vihavainen E, Vandamme P, Swings J, Björkroth J. 2005. Enterococcus devriesei sp. nov., associated with animal sources. Int J Syst Evol Microbiol 55:2479–2484 10.1099/ijs.0.63851-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 38.Tanasupawat S, Sukontasing S, Lee JS. 2008. Enterococcus thailandicus sp. nov., isolated from fermented sausage (‘mum’) in Thailand. Int J Syst Evol Microbiol 58:1630–1634 10.1099/ijs.0.65535-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 39.Švec P, Vandamme P, Bryndová H, Holochová P, Kosina M, Maslanová I, Sedlácek I. 2012. Enterococcus plantarum sp. nov., isolated from plants. Int J Syst Evol Microbiol 62:1499–1505 10.1099/ijs.0.033357-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 40.Niemi RM, Ollinkangas T, Paulin L, Švec P, Vandamme P, Karkman A, Kosina M, Lindström K. 2012. Enterococcus rivorum sp. nov., from water of pristine brooks. Int J Syst Evol Microbiol 62:2169–2173 10.1099/ijs.0.038257-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 41.Sistek V, Maheux AF, Boissinot M, Bernard KA, Cantin P, Cleenwerck I, De Vos P, Bergeron MG. 2012. Enterococcus ureasiticus sp. nov. and Enterococcus quebecensis sp. nov., isolated from water. Int J Syst Evol Microbiol 62:1314–1320 10.1099/ijs.0.029033-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 42.Sedláček I, Holochová P, Mašlaňová I, Kosina M, Spröer C, Bryndová H, Vandamme P, Rudolf I, Hubálek Z, Švec P. 2013. Enterococcus ureilyticus sp. nov. and Enterococcus rotai sp. nov., two urease-producing enterococci from the environment. Int J Syst Evol Microbiol 63:502–510 10.1099/ijs.0.041152-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 43.Mundt JO. 1963. Occurrence of enterococci in animals in a wild environment. Appl Microbiol 11:136–140. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kühn I, Iversen A, Burman LG, Olsson-Liljequist B, Franklin A, Finn M, Aarestrup F, Seyfarth AM, Blanch AR, Vilanova X, Taylor H, Caplin J, Moreno MA, Dominguez L, Herrero IA, Möllby R. 2003. Comparison of enterococcal populations in animals, humans, and the environment: a European study. Int J Food Microbiol 88:133–145 10.1016/S0168-1605(03)00176-4. [DOI] [PubMed] [Google Scholar]
- 45.Devriese LA, Ceyssens K, Haesebrouck F. 1991a. Characteristics of Enterococcus cecorum strains from the intestines of different animal species. Lett Appl Microbiol 12:137–139 10.1111/j.1472-765X.1991.tb00524.x. [DOI] [Google Scholar]
- 46.Devriese LA, Laurier L, De Herdt P, Haesebrouck F. 1992. Enterococcal and streptococcal species isolated from faeces of calves, young cattle and dairy cows. J Appl Bacteriol 72:29–31 10.1111/j.1365-2672.1992.tb04877.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 47.Scupham AJ, Patton TG, Bent E, Bayles DO. 2008. Comparison of the cecal microbiota of domestic and wild turkeys. Microb Ecol 56:322–331 10.1007/s00248-007-9349-4. [PubMed] [DOI] [PubMed] [Google Scholar]
- 48.Gong J, Forster RJ, Yu H, Chambers JR, Wheatcroft R, Sabour PM, Chen S. 2002. Molecular analysis of bacterial populations in the ileum of broiler chickens and comparison with bacteria in the cecum. FEMS Microbiol Ecol 41:171–179 10.1111/j.1574-6941.2002.tb00978.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 49.Devriese LA, Hommez J, Wijfels R, Haesebrouck F. 1991. Composition of the enterococcal and streptococcal intestinal flora of poultry. J Appl Bacteriol 71:46–50 10.1111/j.1365-2672.1991.tb04585.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 50.Kojima A, Morioka A, Kijima M, Ishihara K, Asai T, Fujisawa T, Tamura Y, Takahashi T. 2010. Classification and antimicrobial susceptibilities of Enterococcus species isolated from apparently healthy food-producing animals in Japan. Zoonoses Public Health 57:137–141 10.1111/j.1863-2378.2008.01204.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 51.Iweriebor BC, Obi LC, Okoh AI. 2016. Macrolide, glycopeptide resistance and virulence genes in Enterococcus species isolates from dairy cattle. J Med Microbiol 65:641–648 10.1099/jmm.0.000275. [PubMed] [DOI] [PubMed] [Google Scholar]
- 52.Beukers AG, Zaheer R, Cook SR, Stanford K, Chaves AV, Ward MP, McAllister TA. 2015. Effect of in-feed administration and withdrawal of tylosin phosphate on antibiotic resistance in enterococci isolated from feedlot steers. Front Microbiol 6:483 10.3389/fmicb.2015.00483. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hwang IY, Ku HO, Lim SK, Park CK, Jung GS, Jung SC, Nam HM. 2009. Species distribution and resistance patterns to growth-promoting antimicrobials of enterococci isolated from pigs and chickens in Korea. J Vet Diagn Invest 21:858–862 10.1177/104063870902100616. [PubMed] [DOI] [PubMed] [Google Scholar]
- 54.Vancanneyt M, Snauwaert C, Cleenwerck I, Baele M, Descheemaeker P, Goossens H, Pot B, Vandamme P, Swings J, Haesebrouck F, Devriese LA. 2001. Enterococcus villorum sp. nov., an enteroadherent bacterium associated with diarrhoea in piglets. Int J Syst Evol Microbiol 51:393–400 10.1099/00207713-51-2-393. [PubMed] [DOI] [PubMed] [Google Scholar]
- 55.Liu Y, Wang Y, Schwarz S, Li Y, Shen Z, Zhang Q, Wu C, Shen J. 2013. Transferable multiresistance plasmids carrying cfr in Enterococcus spp. from swine and farm environment. Antimicrob Agents Chemother 57:42–48 10.1128/AAC.01605-12. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Devriese LA, Cruz Colque JI, De Herdt P, Haesebrouck F. 1992. Identification and composition of the tonsillar and anal enterococcal and streptococcal flora of dogs and cats. J Appl Bacteriol 73:421–425 10.1111/j.1365-2672.1992.tb04998.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 57.Rodrigues J, Poeta P, Martins A, Costa D. 2002. The importance of pets as reservoirs of resistant Enterococcus strains, with special reference to vancomycin. J Vet Med B Infect Dis Vet Public Health 49:278–280 10.1046/j.1439-0450.2002.00561.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 58.Jackson CR, Fedorka-Cray PJ, Davis JA, Barrett JB, Frye JG. 2009. Prevalence, species distribution and antimicrobial resistance of enterococci isolated from dogs and cats in the United States. J Appl Microbiol 107:1269–1278 10.1111/j.1365-2672.2009.04310.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 59.Ben Said L, Dziri R, Sassi N, Lozano C, Ben Slama K, Ouzari I, Torres C, Klibi N. 2017. Species distribution, antibiotic resistance and virulence traits in canine and feline enterococci in Tunisia. Acta Vet Hung 65:173–184 10.1556/004.2017.018. [PubMed] [DOI] [PubMed] [Google Scholar]
- 60.Naser SM, Vancanneyt M, De Graef E, Devriese LA, Snauwaert C, Lefebvre K, Hoste B, Svec P, Decostere A, Haesebrouck F, Swings J. 2005. Enterococcus canintestini sp. nov., from faecal samples of healthy dogs. Int J Syst Evol Microbiol 55:2177–2182 10.1099/ijs.0.63752-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 61.Baele M, Devriese LA, Butaye P, Haesebrouck F. 2002. Composition of enterococcal and streptococcal flora from pigeon intestines. J Appl Microbiol 92:348–351 10.1046/j.1365-2672.2002.01537.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 62.Splichalova P, Svec P, Ghosh A, Zurek L, Oravcova V, Radimersky T, Bohus M, Literak I. 2015. Prevalence, diversity and characterization of enterococci from three coraciiform birds. Antonie van Leeuwenhoek 107:1281–1289 10.1007/s10482-015-0422-6. [PubMed] [DOI] [PubMed] [Google Scholar]
- 63.Radhouani H, Poeta P, Gonçalves A, Pacheco R, Sargo R, Igrejas G. 2012. Wild birds as biological indicators of environmental pollution: antimicrobial resistance patterns of Escherichia coli and enterococci isolated from common buzzards (Buteo buteo). J Med Microbiol 61:837–843 10.1099/jmm.0.038364-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 64.Silva N, Igrejas G, Rodrigues P, Rodrigues T, Gonçalves A, Felgar AC, Pacheco R, Gonçalves D, Cunha R, Poeta P. 2011. Molecular characterization of vancomycin-resistant enterococci and extended-spectrum β-lactamase-containing Escherichia coli isolates in wild birds from the Azores Archipelago. Avian Pathol 40:473–479 10.1080/03079457.2011.599061. [PubMed] [DOI] [PubMed] [Google Scholar]
- 65.Marinho C, Silva N, Pombo S, Santos T, Monteiro R, Gonçalves A, Micael J, Rodrigues P, Costa AC, Igrejas G, Poeta P. 2013. Echinoderms from Azores islands: an unexpected source of antibiotic resistant Enterococcus spp. and Escherichia coli isolates. Mar Pollut Bull 69:122–127 10.1016/j.marpolbul.2013.01.017. [PubMed] [DOI] [PubMed] [Google Scholar]
- 66.Medeiros AW, Blaese Amorim D, Tavares M, de Moura TM, Franco AC, d’Azevedo PA, Frazzon J, Frazzon AP. 2017. Enterococcus species diversity in fecal samples of wild marine species as determined by real-time PCR. Can J Microbiol 63:129–136 10.1139/cjm-2016-0427. [PubMed] [DOI] [PubMed] [Google Scholar]
- 67.Farnleitner AH, Ryzinska-Paier G, Reischer GH, Burtscher MM, Knetsch S, Kirschner AK, Dirnböck T, Kuschnig G, Mach RL, Sommer R. 2010. Escherichia coli and enterococci are sensitive and reliable indicators for human, livestock and wildlife faecal pollution in alpine mountainous water resources. J Appl Microbiol 109:1599–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Radhouani H, Igrejas G, Carvalho C, Pinto L, Gonçalves A, Lopez M, Sargo R, Cardoso L, Martinho A, Rego V, Rodrigues R, Torres C, Poeta P. 2011. Clonal lineages, antibiotic resistance and virulence factors in vancomycin-resistant enterococci isolated from fecal samples of red foxes (Vulpes vulpes). J Wildl Dis 47:769–773 10.7589/0090-3558-47.3.769. [PubMed] [DOI] [PubMed] [Google Scholar]
- 69.Lozano C, González-Barrio D, García JT, Ceballos S, Olea PP, Ruiz-Fons F, Torres C. 2015. Detection of vancomycin-resistant Enterococcus faecalis ST6-vanB2 and E. faecium ST915-vanA in faecal samples of wild Rattus rattus in Spain. Vet Microbiol 177:168–174 10.1016/j.vetmic.2015.02.025. [PubMed] [DOI] [PubMed] [Google Scholar]
- 70.Fontana R, Aldegheri M, Ligozzi M, Lopez H, Sucari A, Satta G. 1994. Overproduction of a low-affinity penicillin-binding protein and high-level ampicillin resistance in Enterococcus faecium. Antimicrob Agents Chemother 38:1980–1983 10.1128/AAC.38.9.1980. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ligozzi M, Pittaluga F, Fontana R. 1996. Modification of penicillin-binding protein 5 associated with high-level ampicillin resistance in Enterococcus faecium. Antimicrob Agents Chemother 40:354–357. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Montealegre MC, Roh JH, Rae M, Davlieva MG, Singh KV, Shamoo Y, Murray BE. 2016. Differential penicillin-binding protein 5 (PBP5) levels in the Enterococcus faecium clades with different levels of ampicillin resistance. Antimicrob Agents Chemother 61:e02034-16. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jureen R, Top J, Mohn SC, Harthug S, Langeland N, Willems RJ. 2003. Molecular characterization of ampicillin-resistant Enterococcus faecium isolates from hospitalized patients in Norway. J Clin Microbiol 41:2330–2336 10.1128/JCM.41.6.2330-2336.2003. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Poeta P, Costa D, Igrejas G, Sáenz Y, Zarazaga M, Rodrigues J, Torres C. 2007. Polymorphisms of the pbp5 gene and correlation with ampicillin resistance in Enterococcus faecium isolates of animal origin. J Med Microbiol 56:236–240 10.1099/jmm.0.46778-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 75.Klibi N, Sáenz Y, Zarazaga M, Ben Slama K, Masmoudi A, Ruiz-Larrea F, Boudabous A, Torres C. 2008. Polymorphism in pbp5 gene detected in clinical Enterococcus faecium strains with different ampicillin MICs from a Tunisian hospital. J Chemother 20:436–440 10.1179/joc.2008.20.4.436. [PubMed] [DOI] [PubMed] [Google Scholar]
- 76.Kristich CJ, Rice LB, Arias CA. 2014. Enterococcal infection treatment and antibiotic resistance, In Gilmore MS, Clewell DB, Ike Y, Shankar N (ed), Enterococci: from Commensals to Leading Causes of Drug Resistant Infection. Eye and Ear Infirmary, Boston: MA. [PubMed] [Google Scholar]
- 77.Galloway-Peña JR, Rice LB, Murray BE. 2011. Analysis of PBP5 of early U.S. isolates of Enterococcus faecium: sequence variation alone does not explain increasing ampicillin resistance over time. Antimicrob Agents Chemother 55:3272–3277 10.1128/AAC.00099-11. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pietta E, Montealegre MC, Roh JH, Cocconcelli PS, Murray BE. 2014. Enterococcus faecium PBP5-S/R, the missing link between PBP5-S and PBP5-R. Antimicrob Agents Chemother 58:6978–6981 10.1128/AAC.03648-14. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lebreton F, van Schaik W, McGuire AM, Godfrey P, Griggs A, Mazumdar V, Corander J, Cheng L, Saif S, Young S, Zeng Q, Wortman J, Birren B, Willems RJ, Earl AM, Gilmore MS. 2013. Emergence of epidemic multidrug-resistant Enterococcus faecium from animal and commensal strains. MBio 4:e00534-13 10.1128/mBio.00534-13. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ono S, Muratani T, Matsumoto T. 2005. Mechanisms of resistance to imipenem and ampicillin in Enterococcus faecalis. Antimicrob Agents Chemother 49:2954–2958 10.1128/AAC.49.7.2954-2958.2005. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murray BE, Lopardo HA, Rubeglio EA, Frosolono M, Singh KV. 1992. Intrahospital spread of a single gentamicin-resistant, beta-lactamase-producing strain of Enterococcus faecalis in Argentina. Antimicrob Agents Chemother 36:230–232 10.1128/AAC.36.1.230. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Coudron PE, Markowitz SM, Wong ES. 1992. Isolation of a beta-lactamase-producing, aminoglycoside-resistant strain of Enterococcus faecium. Antimicrob Agents Chemother 36:1125–1126 10.1128/AAC.36.5.1125. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sarti M, Campanile F, Sabia C, Santagati M, Gargiulo R, Stefani S. 2012. Polyclonal diffusion of beta-lactamase-producing Enterococcus faecium. J Clin Microbiol 50:169–172 10.1128/JCM.05640-11. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rice LB, Carias LL, Rudin S, Lakticová V, Wood A, Hutton-Thomas R. 2005. Enterococcus faecium low-affinity pbp5 is a transferable determinant. Antimicrob Agents Chemother 49:5007–5012 10.1128/AAC.49.12.5007-5012.2005. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Novais C, Freitas AR, Silveira E, Antunes P, Silva R, Coque TM, Peixe L. 2013. Spread of multidrug-resistant Enterococcus to animals and humans: an underestimated role for the pig farm environment. J Antimicrob Chemother 68:2746–2754 10.1093/jac/dkt289. [PubMed] [DOI] [PubMed] [Google Scholar]
- 86.Novais C, Tedim AP, Lanza VF, Freitas AR, Silveira E, Escada R, Roberts AP, Al-Haroni M, Baquero F, Peixe L, Coque TM. 2016. Co-diversification of Enterococcus faecium core genomes and PBP5: evidences of pbp5 horizontal transfer. Front Microbiol 7:1581 10.3389/fmicb.2016.01581. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barlow RS, McMillan KE, Duffy LL, Fegan N, Jordan D, Mellor GE. 2017. Antimicrobial resistance status of Enterococcus from Australian cattle populations at slaughter. PLoS One 12:e0177728 10.1371/journal.pone.0177728. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Poeta P, Costa D, Rodrigues J, Torres C. 2006. Antimicrobial resistance and the mechanisms implicated in faecal enterococci from healthy humans, poultry and pets in Portugal. Int J Antimicrob Agents 27:131–137 10.1016/j.ijantimicag.2005.09.018. [PubMed] [DOI] [PubMed] [Google Scholar]
- 89.Damborg P, Top J, Hendrickx AP, Dawson S, Willems RJ, Guardabassi L. 2009. Dogs are a reservoir of ampicillin-resistant Enterococcus faecium lineages associated with human infections. Appl Environ Microbiol 75:2360–2365 10.1128/AEM.02035-08. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gonçalves A, Igrejas G, Radhouani H, Correia S, Pacheco R, Santos T, Monteiro R, Guerra A, Petrucci-Fonseca F, Brito F, Torres C, Poeta P. 2013. Antimicrobial resistance in faecal enterococci and Escherichia coli isolates recovered from Iberian wolf. Lett Appl Microbiol 56:268–274 10.1111/lam.12044. [PubMed] [DOI] [PubMed] [Google Scholar]
- 91.Barros J, Igrejas G, Andrade M, Radhouani H, López M, Torres C, Poeta P. 2011. Gilthead seabream (Sparus aurata) carrying antibiotic resistant enterococci. A potential bioindicator of marine contamination? Mar Pollut Bull 62:1245–1248 10.1016/j.marpolbul.2011.03.021. [PubMed] [DOI] [PubMed] [Google Scholar]
- 92.Gonçalves A, Igrejas G, Radhouani H, Santos T, Monteiro R, Pacheco R, Alcaide E, Zorrilla I, Serra R, Torres C, Poeta P. 2013. Detection of antibiotic resistant enterococci and Escherichia coli in free range Iberian Lynx (Lynx pardinus). Sci Total Environ 456-457:115–119 10.1016/j.scitotenv.2013.03.073. [PubMed] [DOI] [PubMed] [Google Scholar]
- 93.DANMAP. 2014. Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. www.danmap.org.
- 94.Swedres-Svarm. 2015. Consumption of antibiotics and occurrence of antibiotic resistance in Sweden. Solna/Uppsala, Sweden. [Google Scholar]
- 95.NethMap-MARAN. 2015. Monitoring of antimicrobial resistance and antibiotic usage in animals in the Netherlands in 2014. Nigmegen. SWAB. https://www.swab.nl/nethmap [Google Scholar]
- 96.Uttley AH, George RC, Naidoo J, Woodford N, Johnson AP, Collins CH, Morrison D, Gilfillan AJ, Fitch LE, Heptonstall J. 1989. High-level vancomycin-resistant enterococci causing hospital infections. Epidemiol Infect 103:173–181 10.1017/S0950268800030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leclercq R, Derlot E, Duval J, Courvalin P. 1988. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med 319:157–161 10.1056/NEJM198807213190307. [PubMed] [DOI] [PubMed] [Google Scholar]
- 98.European Centre for Disease Prevention and Control. 2017. Antimicrobial resistance surveillance in Europe 2016. Annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). ECDC, Stockholm, Sweden. [Google Scholar]
- 99.Lebreton F, Depardieu F, Bourdon N, Fines-Guyon M, Berger P, Camiade S, Leclercq R, Courvalin P, Cattoir V. 2011. d-Ala-d-Ser VanN-type transferable vancomycin resistance in Enterococcus faecium. Antimicrob Agents Chemother 55:4606–4612 10.1128/AAC.00714-11. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boyd DA, Du T, Hizon R, Kaplen B, Murphy T, Tyler S, Brown S, Jamieson F, Weiss K, Mulvey MR. 2006. VanG-type vancomycin-resistant Enterococcus faecalis strains isolated in Canada. Antimicrob Agents Chemother 50:2217–2221 10.1128/AAC.01541-05. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boyd DA, Willey BM, Fawcett D, Gillani N, Mulvey MR. 2008. Molecular characterization of Enterococcus faecalis N06-0364 with low-level vancomycin resistance harboring a novel d-Ala-d-Ser gene cluster, vanL. Antimicrob Agents Chemother 52:2667–2672 10.1128/AAC.01516-07. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu X, Lin D, Yan G, Ye X, Wu S, Guo Y, Zhu D, Hu F, Zhang Y, Wang F, Jacoby GA, Wang M. 2010. vanM, a new glycopeptide resistance gene cluster found in Enterococcus faecium. Antimicrob Agents Chemother 54:4643–4647 10.1128/AAC.01710-09. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Depardieu F, Foucault ML, Bell J, Dubouix A, Guibert M, Lavigne JP, Levast M, Courvalin P. 2009. New combinations of mutations in VanD-type vancomycin-resistant Enterococcus faecium, Enterococcus faecalis, and Enterococcus avium strains. Antimicrob Agents Chemother 53:1952–1963 10.1128/AAC.01348-08. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.López M, Rezusta A, Seral C, Aspiroz C, Marne C, Aldea MJ, Ferrer I, Revillo MJ, Castillo FJ, Torres C. 2012. Detection and characterization of a ST6 clone of vanB2-Enterococcus faecalis from three different hospitals in Spain. Eur J Clin Microbiol Infect Dis 31:257–260 10.1007/s10096-011-1303-1. [PubMed] [DOI] [PubMed] [Google Scholar]
- 105.Patel R, Piper K, Cockerill FR III, Steckelberg JM, Yousten AA. 2000. The biopesticide Paenibacillus popilliae has a vancomycin resistance gene cluster homologous to the enterococcal VanA vancomycin resistance gene cluster. Antimicrob Agents Chemother 44:705–709 10.1128/AAC.44.3.705-709.2000. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hegstad K, Mikalsen T, Coque TM, Werner G, Sundsfjord A. 2010. Mobile genetic elements and their contribution to the emergence of antimicrobial resistant Enterococcus faecalis and Enterococcus faecium. Clin Microbiol Infect 16:541–554 10.1111/j.1469-0691.2010.03226.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 107.Arthur M, Quintiliani R Jr. 2001. Regulation of VanA- and VanB-type glycopeptide resistance in enterococci. Antimicrob Agents Chemother 45:375–381 10.1128/AAC.45.2.375-381.2001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Arthur M, Courvalin P. 1993. Genetics and mechanisms of glycopeptide resistance in enterococci. Antimicrob Agents Chemother 37:1563–1571 10.1128/AAC.37.8.1563. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.López M, Sáenz Y, Alvarez-Martínez MJ, Marco F, Robredo B, Rojo-Bezares B, Ruiz-Larrea F, Zarazaga M, Torres C. 2010. Tn1546 structures and multilocus sequence typing of vanA-containing enterococci of animal, human and food origin. J Antimicrob Chemother 65:1570–1575 10.1093/jac/dkq192. [PubMed] [DOI] [PubMed] [Google Scholar]
- 110.Werner G, Strommenger B, Witte W. 2008. Acquired vancomycin resistance in clinically relevant pathogens. Future Microbiol 3:547–562 10.2217/17460913.3.5.547. [PubMed] [DOI] [PubMed] [Google Scholar]
- 111.Patel R. 2003. Clinical impact of vancomycin-resistant enterococci. J Antimicrob Chemother 51(Suppl 3):iii13–iii21 10.1093/jac/dkg272. [PubMed] [DOI] [PubMed] [Google Scholar]
- 112.Bager F, Madsen M, Christensen J, Aarestrup FM. 1997. Avoparcin used as a growth promoter is associated with the occurrence of vancomycin-resistant Enterococcus faecium on Danish poultry and pig farms. Prev Vet Med 31:95–112 10.1016/S0167-5877(96)01119-1. [DOI] [PubMed] [Google Scholar]
- 113.Aarestrup FM, Kruse H, Tast E, Hammerum AM, Jensen LB. 2000. Associations between the use of antimicrobial agents for growth promotion and the occurrence of resistance among Enterococcus faecium from broilers and pigs in Denmark, Finland, and Norway. Microb Drug Resist 6:63–70 10.1089/mdr.2000.6.63. [PubMed] [DOI] [PubMed] [Google Scholar]
- 114.Robredo B, Singh KV, Baquero F, Murray BE, Torres C. 1999. From vanA Enterococcus hirae to vanA Enterococcus faecium: a study of feed supplementation with avoparcin and tylosin in young chickens. Antimicrob Agents Chemother 43:1137–1143. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Klare I, Badstübner D, Konstabel C, Böhme G, Claus H, Witte W. 1999. Decreased incidence of VanA-type vancomycin-resistant enterococci isolated from poultry meat and from fecal samples of humans in the community after discontinuation of avoparcin usage in animal husbandry. Microb Drug Resist 5:45–52 10.1089/mdr.1999.5.45. [PubMed] [DOI] [PubMed] [Google Scholar]
- 116.Borgen K, Sørum M, Wasteson Y, Kruse H. 2001. VanA-type vancomycin-resistant enterococci (VRE) remain prevalent in poultry carcasses 3 years after avoparcin was banned. Int J Food Microbiol 64:89–94 10.1016/S0168-1605(00)00435-9. [DOI] [PubMed] [Google Scholar]
- 117.Bortolaia V, Mander M, Jensen LB, Olsen JE, Guardabassi L. 2015. Persistence of vancomycin resistance in multiple clones of Enterococcus faecium isolated from Danish broilers 15 years after the ban of avoparcin. Antimicrob Agents Chemother 59:2926–2929 10.1128/AAC.05072-14. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lauderdale TL, Shiau YR, Wang HY, Lai JF, Huang IW, Chen PC, Chen HY, Lai SS, Liu YF, Ho M. 2007. Effect of banning vancomycin analogue avoparcin on vancomycin-resistant enterococci in chicken farms in Taiwan. Environ Microbiol 9:819–823 10.1111/j.1462-2920.2006.01189.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 119.van Belkun A, van den Braak N, Thomassen R, Verbrugh H, Endtz H. 1996. Vancomycin-resistant enterococci in cats and dogs. Lancet 348:1038–1039 10.1016/S0140-6736(05)64973-2. [DOI] [PubMed] [Google Scholar]
- 120.López M, Tenorio C, Torres C. 2013. Study of vancomycin resistance in faecal enterococci from healthy humans and dogs in Spain a decade after the avoparcin ban in Europe. Zoonoses Public Health 60:160–167 10.1111/j.1863-2378.2012.01502.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 121.Donabedian SM, Perri MB, Abdujamilova N, Gordoncillo MJ, Naqvi A, Reyes KC, Zervos MJ, Bartlett P. 2010. Characterization of vancomycin-resistant Enterococcus faecium isolated from swine in three Michigan counties. J Clin Microbiol 48:4156–4160 10.1128/JCM.02346-09. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gordoncillo MJ, Donabedian S, Bartlett PC, Perri M, Zervos M, Kirkwood R, Febvay C. 2013. Isolation and molecular characterization of vancomycin-resistant Enterococcus faecium from swine in Michigan, USA. Zoonoses Public Health 60:319–326 10.1111/zph.12008. [PubMed] [DOI] [PubMed] [Google Scholar]
- 123.Centers for Disease Control and Prevention (CDC). 1993. Nosocomial enterococci resistant to vancomycin: United States, 1989–1993. MMWR Morb Mortal Wkly Rep 42:597–599. [PubMed] [PubMed] [Google Scholar]
- 124.Goossens H. 1998. Spread of vancomycin-resistant enterococci: differences between the United States and Europe. Infect Control Hosp Epidemiol 19:546–551 10.2307/30141778. [PubMed] [DOI] [PubMed] [Google Scholar]
- 125.Johnsen PJ, Østerhus JI, Sletvold H, Sørum M, Kruse H, Nielsen K, Simonsen GS, Sundsfjord A. 2005. Persistence of animal and human glycopeptide-resistant enterococci on two Norwegian poultry farms formerly exposed to avoparcin is associated with a widespread plasmid-mediated vanA element within a polyclonal enterococcus faecium population. Appl Environ Microbiol 71:159–168 10.1128/AEM.71.1.159-168.2005. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.van den Bogaard AE, Willems R, London N, Top J, Stobberingh EE. 2002. Antibiotic resistance of faecal enterococci in poultry, poultry farmers and poultry slaughterers. J Antimicrob Chemother 49:497–505 10.1093/jac/49.3.497. [PubMed] [DOI] [PubMed] [Google Scholar]
- 127.Sørum M, Johnsen PJ, Aasnes B, Rosvoll T, Kruse H, Sundsfjord A, Simonsen GS. 2006. Prevalence, persistence, and molecular characterization of glycopeptide-resistant enterococci in Norwegian poultry and poultry farmers 3 to 8 years after the ban on avoparcin. Appl Environ Microbiol 72:516–521 10.1128/AEM.72.1.516-521.2006. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Robredo B, Singh KV, Baquero F, Murray BE, Torres C. 2000. Vancomycin-resistant enterococci isolated from animals and food. Int J Food Microbiol 54:197–204 10.1016/S0168-1605(99)00195-6. [DOI] [PubMed] [Google Scholar]
- 129.Nilsson O, Greko C, Top J, Franklin A, Bengtsson B. 2009. Spread without known selective pressure of a vancomycin-resistant clone of Enterococcus faecium among broilers. J Antimicrob Chemother 63:868–872 10.1093/jac/dkp045. [PubMed] [DOI] [PubMed] [Google Scholar]
- 130.Ghidán A, Kaszanyitzky EJ, Dobay O, Nagy K, Amyes SG, Rozgonyi F. 2008a. Distribution and genetic relatedness of vancomycin-resistant enterococci (VRE) isolated from healthy slaughtered chickens in Hungary from 2001 to 2004. Acta Vet Hung 56:13–25 10.1556/AVet.56.2008.1.3. [PubMed] [DOI] [PubMed] [Google Scholar]
- 131.Manson JM, Smith JM, Cook GM. 2004. Persistence of vancomycin-resistant enterococci in New Zealand broilers after discontinuation of avoparcin use. Appl Environ Microbiol 70:5764–5768 10.1128/AEM.70.10.5764-5768.2004. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kolar M, Pantucek R, Bardon J, Cekanova L, Kesselova M, Sauer P, Vagnerova I, Koukalová D. 2005. Occurrence of vancomycin-resistant enterococci in humans and animals in the Czech Republic between 2002 and 2004. J Med Microbiol 54:965–967 10.1099/jmm.0.45824-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 133.Jung WK, Lim JY, Kwon NH, Kim JM, Hong SK, Koo HC, Kim SH, Park YH. 2007. Vancomycin-resistant enterococci from animal sources in Korea. Int J Food Microbiol 113:102–107 10.1016/j.ijfoodmicro.2006.07.023. [PubMed] [DOI] [PubMed] [Google Scholar]
- 134.Tremblay CL, Letellier A, Quessy S, Boulianne M, Daignault D, Archambault M. 2011. Multiple-antibiotic resistance of Enterococcus faecalis and Enterococcus faecium from cecal contents in broiler chicken and turkey flocks slaughtered in Canada and plasmid colocalization of tetO and ermB genes. J Food Prot 74:1639–1648 10.4315/0362-028X.JFP-10-451. [PubMed] [DOI] [PubMed] [Google Scholar]
- 135.Boulianne M, Arsenault J, Daignault D, Archambault M, Letellier A, Dutil L. 2016. Drug use and antimicrobial resistance among Escherichia coli and Enterococcus spp. isolates from chicken and turkey flocks slaughtered in Quebec, Canada. Can J Vet Res 80:49–59. [PubMed] [PMC free article] [PubMed] [Google Scholar]
- 136.Poeta P, Costa D, Rodrigues J, Torres C. 2005. Study of faecal colonization by vanA-containing Enterococcus strains in healthy humans, pets, poultry and wild animals in Portugal. J Antimicrob Chemother 55:278–280 10.1093/jac/dkh549. [PubMed] [DOI] [PubMed] [Google Scholar]
- 137.Ghidán A, Dobay O, Kaszanyitzky EJ, Samu P, Amyes SG, Nagy K, Rozgonyi F. 2008. Vancomycin resistant enterococci (VRE) still persist in slaughtered poultry in hungary 8 years after the ban on avoparcin. Acta Microbiol Immunol Hung 55:409–417 10.1556/AMicr.55.2008.4.5. [PubMed] [DOI] [PubMed] [Google Scholar]
- 138.Tzavaras I, Siarkou VI, Zdragas A, Kotzamanidis C, Vafeas G, Bourtzi-Hatzopoulou E, Pournaras S, Sofianou D. 2012. Diversity of vanA-type vancomycin-resistant Enterococcus faecium isolated from broilers, poultry slaughterers and hospitalized humans in Greece. J Antimicrob Chemother 67:1811–1818 10.1093/jac/dks166. [PubMed] [DOI] [PubMed] [Google Scholar]
- 139.Sting R, Richter A, Popp C, Hafez HM. 2013. Occurrence of vancomycin-resistant enterococci in turkey flocks. Poult Sci 92:346–351 10.3382/ps.2012-02652. [PubMed] [DOI] [PubMed] [Google Scholar]
- 140.Obeng AS, Rickard H, Ndi O, Sexton M, Barton M. 2013. Comparison of antimicrobial resistance patterns in enterococci from intensive and free range chickens in Australia. Avian Pathol 42:45–54 10.1080/03079457.2012.757576. [PubMed] [DOI] [PubMed] [Google Scholar]
- 141.Maasjost J, Mühldorfer K, Hafez HM, Hafez HM, Cortez de Jäckel S. 2015. Antimicrobial susceptibility patterns of Enterococcus faecalis an Enterococcus faecium isolated from poultry flocks in Germany. Avian Dis 59:143–148 10.1637/10928-090314-RegR. [PubMed] [DOI] [PubMed] [Google Scholar]
- 142.Stępień-Pyśniak D, Marek A, Banach T, Adaszek Ł, Pyzik E, Wilczyński J, Winiarczyk S. 2016. Prevalence and antibiotic resistance of Enterococcus strains isolated from poultry. Acta Vet Hung 64:148–163 10.1556/004.2016.016. [PubMed] [DOI] [PubMed] [Google Scholar]
- 143.Bertelloni F, Salvadori C, Moni A, Cerri D, Mani P, Ebani VV. 2015. Antimicrobial resistance in Enterococcus spp. isolated from laying hens of backyard poultry floks. Ann Agric Environ Med 22:665–669 10.5604/12321966.1185771. [PubMed] [DOI] [PubMed] [Google Scholar]
- 144.Nilsson O, Greko C, Bengtsson B. 2009. Environmental contamination by vancomycin resistant enterococci (VRE) in Swedish broiler production. Acta Vet Scand 51:49 10.1186/1751-0147-51-49. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Torres C, Tenorio C, Portillo A, García M, Martínez C, Del Campo R, Ruiz-Larrea F, Zarazaga M. 2003. Intestinal colonization by vanA- or vanB2-containing enterococcal isolates of healthy animals in Spain. Microb Drug Resist 9(Suppl 1):S47–S52 10.1089/107662903322541892. [PubMed] [DOI] [PubMed] [Google Scholar]
- 146.Herrero IA, Teshager T, Garde J, Moreno MA, Domínguez L. 2000. Prevalence of vancomycin-resistant Enterococcus faecium (VREF) in pig faeces from slaughterhouses in Spain. Prev Vet Med 47:255–262 10.1016/S0167-5877(00)00171-9. [DOI] [PubMed] [Google Scholar]
- 147.Iweriebor BC, Obi LC, Okoh AI. 2015. Virulence and antimicrobial resistance factors of Enterococcus spp. isolated from fecal samples from piggery farms in Eastern Cape, South Africa. BMC Microbiol 15:136 10.1186/s12866-015-0468-7. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fard RM, Heuzenroeder MW, Barton MD. 2011. Antimicrobial and heavy metal resistance in commensal enterococci isolated from pigs. Vet Microbiol 148:276–282 10.1016/j.vetmic.2010.09.002. [PubMed] [DOI] [PubMed] [Google Scholar]
- 149.Bustamante W, Alpízar A, Hernández S, Pacheco A, Vargas N, Herrera ML, Vargas A, Caballero M, García F. 2003. Predominance of vanA genotype among vancomycin-resistant Enterococcus isolates from poultry and swine in Costa Rica. Appl Environ Microbiol 69:7414–7419 10.1128/AEM.69.12.7414-7419.2003. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kühn I, Iversen A, Finn M, Greko C, Burman LG, Blanch AR, Vilanova X, Manero A, Taylor H, Caplin J, Domínguez L, Herrero IA, Moreno MA, Möllby R. 2005. Occurrence and relatedness of vancomycin-resistant enterococci in animals, humans, and the environment in different European regions. Appl Environ Microbiol 71:5383–5390 10.1128/AEM.71.9.5383-5390.2005. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.García-Migura L, Pleydell E, Barnes S, Davies RH, Liebana E. 2005. Characterization of vancomycin-resistant Enterococcusfaecium isolates from broiler poultry and pig farms in England and Wales. J Clin Microbiol 43:3283–3289 10.1128/JCM.43.7.3283-3289.2005. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kempf I, Hellard G, Perrin-Guyomard A, Gicquel-Bruneau M, Sanders P, Leclercq R. 2008. Prevalence of high-level vancomycin-resistant enterococci in French broilers and pigs. Int J Antimicrob Agents 32:463–464 10.1016/j.ijantimicag.2008.04.029. [PubMed] [DOI] [PubMed] [Google Scholar]
- 153.Liu Y, Liu K, Lai J, Wu C, Shen J, Wang Y. 2013. Prevalence and antimicrobial resistance of Enterococcus species of food animal origin from Beijing and Shandong Province, China. J Appl Microbiol 114:555–563 10.1111/jam.12054. [PubMed] [DOI] [PubMed] [Google Scholar]
- 154.Ngbede EO, Raji MA, Kwanashie CN, Kwaga JKP. 2017. Antimicrobial resistance and virulence profile of enterococci isolated from poultry and cattle sources in Nigeria. Trop Anim Health Prod 49:451–458 10.1007/s11250-016-1212-5. [PubMed] [DOI] [PubMed] [Google Scholar]
- 155.Bekele B, Ashenafi M. 2010. Distribution of drug resistance among enterococci and Salmonella from poultry and cattle in Ethiopia. Trop Anim Health Prod 42:857–864 10.1007/s11250-009-9499-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 156.Ho PL, Lai E, Chan PY, Lo WU, Chow KH. 2013. Rare occurrence of vancomycin-resistant Enterococcus faecium among livestock animals in China. J Antimicrob Chemother 68:2948–2949 10.1093/jac/dkt293. [PubMed] [DOI] [PubMed] [Google Scholar]
- 157.Eisner A, Feierl G, Gorkiewicz G, Dieber F, Kessler HH, Marth E, Köfer J. 2005. High prevalence of VanA-type vancomycin-resistant enterococci in Austrian poultry. Appl Environ Microbiol 71:6407–6409 10.1128/AEM.71.10.6407-6409.2005. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Haenni M, Saras E, Châtre P, Meunier D, Martin S, Lepage G, Ménard MF, Lebreton P, Rambaud T, Madec JY. 2009. vanA in Enterococcus faecium, Enterococcus faecalis, and Enterococcus casseliflavus detected in French cattle. Foodborne Pathog Dis 6:1107–1111 10.1089/fpd.2009.0303. [PubMed] [DOI] [PubMed] [Google Scholar]
- 159.de Niederhäusern S, Sabia C, Messi P, Guerrieri E, Manicardi G, Bondi M. 2007. VanA-type vancomycin-resistant enterococci in equine and swine rectal swabs and in human clinical samples. Curr Microbiol 55:240–246 10.1007/s00284-007-0115-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 160.Ramos S, Igrejas G, Rodrigues J, Capelo-Martínez JL, Poeta P. 2012. Genetic characterisation of antibiotic resistance and virulence factors in vanA-containing enterococci from cattle, sheep and pigs subsequent to the discontinuation of the use of avoparcin. Vet J 193:301–303 10.1016/j.tvjl.2011.12.007. [PubMed] [DOI] [PubMed] [Google Scholar]
- 161.Hershberger E, Oprea SF, Donabedian SM, Perri M, Bozigar P, Bartlett P, Zervos MJ. 2005. Epidemiology of antimicrobial resistance in enterococci of animal origin. J Antimicrob Chemother 55:127–130 10.1093/jac/dkh508. [PubMed] [DOI] [PubMed] [Google Scholar]
- 162.Song JY, Hwang IS, Eom JS, Cheong HJ, Bae WK, Park YH, Kim WJ. 2005. Prevalence and molecular epidemiology of vancomycin-resistant enterococci (VRE) strains isolated from animals and humans in Korea. Korean J Intern Med (Korean Assoc Intern Med) 20:55–62 10.3904/kjim.2005.20.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lim SK, Kim TS, Lee HS, Nam HM, Joo YS, Koh HB. 2006. Persistence of vanA-type Enterococcus faecium in Korean livestock after ban on avoparcin. Microb Drug Resist 12:136–139 10.1089/mdr.2006.12.136. [PubMed] [DOI] [PubMed] [Google Scholar]
- 164.Gonçalves A, Poeta P, Silva N, Araújo C, López M, Ruiz E, Uliyakina I, Direitinho J, Igrejas G, Torres C. 2010. Characterization of vancomycin-resistant enterococci isolated from fecal samples of ostriches by molecular methods. Foodborne Pathog Dis 7:1133–1136 10.1089/fpd.2010.0548. [PubMed] [DOI] [PubMed] [Google Scholar]
- 165.Araújo C, Torres C, Gonçalves A, Carneiro C, López M, Radhouani H, Pardal M, Igrejas G, Poeta P. 2011. Genetic detection and multilocus sequence typing of vanA-containing Enterococcus strains from mullets fish (Liza ramada). Microb Drug Resist 17:357–361 10.1089/mdr.2010.0171. [PubMed] [DOI] [PubMed] [Google Scholar]
- 166.Yoshimura H, Ishimaru M, Endoh YS, Suginaka M, Yamatani S. 1998. Isolation of glycopeptide-resistant enterococci from chicken in Japan. Antimicrob Agents Chemother 42:3333. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Butaye P, Van Damme K, Devriese LA, Van Damme L, Bael M, Lauwers S, Haesebrouck F. 2000. In vitro susceptibility of Enterococcus faecium isolated from food to growth-promoting and therapeutic antibiotics. Int J Food Microbiol 54:181–187 10.1016/S0168-1605(99)00198-1. [DOI] [PubMed] [Google Scholar]
- 168.Harada T, Kawahara R, Kanki M, Taguchi M, Kumeda Y. 2012. Isolation and characterization of vanA genotype vancomycin-resistant Enterococcus cecorum from retail poultry in Japan. Int J Food Microbiol 153:372–377 10.1016/j.ijfoodmicro.2011.11.029. [PubMed] [DOI] [PubMed] [Google Scholar]
- 169.Donado-Godoy P, Byrne BA, León M, Castellanos R, Vanegas C, Coral A, Arevalo A, Clavijo V, Vargas M, Romero Zuñiga JJ, Tafur M, Pérez-Gutierrez E, Smith WA. 2015. Prevalence, resistance patterns, and risk factors for antimicrobial resistance in bacteria from retail chicken meat in Colombia. J Food Prot 78:751–759 10.4315/0362-028X.JFP-14-349. [PubMed] [DOI] [PubMed] [Google Scholar]
- 170.Kasimoglu-Dogru A, Gencay YE, Ayaz ND. 2010. Prevalence and antibiotic resistance profiles of Enterococcus species in chicken at slaughter level; absence of vanA and vanB genes in E. faecalis and E. faecium. Res Vet Sci 89:153–158 10.1016/j.rvsc.2010.02.005. [PubMed] [DOI] [PubMed] [Google Scholar]
- 171.Harwood VJ, Brownell M, Perusek W, Whitlock JE. 2001. Vancomycin-resistant Enterococcus spp. isolated from wastewater and chicken feces in the United States. Appl Environ Microbiol 67:4930–4933 10.1128/AEM.67.10.4930-4933.2001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wilson IG, McAfee GG. 2002. Vancomycin-resistant enterococci in shellfish, unchlorinated waters, and chicken. Int J Food Microbiol 79:143–151 10.1016/S0168-1605(02)00063-6. [DOI] [PubMed] [Google Scholar]
- 173.Novais C, Coque TM, Costa MJ, Sousa JC, Baquero F, Peixe LV. 2005. High occurrence and persistence of antibiotic-resistant enterococci in poultry food samples in Portugal. J Antimicrob Chemother 56:1139–1143 10.1093/jac/dki360. [PubMed] [DOI] [PubMed] [Google Scholar]
- 174.Lemcke R, Bülte M. 2000. Occurrence of the vancomycin-resistant genes vanA, vanB, vanCl, vanC2 and vanC3 in Enterococcus strains isolated from poultry and pork. Int J Food Microbiol 60:185–194 10.1016/S0168-1605(00)00310-X. [DOI] [PubMed] [Google Scholar]
- 175.Del Grosso M, Caprioli A, Chinzari P, Fontana MC, Pezzotti G, Manfrin A, Giannatale ED, Goffredo E, Pantosti A. 2000. Detection and characterization of vancomycin-resistant enterococci in farm animals and raw meat products in Italy. Microb Drug Resist 6:313–318 10.1089/mdr.2000.6.313. [PubMed] [DOI] [PubMed] [Google Scholar]
- 176.Gambarotto K, Ploy MC, Dupron F, Giangiobbe M, Denis F. 2001. Occurrence of vancomycin-resistant enterococci in pork and poultry products from a cattle-rearing area of France. J Clin Microbiol 39:2354–2355 10.1128/JCM.39.6.2354-2355.2001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nomura T, Tanimoto K, Shibayama K, Arakawa Y, Fujimoto S, Ike Y, Tomita H. 2012. Identification of VanN-type vancomycin resistance in an Enterococcus faecium isolate from chicken meat in Japan. Antimicrob Agents Chemother 56:6389–6392 10.1128/AAC.00747-12. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Peters J, Mac K, Wichmann-Schauer H, Klein G, Ellerbroek L. 2003. Species distribution and antibiotic resistance patterns of enterococci isolated from food of animal origin in Germany. Int J Food Microbiol 88:311–314 10.1016/S0168-1605(03)00193-4. [DOI] [PubMed] [Google Scholar]
- 179.Delpech G, Pourcel G, Schell C, De Luca M, Basualdo J, Bernstein J, Grenovero S, Sparo M. 2012. Antimicrobial resistance profiles of Enterococcus faecalis and Enterococcus faecium isolated from artisanal food of animal origin in Argentina. Foodborne Pathog Dis 9:939–944 10.1089/fpd.2012.1192. [PubMed] [DOI] [PubMed] [Google Scholar]
- 180.Talebi M, Sadeghi J, Rahimi F, Pourshafie MR. 2015. Isolation and biochemical fingerprinting of vancomycin-resistant Enterococcus faecium from meat, chicken and cheese. Jundishapur J Microbiol 8:e15815 10.5812/jjm.8(4)2015.15815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gousia P, Economou V, Bozidis P, Papadopoulou C. 2015. Vancomycin-resistance phenotypes, vancomycin-resistance genes, and resistance to antibiotics of enterococci isolated from food of animal origin. Foodborne Pathog Dis 12:214–220 10.1089/fpd.2014.1832. [PubMed] [DOI] [PubMed] [Google Scholar]
- 182.Pesavento G, Calonico C, Ducci B, Magnanini A, Lo Nostro A. 2014. Prevalence and antibiotic resistance of Enterococcus spp. isolated from retail cheese, ready-to-eat salads, ham, and raw meat. Food Microbiol 41:1–7 10.1016/j.fm.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 183.Sánchez Valenzuela A, Lavilla Lerma L, Benomar N, Gálvez A, Pérez Pulido R, Abriouel H. 2013. Phenotypic and molecular antibiotic resistance profile of Enterococcus faecalis and Enterococcus faecium isolated from different traditional fermented foods. Foodborne Pathog Dis 10:143–149 10.1089/fpd.2012.1279. [PubMed] [DOI] [PubMed] [Google Scholar]
- 184.Pantosti A, Del Grosso M, Tagliabue S, Macrì A, Caprioli A. 1999.Decrease of vancomycin-resistant enterococci in poultry meat after avoparcin ban. Lancet 354:741–742 10.1016/S0140-6736(99)02395-8. [DOI] [PubMed] [Google Scholar]
- 185.Hayes JR, English LL, Carter PJ, Proescholdt T, Lee KY, Wagner DD, White DG. 2003. Prevalence and antimicrobial resistance of Enterococcus species isolated from retail meats. Appl Environ Microbiol 69:7153–7160 10.1128/AEM.69.12.7153-7160.2003. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hiroi M, Kawamori F, Harada T, Sano Y, Miwa N, Sugiyama K, Hara-Kudo Y, Masuda T. 2012. Antibiotic resistance in bacterial pathogens from retail raw meats and food-producing animals in Japan. J Food Prot 75:1774–1782 10.4315/0362-028X.JFP-11-479. [PubMed] [DOI] [PubMed] [Google Scholar]
- 187.Aslam M, Diarra MS, Checkley S, Bohaychuk V, Masson L. 2012. Characterization of antimicrobial resistance and virulence genes in Enterococcus spp. isolated from retail meats in Alberta, Canada. Int J Food Microbiol 156:222–230 10.1016/j.ijfoodmicro.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 188.López M, Sáenz Y, Rojo-Bezares B, Martínez S, del Campo R, Ruiz-Larrea F, Zarazaga M, Torres C. 2009. Detection of vanA and vanB2-containing enterococci from food samples in Spain, including Enterococcus faecium strains of CC17 and the new singleton ST425. Int J Food Microbiol 133:172–178 10.1016/j.ijfoodmicro.2009.05.020. [PubMed] [DOI] [PubMed] [Google Scholar]
- 189.Jahan M, Krause DO, Holley RA. 2013. Antimicrobial resistance of Enterococcus species from meat and fermented meat products isolated by a PCR-based rapid screening method. Int J Food Microbiol 163:89–95 10.1016/j.ijfoodmicro.2013.02.017. [PubMed] [DOI] [PubMed] [Google Scholar]
- 190.Guerrero-Ramos E, Molina-González D, Blanco-Morán S, Igrejas G, Poeta P, Alonso-Calleja C, Capita R. 2016a. Prevalence, antimicrobial resistance, and genotypic characterization of vancomycin-resistant enterococci in meat preparations. J Food Prot 79:748–756 10.4315/0362-028X.JFP-15-390. [PubMed] [DOI] [PubMed] [Google Scholar]
- 191.Jamet E, Akary E, Poisson MA, Chamba JF, Bertrand X, Serror P. 2012. Prevalence and characterization of antibiotic resistant Enterococcus faecalis in French cheeses. Food Microbiol 31:191–198 10.1016/j.fm.2012.03.009. [PubMed] [DOI] [PubMed] [Google Scholar]
- 192.de Garnica ML, Valdezate S, Gonzalo C, Saez-Nieto JA. 2013. Presence of the vanC1 gene in a vancomycin-resistant Enterococcus faecalis strain isolated from ewe bulk tank milk. J Med Microbiol 62:494–495 10.1099/jmm.0.052274-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 193.Osman KM, Ali MN, Radwan I, ElHofy F, Abed AH, Orabi A, Fawzy NM. 2016. Dispersion of the vancomycin resistance genes vanA and vanC of Enterococcus isolated from Nile Tilapia on retail sale: a public health hazard. Front Microbiol 7:1354 10.3389/fmicb.2016.01354. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tansuphasiri U, Khaminthakul D, Pandii W. 2006. Antibiotic resistance of enterococci isolated from frozen foods and environmental water. Southeast Asian J Trop Med Public Health 37:162–170. [PubMed] [PubMed] [Google Scholar]
- 195.Simjee S, White DG, McDermott PF, Wagner DD, Zervos MJ, Donabedian SM, English LL, Hayes JR, Walker RD. 2002. Characterization of Tn1546 in vancomycin-resistant Enterococcus faecium isolated from canine urinary tract infections: evidence of gene exchange between human and animal enterococci. J Clin Microbiol 40:4659–4665 10.1128/JCM.40.12.4659-4665.2002. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Herrero IA, Fernández-Garayzábal JF, Moreno MA, Domínguez L. 2004. Dogs should be included in surveillance programs for vancomycin-resistant enterococci. J Clin Microbiol 42:1384–1385 10.1128/JCM.42.3.1384-1385.2004. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ghosh A, Dowd SE, Zurek L. 2011. Dogs leaving the ICU carry a very large multi-drug resistant enterococcal population with capacity for biofilm formation and horizontal gene transfer. PLoS One 6:e22451 10.1371/journal.pone.0022451. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kataoka Y, Umino Y, Ochi H, Harada K, Sawada T. 2014. Antimicrobial susceptibility of enterococcal species isolated from antibiotic-treated dogs and cats. J Vet Med Sci 76:1399–1402 10.1292/jvms.13-0576. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Abdel-Moein KA, El-Hariri MD, Wasfy MO, Samir A. 2017. Occurrence of ampicillin-resistant Enterococcus faecium carrying esp gene in pet animals: an upcoming threat for pet lovers. J Glob Antimicrob Resist 9:115–117 10.1016/j.jgar.2017.02.011. [PubMed] [DOI] [PubMed] [Google Scholar]
- 200.Oliveira M, Tavares M, Gomes D, Touret T, São Braz B, Tavares L, Semedo-Lemsaddek T. 2016. Virulence traits and antibiotic resistance among enterococci isolated from dogs with periodontal disease. Comp Immunol Microbiol Infect Dis 46:27–31 10.1016/j.cimid.2016.04.002. [PubMed] [DOI] [PubMed] [Google Scholar]
- 201.Gulhan T, Boynukara B, Ciftci A, Sogut MU, Findik A. 2015. Characterization of Enterococcus faecalis isolates originating from different sources for their virulence factors and genes, antibiotic resistance patterns, genotypes and biofilm production. Majallah-i Tahqiqat-i Dampizishki-i Iran 16:261–266. [PMC free article] [PubMed] [Google Scholar]
- 202.Moura I, Radhouani H, Torres C, Poeta P, Igrejas G. 2010. Detection and genetic characterisation of vanA-containing Enterococcus strains in healthy Lusitano horses. Equine Vet J 42:181–183 10.2746/042516409X480386. [PubMed] [DOI] [PubMed] [Google Scholar]
- 203.Mallon DJ, Corkill JE, Hazel SM, Wilson JS, French NP, Bennett M, Hart CA. 2002. Excretion of vancomycin-resistant enterococci by wild mammals. Emerg Infect Dis 8:636–638 10.3201/eid0806.010247. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lozano C, González-Barrio D, García JT, Ceballos S, Olea PP, Ruiz-Fons F, Torres C. 2015. Detection of vancomycin-resistant Enterococcus faecalis ST6-vanB2 and E. faecium ST915-vanA in faecal samples of wild Rattus rattus in Spain. Vet Microbiol 177:168–174 10.1016/j.vetmic.2015.02.025. [PubMed] [DOI] [PubMed] [Google Scholar]
- 205.Nowakiewicz A, Ziółkowska G, Zięba P, Kostruba A. 2014. Undomesticated animals as a reservoir of multidrug-resistant Enterococcus in eastern Poland. J Wildl Dis 50:645–650 10.7589/2013-09-240. [PubMed] [DOI] [PubMed] [Google Scholar]
- 206.Lozano C, González-Barrio D, Camacho MC, Lima-Barbero JF, de la Puente J, Höfle U, Torres C. 2016. Characterization of fecal vancomycin-resistant enterococci with acquired and intrinsic resistance mechanisms in wild animals, Spain. Microb Ecol 72:813–820 10.1007/s00248-015-0648-x. [DOI] [PubMed] [Google Scholar]
- 207.Silva N, Igrejas G, Rodrigues P, Rodrigues T, Gonçalves A, Felgar AC, Pacheco R, Gonçalves D, Cunha R, Poeta P. 2011. Molecular characterization of vancomycin-resistant enterococci and extended-spectrum β-lactamase-containing Escherichia coli isolates in wild birds from the Azores Archipelago. Avian Pathol 40:473–479 10.1080/03079457.2011.599061. [PubMed] [DOI] [PubMed] [Google Scholar]
- 208.Silva N, Igrejas G, Felgar A, Gonçalves A, Pacheco R, Poeta P. 2012. Molecular characterization of vanA-containing Enterococcus from migratory birds: song thrush (Turdus philomelos). Braz J Microbiol 43:1026–1029 10.1590/S1517-83822012000300026. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Santos T, Silva N, Igrejas G, Rodrigues P, Micael J, Rodrigues T, Resendes R, Gonçalves A, Marinho C, Gonçalves D, Cunha R, Poeta P. 2013. Dissemination of antibiotic resistant Enterococcus spp. and Escherichia coli from wild birds of Azores Archipelago. Anaerobe 24:25–31 10.1016/j.anaerobe.2013.09.004. [PubMed] [DOI] [PubMed] [Google Scholar]
- 210.Klibi N, Ben Amor I, Rahmouni M, Dziri R, Douja G, Ben Said L, Lozano C, Boudabous A, Ben Slama K, Mansouri R, Torres C. 2015. Diversity of species and antibiotic resistance among fecal enterococci from wild birds in Tunisia. Detection of vanA-containing Enterococcus faecium isolates. Eur J Wildl Res 61:319–323 10.1007/s10344-014-0884-2. [DOI] [Google Scholar]
- 211.Oravcova V, Zurek L, Townsend A, Clark AB, Ellis JC, Cizek A, Literak I. 2014. American crows as carriers of vancomycin-resistant enterococci with vanA gene. Environ Microbiol 16:939–949 10.1111/1462-2920.12213. [PubMed] [DOI] [PubMed] [Google Scholar]
- 212.Roberts MC, No DB, Marzluff JM, Delap JH, Turner R. 2016. Vancomycin resistant Enterococcus spp. from crows and their environment in metropolitan Washington State, USA: is there a correlation between VRE positive crows and the environment? Vet Microbiol 194:48–54 10.1016/j.vetmic.2016.01.022. [PubMed] [DOI] [PubMed] [Google Scholar]
- 213.Oravcova V, Janecko N, Ansorge A, Masarikova M, Literak I. 2014. First record of vancomycin-resistant Enterococcus faecium in Canadian wildlife. Environ Microbiol Rep 6:210–211 10.1111/1758-2229.12141. [PubMed] [DOI] [PubMed] [Google Scholar]
- 214.Oravcova V, Hadelova D, Literak I. 2016. Vancomycin-resistant Enterococcus faecium with vanA gene isolated for the first time from wildlife in Slovakia. Vet Microbiol 194:43–47 10.1016/j.vetmic.2015.11.027. [PubMed] [DOI] [PubMed] [Google Scholar]
- 215.Poeta P, Costa D, Igrejas G, Rojo-Bezares B, Sáenz Y, Zarazaga M, Ruiz-Larrea F, Rodrigues J, Torres C. 2007. Characterization of vanA-containing Enterococcus faecium isolates carrying Tn5397-like and Tn916/Tn1545-like transposons in wild boars (Sus Scrofa). Microb Drug Resist 13:151–156 10.1089/mdr.2007.759. [PubMed] [DOI] [PubMed] [Google Scholar]
- 216.Radhouani H, Poeta P, Pinto L, Miranda J, Coelho C, Carvalho C, Rodrigues J, López M, Torres C, Vitorino R, Domingues P, Igrejas G. 2010. Proteomic characterization of vanA-containing Enterococcus recovered from seagulls at the Berlengas Natural Reserve, W Portugal. Proteome Sci 8:48 10.1186/1477-5956-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Barros J, Andrade M, Radhouani H, López M, Igrejas G, Poeta P, Torres C. 2012. Detection of vanA-containing Enterococcus species in faecal microbiota of gilthead seabream (Sparus aurata). Microbes Environ 27:509–511 10.1264/jsme2.ME11346. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Figueiredo N, Radhouani H, Gonçalves A, Rodrigues J, Carvalho C, Igrejas G, Poeta P. 2009. Genetic characterization of vancomycin-resistant enterococci isolates from wild rabbits. J Basic Microbiol 49:491–494 10.1002/jobm.200800387. [PubMed] [DOI] [PubMed] [Google Scholar]
- 219.da Silva VL, Caçador NC, da Silva CS, Fontes CO, Garcia GD, Nicoli JR, Diniz CG. 2012. Occurrence of multidrug-resistant and toxic-metal tolerant enterococci in fresh feces from urban pigeons in Brazil. Microbes Environ 27:179–185 10.1264/jsme2.ME11296. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Radhouani H, Pinto L, Coelho C, Sargo R, Araújo C, López M, Torres C, Igrejas G, Poeta P. 2010. MLST and a genetic study of antibiotic resistance and virulence factors in vanA-containing Enterococcus from buzzards (Buteo buteo). Lett Appl Microbiol 50:537–541 10.1111/j.1472-765X.2010.02807.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 221.Silva V, Igrejas G, Carvalho I, Peixoto F, Cardoso L, Pereira JE, Del Campo R, Poeta P. 2018. Genetic characterization of vanA-Enterococcus faecium isolates from wild red-legged partridges in Portugal. Microb Drug Resist 24:89–94 10.1089/mdr.2017.0040. [PubMed] [DOI] [PubMed] [Google Scholar]
- 222.Radhouani H, Igrejas G, Carvalho C, Pinto L, Gonçalves A, Lopez M, Sargo R, Cardoso L, Martinho A, Rego V, Rodrigues R, Torres C, Poeta P. 2011. Clonal lineages, antibiotic resistance and virulence factors in vancomycin-resistant enterococci isolated from fecal samples of red foxes (Vulpes vulpes). J Wildl Dis 47:769–773 10.7589/0090-3558-47.3.769. [PubMed] [DOI] [PubMed] [Google Scholar]
- 223.Gonçalves A, Igrejas G, Radhouani H, López M, Guerra A, Petrucci-Fonseca F, Alcaide E, Zorrilla I, Serra R, Torres C, Poeta P. 2011. Detection of vancomycin-resistant enterococci from faecal samples of Iberian wolf and Iberian lynx, including Enterococcus faecium strains of CC17 and the new singleton ST573. Sci Total Environ 410-411:266–268 10.1016/j.scitotenv.2011.09.074. [PubMed] [DOI] [PubMed] [Google Scholar]
- 224.Katakweba AA, Møller KS, Muumba J, Muhairwa AP, Damborg P, Rosenkrantz JT, Minga UM, Mtambo MM, Olsen JE. 2015. Antimicrobial resistance in faecal samples from buffalo, wildebeest and zebra grazing together with and without cattle in Tanzania. J Appl Microbiol 118:966–975 10.1111/jam.12738. [PubMed] [DOI] [PubMed] [Google Scholar]
- 225.Tejedor Junco MT, González-Martin M, Rodríguez González NF, Gutierrez C. 2015. Identification, antimicrobial susceptibility, and virulence factors of Enterococcus spp. strains isolated from Camels in Canary Islands, Spain. Vet Ital 51:179–183. [PubMed] [DOI] [PubMed] [Google Scholar]
- 226.Guerrero-Ramos E, Cordero J, Molina-González D, Poeta P, Igrejas G, Alonso-Calleja C, Capita R. 2016. Antimicrobial resistance and virulence genes in enterococci from wild game meat in Spain. Food Microbiol 53(Pt B):156–164 10.1016/j.fm.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 227.Klare I, Fleige C, Geringer U, Thürmer A, Bender J, Mutters NT, Mischnik A, Werner G. 2015. Increased frequency of linezolid resistance among clinical Enterococcus faecium isolates from German hospital patients. J Glob Antimicrob Resist 3:128–131 10.1016/j.jgar.2015.02.007. [PubMed] [DOI] [PubMed] [Google Scholar]
- 228.Herrero IA, Issa NC, Patel R. 2002. Nosocomial spread of linezolid-resistant, vancomycin-resistant Enterococcus faecium. N Engl J Med 346:867–869 10.1056/NEJM200203143461121. [PubMed] [DOI] [PubMed] [Google Scholar]
- 229.Si H, Zhang WJ, Chu S, Wang XM, Dai L, Hua X, Dong Z, Schwarz S, Liu S. 2015. Novel plasmid-borne multidrug resistance gene cluster including lsa(E) from a linezolid-resistant Enterococcus faecium isolate of swine origin. Antimicrob Agents Chemother 59:7113–7116 10.1128/AAC.01394-15. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mendes RE, Deshpande LM, Jones RN. 2014. Linezolid update: stable in vitro activity following more than a decade of clinical use and summary of associated resistance mechanisms. Drug Resist Updat 17:1–12 10.1016/j.drup.2014.04.002. [PubMed] [DOI] [PubMed] [Google Scholar]
- 231.Shen J, Wang Y, Schwarz S. 2013. Presence and dissemination of the multiresistance gene cfr in Gram-positive and Gram-negative bacteria. J Antimicrob Chemother 68:1697–1706 10.1093/jac/dkt092. [PubMed] [DOI] [PubMed] [Google Scholar]
- 232.Liu Y, Wang Y, Wu C, Shen Z, Schwarz S, Du XD, Dai L, Zhang W, Zhang Q, Shen J. 2012. First report of the multidrug resistance gene cfr in Enterococcus faecalis of animal origin. Antimicrob Agents Chemother 56:1650–1654 10.1128/AAC.06091-11. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Liu Y, Wang Y, Schwarz S, Wang S, Chen L, Wu C, Shen J. 2014. Investigation of a multiresistance gene cfr that fails to mediate resistance to phenicols and oxazolidinones in Enterococcus faecalis. J Antimicrob Chemother 69:892–898 10.1093/jac/dkt459. [PubMed] [DOI] [PubMed] [Google Scholar]
- 234.Filsner PHLN, de Almeida LM, Moreno M, Moreno LZ, Matajira CEC, Silva KC, Pires C, Cerdeira LT, Sacramento AG, Mamizuka EM, Lincopan N, Moreno AM. 2017. Identification of the cfr methyltransferase gene in Enterococcus faecalis isolated from swine: first report in Brazil. J Glob Antimicrob Resist 8:192–193 10.1016/j.jgar.2017.02.002. [PubMed] [DOI] [PubMed] [Google Scholar]
- 235.Liu Y, Wang Y, Dai L, Wu C, Shen J. 2014. First report of multiresistance gene cfr in Enterococcus species casseliflavus and gallinarum of swine origin. Vet Microbiol 170:352–357 10.1016/j.vetmic.2014.02.037. [PubMed] [DOI] [PubMed] [Google Scholar]
- 236.Wang Y, Lv Y, Cai J, Schwarz S, Cui L, Hu Z, Zhang R, Li J, Zhao Q, He T, Wang D, Wang Z, Shen Y, Li Y, Feßler AT, Wu C, Yu H, Deng X, Xia X, Shen J. 2015. A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J Antimicrob Chemother 70:2182–2190 10.1093/jac/dkv116. [PubMed] [DOI] [PubMed] [Google Scholar]
- 237.He T, Shen Y, Schwarz S, Cai J, Lv Y, Li J, Feßler AT, Zhang R, Wu C, Shen J, Wang Y. 2016. Genetic environment of the transferable oxazolidinone/phenicol resistance gene optrA in Enterococcus faecalis isolates of human and animal origin. J Antimicrob Chemother 71:1466–1473 10.1093/jac/dkw016. [PubMed] [DOI] [PubMed] [Google Scholar]
- 238.Tamang MD, Moon DC, Kim SR, Kang HY, Lee K, Nam HM, Jang GC, Lee HS, Jung SC, Lim SK. 2017. Detection of novel oxazolidinone and phenicol resistance gene optrA in enterococcal isolates from food animals and animal carcasses. Vet Microbiol 201:252–256 10.1016/j.vetmic.2017.01.035. [PubMed] [DOI] [PubMed] [Google Scholar]
- 239.Cavaco LM, Korsgaard H, Kaas RS, Seyfarth AM, Leekitcharoenphon P, Hendriksen RS. 2017. First detection of linezolid resistance due to the optrA gene in enterococci isolated from food products in Denmark. J Glob Antimicrob Resist 9:128–129 10.1016/j.jgar.2017.04.001. [PubMed] [DOI] [PubMed] [Google Scholar]
- 240.Cavaco LM, Bernal JF, Zankari E, Léon M, Hendriksen RS, Perez-Gutierrez E, Aarestrup FM, Donado-Godoy P. 2017. Detection of linezolid resistance due to the optrA gene in Enterococcus faecalis from poultry meat from the American continent (Colombia). J Antimicrob Chemother 72:678–683. [PubMed] [DOI] [PubMed] [Google Scholar]
- 241.Freitas AR, Elghaieb H, León-Sampedro R, Abbassi MS, Novais C, Coque TM, Hassen A, Peixe L. 2017. Detection of optrA in the African continent (Tunisia) within a mosaic Enterococcus faecalis plasmid from urban wastewaters. J Antimicrob Chemother 72:3245–3251 10.1093/jac/dkx321. [PubMed] [DOI] [PubMed] [Google Scholar]
- 242.Diaz L, Kiratisin P, Mendes RE, Panesso D, Singh KV, Arias CA. 2012. Transferable plasmid-mediated resistance to linezolid due to cfr in a human clinical isolate of Enterococcus faecalis. Antimicrob Agents Chemother 56:3917–3922 10.1128/AAC.00419-12. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Deshpande LM, Ashcraft DS, Kahn HP, Pankey G, Jones RN, Farrell DJ, Mendes RE. 2015. Detection of a new cfr-like gene, cfr(B), in Enterococcus faecium recovered from human specimens in the United States: report from the SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother 59:6256–6261 10.1128/AAC.01473-15. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Bender JK, Fleige C, Klare I, Fiedler S, Mischnik A, Mutters NT, Dingle KE, Werner G. 2016. Detection of a cfr(B) variant in German Enterococcus faecium clinical isolates and the impact on linezolid resistance in Enterococcus spp. PLoS One 11:e0167042 10.1371/journal.pone.0167042. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lazaris A, Coleman DC, Kearns AM, Pichon B, Kinnevey PM, Earls MR, Boyle B, O’Connell B, Brennan GI, Shore AC. 2017. Novel multiresistance cfr plasmids in linezolid-resistant methicillin-resistant Staphylococcus epidermidis and vancomycin-resistant Enterococcus faecium (VRE) from a hospital outbreak: co-location of cfr and optrA in VRE. J Antimicrob Chemother 72:3252–3257 10.1093/jac/dkx292. [PubMed] [DOI] [PubMed] [Google Scholar]
- 246.Morroni G, Brenciani A,Simoni S, Vignaroli C, Mingoia M, Giovanetti E. 2017. Nationwide surveillance of novel oxazolidinone resistance gene optrA in Enterococcus isolates in China from 20004 to 2014. Front Microbiol 8:1631. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Cai J, Wang Y, Schwarz S, Lv H, Li Y, Liao K, Yu S, Zhao K, Gu D, Wang X, Zhang R, Shen J. 2015. Enterococcal isolates carrying the novel oxazolidinone resistance gene optrA from hospitals in Zhejiang, Guangdong, and Henan, China, 2010–2014. Clin Microbiol Infect 21:1095.e1–1095.e4 10.1016/j.cmi.2015.08.007. [PubMed] [DOI] [PubMed] [Google Scholar]
- 248.Cui L, Wang Y, Lv Y, Wang S, Song Y, Li Y, Liu J, Xue F, Yang W, Zhang J. 2016. Nationwide surveillance of novel oxazolidinone resistance gene optrA in Enterococcus isolates in China from 2004 to 2014. Antimicrob Agents Chemother 60:7490–7493. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Del Campo R, Galán JC, Tenorio C, Ruiz-Garbajosa P, Zarazaga M, Torres C, Baquero F. 2005. New aac(6′)-I genes in Enterococcus hirae and Enterococcus durans: effect on beta-lactam/aminoglycoside synergy. J Antimicrob Chemother 55:1053–1055 10.1093/jac/dki138. [PubMed] [DOI] [PubMed] [Google Scholar]
- 250.Galimand M, Schmitt E, Panvert M, Desmolaize B, Douthwaite S, Mechulam Y, Courvalin P. 2011. Intrinsic resistance to aminoglycosides in Enterococcus faecium is conferred by the 16S rRNA m5C1404-specific methyltransferase EfmM. RNA 17:251–262 10.1261/rna.2233511. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Bager F, Emborg HD (ed). 1998. DANMAP 1997. Consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. National Food Institute, Technical University of Denmark, Søborg, Denmark. [Google Scholar]
- 252.Donabedian SM, Thal LA, Hershberger E, Perri MB, Chow JW, Bartlett P, Jones R, Joyce K, Rossiter S, Gay K, Johnson J, Mackinson C, Debess E, Madden J, Angulo F, Zervos MJ. 2003. Molecular characterization of gentamicin-resistant enterococci in the United States: evidence of spread from animals to humans through food. J Clin Microbiol 41:1109–1113 10.1128/JCM.41.3.1109-1113.2003. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Werner G, Coque TM, Franz CM, Grohmann E, Hegstad K, Jensen L, van Schaik W, Weaver K. 2013. Antibiotic resistant enterococci: tales of a drug resistance gene trafficker. Int J Med Microbiol 303:360–379 10.1016/j.ijmm.2013.03.001. [PubMed] [DOI] [PubMed] [Google Scholar]
- 254.Klibi N, Ben Lagha A, Ben Slama K, Boudabous A, Torres C. 2013. Faecal enterococci from camels in Tunisia: species, antibiotic resistance and virulent genes. Vet Rec 172:213 10.1136/vr.100910. [PubMed] [DOI] [PubMed] [Google Scholar]
- 255.Muñoz-Atienza E, Gómez-Sala B, Araújo C, Campanero C, del Campo R, Hernández PE, Herranz C, Cintas LM. 2013. Antimicrobial activity, antibiotic susceptibility and virulence factors of lactic acid bacteria of aquatic origin intended for use as probiotics in aquaculture. BMC Microbiol 13:15 10.1186/1471-2180-13-15. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Araújo C, Muñoz-Atienza E, Hernández PE, Herranz C, Cintas LM, Igrejas G, Poeta P. 2015. Evaluation of Enterococcus spp. from rainbow trout (Oncorhynchus mykiss, Walbaum), feed, and rearing environment against fish pathogens. Foodborne Pathog Dis 12:311–322 10.1089/fpd.2014.1906. [PubMed] [DOI] [PubMed] [Google Scholar]
- 257.Di Cesare A, Pasquaroli S, Vignaroli C, Paroncini P, Luna GM, Manso E, Biavasco F. 2014. The marine environment as a reservoir of enterococci carrying resistance and virulence genes strongly associated with clinical strains. Environ Microbiol Rep 6:184–190 10.1111/1758-2229.12125. [PubMed] [DOI] [PubMed] [Google Scholar]
- 258.Li P, Wu D, Liu K, Suolang S, He T, Liu X, Wu C, Wang Y, Lin D. 2014. Investigation of antimicrobial resistance in Escherichia coli and enterococci isolated from Tibetan pigs. PLoS One 9:e95623 10.1371/journal.pone.0095623. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Pantozzi FL, Ibar MP, Nievas VF, Vigo GB, Moredo FA, Giacoboni GI. 2014. Wild-type minimal inhibitory concentration distributions in bacteria of animal origin in Argentina. Rev Argent Microbiol 46:34–40 10.1016/S0325-7541(14)70045-8. [DOI] [PubMed] [Google Scholar]
- 260.Usui M, Ozawa S, Onozato H, Kuge R, Obata Y, Uemae T, Ngoc PT, Heriyanto A, Chalemchaikit T, Makita K, Muramatsu Y, Tamura Y. 2014. Antimicrobial susceptibility of indicator bacteria isolated from chickens in Southeast Asian countries (Vietnam, Indonesia and Thailand). J Vet Med Sci 76:685–692 10.1292/jvms.13-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Klibi N, Aouini R, Borgo F, Ben Said L, Ferrario C, Dziri R, Boudabous A, Torres C, Ben Slama K. 2015. Antibiotic resistance and virulence of faecal enterococci isolated from food-producing animals in Tunisia. Ann Microbiol 65:695–702 10.1007/s13213-014-0908-x. [DOI] [Google Scholar]
- 262.Ayeni FA, Odumosu BT, Oluseyi AE, Ruppitsch W. 2016. Identification and prevalence of tetracycline resistance in enterococci isolated from poultry in Ilishan, Ogun State, Nigeria. J Pharm Bioallied Sci 8:69–73 10.4103/0975-7406.171729. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Boss R, Overesch G, Baumgartner A. 2016. Antimicrobial resistance of Escherichia coli, enterococci, Pseudomonas aeruginosa, and Staphylococcus aureus from raw fish and seafood imported into Switzerland. J Food Prot 79:1240–1246 10.4315/0362-028X.JFP-15-463. [PubMed] [DOI] [PubMed] [Google Scholar]
- 264.Wu X, Hou S, Zhang Q, Ma Y, Zhang Y, Kan W, Zhao X. 2016. Prevalence of virulence and resistance to antibiotics in pathogenic enterococci isolated from mastitic cows. J Vet Med Sci 78:1663–1668 10.1292/jvms.15-0718. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Beshiru A, Igbinosa IH, Omeje FI, Ogofure AG, Eyong MM, Igbinosa EO. 2017. Multi-antibiotic resistant and putative virulence gene signatures in Enterococcus species isolated from pig farms environment. Microb Pathog 104:90–96 10.1016/j.micpath.2017.01.020. [PubMed] [DOI] [PubMed] [Google Scholar]
- 266.Carvalho I, Campo RD, Sousa M, Silva N, Carrola J, Marinho C, Santos T, Carvalho S, Nóvoa M, Quaresma M, Pereira JE, Cobo M, Igrejas G, Poeta P. 2017. Antimicrobial-resistant Escherichia coli and Enterococcus spp. isolated from Miranda donkey (Equus asinus): an old problem from a new source with a different approach. J Med Microbiol 66:191–202 10.1099/jmm.0.000423. [PubMed] [DOI] [PubMed] [Google Scholar]
- 267.Kataoka Y, Ito C, Kawashima A, Ishii M, Yamashiro S, Harada K, Ochi H, Sawada T. 2013. Identification and antimicrobial susceptibility of enterococci isolated from dogs and cats subjected to differing antibiotic pressures. J Vet Med Sci 75:749–753 10.1292/jvms.12-0243. [PubMed] [DOI] [PubMed] [Google Scholar]
- 268.Chung YS, Kwon KH, Shin S, Kim JH, Park YH, Yoon JW. 2014. Characterization of veterinary hospital-associated isolates of Enterococcus species in Korea. J Microbiol Biotechnol 24:386–393 10.4014/jmb.1310.10088. [PubMed] [DOI] [PubMed] [Google Scholar]
- 269.Iseppi R, Messi P, Anacarso I, Bondi M, Sabia C, Condò C, de Niederhausern S. 2015. Antimicrobial resistance and virulence traits in Enterococcus strains isolated from dogs and cats. New Microbiol 38:369–378. [PubMed] [PubMed] [Google Scholar]
- 270.Radhouani H, Igrejas G, Gonçalves A, Pacheco R, Monteiro R, Sargo R, Brito F, Torres C, Poeta P. 2013. Antimicrobial resistance and virulence genes in Escherichia coli and enterococci from red foxes (Vulpes vulpes). Anaerobe 23:82–86 10.1016/j.anaerobe.2013.06.013. [PubMed] [DOI] [PubMed] [Google Scholar]
- 271.Semedo-Lemsaddek T, Nóbrega CS, Ribeiro T, Pedroso NM, Sales-Luís T, Lemsaddek A, Tenreiro R, Tavares L, Vilela CL, Oliveira M. 2013. Virulence traits and antibiotic resistance among enterococci isolated from Eurasian otter (Lutra lutra). Vet Microbiol 163:378–382 10.1016/j.vetmic.2012.12.032. [PubMed] [DOI] [PubMed] [Google Scholar]
- 272.Doud CW, Scott HM, Zurek L. 2014. Role of house flies in the ecology of Enterococcus faecalis from wastewater treatment facilities. Microb Ecol 67:380–391 10.1007/s00248-013-0337-6. [PubMed] [DOI] [PubMed] [Google Scholar]
- 273.Santestevan NA, de Angelis Zvoboda D, Prichula J, Pereira RI, Wachholz GR, Cardoso LA, de Moura TM, Medeiros AW, de Amorin DB, Tavares M, d’Azevedo PA, Franco AC, Frazzon J, Frazzon AP. 2015. Antimicrobial resistance and virulence factor gene profiles of Enterococcus spp. isolates from wild Arctocephalus australis (South American fur seal) and Arctocephalus tropicalis (Subantarctic fur seal). World J Microbiol Biotechnol 31:1935–1946 10.1007/s11274-015-1938-7. [PubMed] [DOI] [PubMed] [Google Scholar]
- 274.Prichula J, Pereira RI, Wachholz GR, Cardoso LA, Tolfo NC, Santestevan NA, Medeiros AW, Tavares M, Frazzon J, d’Azevedo PA, Frazzon AP. 2016. Resistance to antimicrobial agents among enterococci isolated from fecal samples of wild marine species in the southern coast of Brazil. Mar Pollut Bull 105:51–57 10.1016/j.marpolbul.2016.02.071. [PubMed] [DOI] [PubMed] [Google Scholar]
- 275.Ben Said L, Hamdaoui M, Klibi A, Ben Slama K, Torres C, Klibi N. 2017. Diversity of species and antibiotic resistance in enterococci isolated from seafood in Tunisia. Ann Microbiol 67:135–141 10.1007/s13213-016-1246-y. [DOI] [Google Scholar]
- 276.Chaiwong T, Srivoramas T, Panya M, Wanram S, Panomket P. 2014. Antibiotic resistance patterns of Enterococcus spp. isolated from Musca domestica and Chrysomya megacephala in ubon Ratchathani. J Med Assoc Thai 97(Suppl 4):S1–S6. [PubMed] [PubMed] [Google Scholar]
- 277.Chopra I, Roberts M. 2001. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev 65:232–260 10.1128/MMBR.65.2.232-260.2001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Roberts MC, Sutcliffe J, Courvalin P, Jensen LB, Rood J, Seppala H. 1999. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob Agents Chemother 43:2823–2830. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Roberts MC. 2005. Update on acquired tetracycline resistance genes. FEMS Microbiol Lett 245:195–203 10.1016/j.femsle.2005.02.034. [PubMed] [DOI] [PubMed] [Google Scholar]
- 280.Franke AE, Clewell DB. 1980. Evidence for conjugal transfer of a Streptococcus faecalis tranposon (Tn916) from a chromosomal site in the absence of plasmid DNA. Cold Spring Harb Symp Quant Biol 46:7780. [DOI] [PubMed] [Google Scholar]
- 281.Aarestrup FM, Seyfarth AM, Emborg HD, Pedersen K, Hendriksen RS, Bager F. 2001. Effect of abolishment of the use of antimicrobial agents for growth promotion on occurrence of antimicrobial resistance in fecal enterococci from food animals in Denmark. Antimicrob Agents Chemother 45:2054–2059 10.1128/AAC.45.7.2054-2059.2001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Portillo A, Ruiz-Larrea F, Zarazaga M, Alonso A, Martinez JL, Torres C. 2000. Macrolide resistance genes in Enterococcus spp. Antimicrob Agents Chemother 44:967–971 10.1128/AAC.44.4.967-971.2000. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Bozdogan B, Berrezouga L, Kuo MS, Yurek DA, Farley KA, Stockman BJ, Leclercq R. 1999. A new resistance gene, linB, conferring resistance to lincosamides by nucleotidylation in Enterococcus faecium HM1025. Antimicrob Agents Chemother 43:925–929. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Roberts MC. 1996. Tetracycline resistance determinants: mechanisms of action, regulation of expression, genetic mobility, and distribution. FEMS Microbiol Rev 19:1–24 10.1111/j.1574-6976.1996.tb00251.x. [DOI] [PubMed] [Google Scholar]
- 285.Hooper DC. 2002. Fluoroquinolone resistance among Gram-positive cocci. Lancet Infect Dis 2:530–538 10.1016/S1473-3099(02)00369-9. [DOI] [PubMed] [Google Scholar]
- 286.López M, Tenorio C, Del Campo R, Zarazaga M, Torres C. 2011. Characterization of the mechanisms of fluoroquinolone resistance in vancomycin-resistant enterococci of different origins. J Chemother 23:87–91 10.1179/joc.2011.23.2.87. [PubMed] [DOI] [PubMed] [Google Scholar]
- 287.Arsène S, Leclercq R. 2007. Role of a qnr-like gene in the intrinsic resistance of Enterococcus faecalis to fluoroquinolones. Antimicrob Agents Chemother 51:3254–3258 10.1128/AAC.00274-07. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Jonas BM, Murray BE, Weinstock GM. 2001. Characterization of emeA, a NorA homolog and multidrug resistance efflux pump, in Enterococcus faecalis. Antimicrob Agents Chemother 45:3574–3579 10.1128/AAC.45.12.3574-3579.2001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Oyamada Y, Ito H, Fujimoto K, Asada R, Niga T, Okamoto R, Inoue M, Yamagishi J. 2006. Combination of known and unknown mechanisms confers high-level resistance to fluoroquinolones in Enterococcus faecium. J Med Microbiol 55:729–736 10.1099/jmm.0.46303-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 290.Werner G, Fleige C, Ewert B, Laverde-Gomez JA, Klare I, Witte W. 2010. High-level ciprofloxacin resistance among hospital-adapted Enterococcus faecium (CC17). Int J Antimicrob Agents 35:119–125 10.1016/j.ijantimicag.2009.10.012. [PubMed] [DOI] [PubMed] [Google Scholar]
- 291.Guzman Prieto AM, van Schaik W, Rogers MR, Coque TM, Baquero F, Corander J, Willems RJ. 2016. Global emergence and dissemination of enterococci as nosocomial pathogens: attack of the clones? Front Microbiol 7:788 10.3389/fmicb.2016.00788. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.van den Bogaard AE, Jensen LB, Stobberingh EE. 1997. Vancomycin-resistant enterococci in turkeys and farmers. N Engl J Med 337:1558–1559 10.1056/NEJM199711203372117. [PubMed] [DOI] [PubMed] [Google Scholar]
- 293.Klein G, Pack A, Reuter G. 1998. Antibiotic resistance patterns of enterococci and occurrence of vancomycin-resistant enterococci in raw minced beef and pork in Germany. Appl Environ Microbiol 64:1825–1830. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Butaye P, Devriese LA, Goossens H, Ieven M, Haesebrouck F. 1999. Enterococci with acquired vancomycin resistance in pigs and chickens of different age groups. Antimicrob Agents Chemother 43:365–366. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Klare I, Heier H, Claus H, Böhme G, Marin S, Seltmann G, Hakenbeck R, Antanassova V, Witte W. 1995. Enterococcus faecium strains with vanA-mediated high-level glycopeptide resistance isolated from animal foodstuffs and fecal samples of humans in the community. Microb Drug Resist 1:265–272 10.1089/mdr.1995.1.265. [PubMed] [DOI] [PubMed] [Google Scholar]
- 296.Wegener HC, Madsen M, Nielsen N, Aarestrup FM. 1997. Isolation of vancomycin resistant Enterococcus faecium from food. Int J Food Microbiol 35:57–66 10.1016/S0168-1605(96)01221-4. [DOI] [PubMed] [Google Scholar]
- 297.Balzereit-Scheuerlein F, Stephan R. 2001. Prevalence of colonisation and resistance patterns of vancomycin-resistant enterococci in healthy, non-hospitalised persons in Switzerland. Swiss Med Wkly 131:280–282. [PubMed] [DOI] [PubMed] [Google Scholar]
- 298.Van der Auwera P, Pensart N, Korten V, Murray BE, Leclercq R. 1996. Influence of oral glycopeptides on the fecal flora of human volunteers: selection of highly glycopeptide-resistant enterococci. J Infect Dis 173:1129–1136 10.1093/infdis/173.5.1129. [PubMed] [DOI] [PubMed] [Google Scholar]
- 299.del Campo R, Ruiz-Garbajosa P, Sánchez-Moreno MP, Baquero F, Torres C, Cantón R, Coque TM. 2003. Antimicrobial resistance in recent fecal enterococci from healthy volunteers and food handlers in Spain: genes and phenotypes. Microb Drug Resist 9:47–60 10.1089/107662903764736346. [PubMed] [DOI] [PubMed] [Google Scholar]
- 300.Bates J, Jordens Z, Selkon JB. 1993. Evidence for an animal origin of vancomycin-resistant enterococci. Lancet 342:490–491 10.1016/0140-6736(93)91613-Q. [DOI] [PubMed] [Google Scholar]
- 301.Nannini E, Murray BE. 2006. Vancomycin resistant enterococci, p 155–188. In Fong IW, Drlica K (ed), Reemergence of Established Pathogens in the 21st Century. Kluwer Academic, New York, NY. [Google Scholar]
- 302.Freitas AR, Novais C, Ruiz-Garbajosa P, Coque TM, Peixe L. 2009. Dispersion of multidrug-resistant Enterococcus faecium isolates belonging to major clonal complexes in different Portuguese settings. Appl Environ Microbiol 75:4904–4908 10.1128/AEM.02945-08. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Freitas AR, Novais C, Ruiz-Garbajosa P, Coque TM, Peixe L. 2009. Clonal expansion within clonal complex 2 and spread of vancomycin-resistant plasmids among different genetic lineages of Enterococcus faecalis from Portugal. J Antimicrob Chemother 63:1104–1111 10.1093/jac/dkp103. [PubMed] [DOI] [PubMed] [Google Scholar]
- 304.Freitas AR, Coque TM, Novais C, Hammerum AM, Lester CH, Zervos MJ, Donabedian S, Jensen LB, Francia MV, Baquero F, Peixe L. 2011. Human and swine hosts share vancomycin-resistant Enterococcus faecium CC17 and CC5 and Enterococcus faecalis CC2 clonal clusters harboring Tn1546 on indistinguishable plasmids. J Clin Microbiol 49:925–931 10.1128/JCM.01750-10. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Robredo B, Singh KV, Torres C, Murray BE. 2000. Streptogramin resistance and shared pulsed-field gel electrophoresis patterns in vanA-containing Enterococcus faecium and Enterococcus hirae isolated from humans and animals in Spain. Microb Drug Resist 6:305–311 10.1089/mdr.2000.6.305. [PubMed] [DOI] [PubMed] [Google Scholar]
- 306.Daniel DS, Lee SM, Dykes GA, Rahman S. 2015. Public health risks of multiple-drug-resistant Enterococcus spp. in Southeast Asia. Appl Environ Microbiol 81:6090–6097 10.1128/AEM.01741-15. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Seo KS, Lim JY, Yoo HS, Bae WK, Park YH. 2005. Comparison of vancomycin-resistant enterococci isolates from human, poultry and pigs in Korea. Vet Microbiol 106:225–233 10.1016/j.vetmic.2004.11.017. [PubMed] [DOI] [PubMed] [Google Scholar]
- 308.Howden BP, Holt KE, Lam MM, Seemann T, Ballard S, Coombs GW, Tong SY, Grayson ML, Johnson PD, Stinear TP. 2013. Genomic insights to control the emergence of vancomycin-resistant enterococci. MBio 4:e00412-13 10.1128/mBio.00412-13. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Hammerum AM, Lester CH, Neimann J, Porsbo LJ, Olsen KE, Jensen LB, Emborg HD, Wegener HC, Frimodt-Moller N. 2004. A vancomycin-resistant Enterococcus faecium isolate from a Danish healthy volunteer, detected 7 years after the ban of avoparcin, is possibly related to pig isolates. J Antimicrob Chemother 53:547–549 10.1093/jac/dkh101. [PubMed] [DOI] [PubMed] [Google Scholar]
- 310.van den Bogaard AE, Stobberingh EE. 2000. Epidemiology of resistanceto antibiotics. Links between animals and humans. Int J Antimicrob Agents 14:327–335 10.1016/S0924-8579(00)00145-X. [DOI] [PubMed] [Google Scholar]
- 311.Willems RJ, Top J, van Den Braak N, van Belkum A, Endtz H, Mevius D, Stobberingh E, van Den Bogaard A, van Embden JD. 2000. Host specificity of vancomycin-resistant Enterococcus faecium. J Infect Dis 182:816–823 10.1086/315752. [PubMed] [DOI] [PubMed] [Google Scholar]
- 312.Homan WL, Tribe D, Poznanski S, Li M, Hogg G, Spalburg E, Van Embden JD, Willems RJ. 2002. Multilocus sequence typing scheme for Enterococcus faecium. J Clin Microbiol 40:1963–1971 10.1128/JCM.40.6.1963-1971.2002. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Willems RJ, Top J, van Schaik W, Leavis H, Bonten M, Sirén J, Hanage WP, Corander J. 2012. Restricted gene flow among hospital subpopulations of Enterococcus faecium. MBio 3:e00151-12 10.1128/mBio.00151-12. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Tedim AP, Ruiz-Garbajosa P, Corander J, Rodríguez CM, Cantón R, Willems RJ, Baquero F, Coque TM. 2015. Population biology of intestinal enterococcus isolates from hospitalized and nonhospitalized individuals in different age groups. Appl Environ Microbiol 81:1820–1831 10.1128/AEM.03661-14. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Bruinsma N, Willems RJ, van den Bogaard AE, van Santen-Verheuvel M, London N, Driessen C, Stobberingh EE. 2002. Different levels of genetic homogeneity in vancomycin-resistant and -susceptible Enterococcus faecium isolates from different human and animal sources analyzed by amplified-fragment length polymorphism. Antimicrob Agents Chemother 46:2779–2783 10.1128/AAC.46.9.2779-2783.2002. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Willems RJ, Top J, van Santen M, Robinson DA, Coque TM, Baquero F, Grundmann H, Bonten MJ. 2005. Global spread of vancomycin-resistant Enterococcus faecium from distinct nosocomial genetic complex. Emerg Infect Dis 11:821–828 10.3201/1106.041204. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Hwang IY, Ku HO, Lim SK, Lee KJ, Park CK, Jung GS, Jung SC, Park YH, Nam HM. 2010. Distribution of streptogramin resistance genes and genetic relatedness among quinupristin/dalfopristin-resistant Enterococcus faecium recovered from pigs and chickens in Korea. Res Vet Sci 89:1–4 10.1016/j.rvsc.2010.01.011. [PubMed] [DOI] [PubMed] [Google Scholar]
- 318.Donabedian SM, Perri MB, Vager D, Hershberger E, Malani P, Simjee S, Chow J, Vergis EN, Muder RR, Gay K, Angulo FJ, Bartlett P, Zervos MJ. 2006. Quinupristin-dalfopristin resistance in Enterococcus faecium isolates from humans, farm animals, and grocery store meat in the United States. J Clin Microbiol 44:3361–3365 10.1128/JCM.02412-05. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.De Graef EM, Decostere A, De Leener E, Goossens H, Baele M, Haesebrouck F. 2007. Prevalence and mechanism of resistance against macrolides, lincosamides, and streptogramins among Enterococcus faecium isolates from food-producing animals and hospital patients in Belgium. Microb Drug Resist 13:135–141 10.1089/mdr.2007.718. [PubMed] [DOI] [PubMed] [Google Scholar]
- 320.Cha JO, Jung YH, Lee HR, Yoo JI, Lee YS. 2012. Comparison of genetic epidemiology of vancomycin-resistant Enterococcus faecium isolates from humans and poultry. J Med Microbiol 61:1121–1128 10.1099/jmm.0.037549-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 321.Stobberingh E, van den Bogaard A, London N, Driessen C, Top J, Willems R. 1999. Enterococci with glycopeptide resistance in turkeys, turkey farmers, turkey slaughterers, and (sub)urban residents in the south of The Netherlands: evidence for transmission of vancomycin resistance from animals to humans? Antimicrob Agents Chemother 43:2215–2221. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Simonsen GS, Haaheim H, Dahl KH, Kruse H, Løvseth A, Olsvik O, Sundsfjord A. 1998. Transmission of VanA-type vancomycin-resistant enterococci and vanA resistance elements between chicken and humans at avoparcin-exposed farms. Microb Drug Resist 4:313–318 10.1089/mdr.1998.4.313. [PubMed] [DOI] [PubMed] [Google Scholar]
- 323.De Leener E, Martel A, De Graef EM, Top J, Butaye P, Haesebrouck F, Willems R, Decostere A. 2005. Molecular analysis of human, porcine, and poultry Enterococcus faecium isolates and their erm(B) genes. Appl Environ Microbiol 71:2766–2770 10.1128/AEM.71.5.2766-2770.2005. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Aarestrup FM, Carstensen B. 1998. Effect of tylosin used as a growth promoter on the occurrence of macrolide-resistant enterococci and staphylococci in pigs. Microb Drug Resist 4:307–312 10.1089/mdr.1998.4.307. [PubMed] [DOI] [PubMed] [Google Scholar]
- 325.Bager F, Madsen M, Christensen J, Aarestrup FM. 1997. Avoparcin used as a growth promoter is associated with the occurrence of vancomycin-resistant Enterococcus faecium on Danish poultry and pig farms. Prev Vet Med 31:95–112 10.1016/S0167-5877(96)01119-1. [DOI] [PubMed] [Google Scholar]
- 326.Boerlin P, Wissing A, Aarestrup FM, Frey J, Nicolet J. 2001. Antimicrobial growth promoter ban and resistance to macrolides and vancomycin in enterococci from pigs. J Clin Microbiol 39:4193–4195 10.1128/JCM.39.11.4193-4195.2001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Novais C, Coque TM, Boerlin P, Herrero I, Moreno MA, Dominguez L, Peixe L. 2005. Vancomycin-resistant Enterococcus faecium clone in swine, Europe. Emerg Infect Dis 11:1985–1987 10.3201/eid1112.050822. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Novais C, Freitas AR, Sousa JC, Baquero F, Coque TM, Peixe LV. 2008. Diversity of Tn1546 and its role in the dissemination of vancomycin-resistant enterococci in Portugal. Antimicrob Agents Chemother 52:1001–1008 10.1128/AAC.00999-07. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Hasman H, Aarestrup FM. 2002. tcrB, a gene conferring transferable copper resistance in Enterococcus faecium: occurrence, transferability, and linkage to macrolide and glycopeptide resistance. Antimicrob Agents Chemother 46:1410–1416 10.1128/AAC.46.5.1410-1416.2002. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Amachawadi RG, Shelton NW, Shi X, Vinasco J, Dritz SS, Tokach MD, Nelssen JL, Scott HM, Nagaraja TG. 2011. Selection of fecal enterococci exhibiting tcrB-mediated copper resistance in pigs fed diets supplemented with copper. Appl Environ Microbiol 77:5597–5603 10.1128/AEM.00364-11. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Silveira E, Freitas AR, Antunes P, Barros M, Campos J, Coque TM, Peixe L, Novais C. 2014. Co-transfer of resistance to high concentrations of copper and first-line antibiotics among Enterococcus from different origins (humans, animals, the environment and foods) and clonal lineages. J Antimicrob Chemother 69:899–906 10.1093/jac/dkt479. [PubMed] [DOI] [PubMed] [Google Scholar]
- 332.Freitas AR, Tedim AP, Novais C, Ruiz-Garbajosa P, Werner G, Laverde-Gomez JA, Cantón R, Peixe L, Baquero F, Coque TM. 2010. Global spread of the hyl(Efm) colonization-virulence gene in megaplasmids of the Enterococcus faecium CC17 polyclonal subcluster. Antimicrob Agents Chemother 54:2660–2665 10.1128/AAC.00134-10. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Getachew Y, Hassan L, Zakaria Z, Abdul Aziz S. 2013. Genetic variability of vancomycin-resistant Enterococcus faecium and Enterococcus faecalis isolates from humans, chickens, and pigs in Malaysia. Appl Environ Microbiol 79:4528–4533 10.1128/AEM.00650-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Damborg P, Sørensen AH, Guardabassi L. 2008. Monitoring of antimicrobial resistance in healthy dogs: first report of canine ampicillin-resistant Enterococcus faecium clonal complex 17. Vet Microbiol 132:190–196 10.1016/j.vetmic.2008.04.026. [PubMed] [DOI] [PubMed] [Google Scholar]
- 335.de Regt MJ, van Schaik W, van Luit-Asbroek M, Dekker HA, van Duijkeren E, Koning CJ, Bonten MJ, Willems RJ. 2012. Hospital and community ampicillin-resistant Enterococcus faecium are evolutionarily closely linked but have diversified through niche adaptation. PLoS One 7:e30319 10.1371/journal.pone.0030319. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.van den Bunt G, Top J, Hordijk J, de Greeff SC, Mughini-Gras L, Corander J, van Pelt W, Bonten MJM, Fluit AC, Willems RJL. Intestinal carriage of ampicillin- and vancomycin-resistant Enterococcus faecium in humans, dogs and cats in the Netherlands. J Antimicrob Chemother. Epub ahead of print. 10.1093/jac/dkx455. [DOI] [PubMed] [Google Scholar]
- 337.Marques C, Belas A, Franco A, Aboim C, Gama LT, Pomba C. 2018. Increase in antimicrobial resistance and emergence of major international high-risk clonal lineages in dogs and cats with urinary tract infection: 16 year retrospective study. J Antimicrob Chemother 73:377–384 10.1093/jac/dkx401. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Ruiz-Garbajosa P, Bonten MJM, Robinson DA, Top J, Nallapareddy SR, Torres C, Coque TM, Cantón R, Baquero F, Murray BE, del Campo R, Willems RJL. 2006. Multilocus sequence typing scheme for Enterococcus faecalis reveals hospital-adapted genetic complexes in a background of high rates of recombination. J Clin Microbiol 44:2220–2228 10.1128/JCM.02596-05. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.McBride SM, Fischetti VA, Leblanc DJ, Moellering RC Jr, Gilmore MS. 2007. Genetic diversity among Enterococcus faecalis. PLoS One 2:e582 10.1371/journal.pone.0000582. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Kuch A, Willems RJL, Werner G, Coque TM, Hammerum AM, Sundsfjord A, Klare I, Ruiz-Garbajosa P, Simonsen GS, van Luit-Asbroek M, Hryniewicz W, Sadowy E. 2012. Insight into antimicrobial susceptibility and population structure of contemporary human Enterococcus faecalis isolates from Europe. J Antimicrob Chemother 67:551–558 10.1093/jac/dkr544. [PubMed] [DOI] [PubMed] [Google Scholar]
- 341.Chen MY, Lira F, Liang HQ, Wu RT, Duan JH, Liao XP, Martínez JL, Liu YH, Sun J. 2016. Multilevel selection of bcrABDR-mediated bacitracin resistance in Enterococcus faecalis from chicken farms. Sci Rep 6:34895 10.1038/srep34895. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Petersen A, Christensen H, Philipp H-C, Bisgaard M. 2009. Clonality of Enterococcus faecalis associated with amyloid arthropathy in chickens evaluated by multilocus sequence typing (MLST). Vet Microbiol 134:392–395 10.1016/j.vetmic.2008.08.014. [PubMed] [DOI] [PubMed] [Google Scholar]
- 343.Ruiz-Garbajosa P, Cantón R, Pintado V, Coque TM, Willems R, Baquero F, del Campo R. 2006. Genetic and phenotypic differences among Enterococcus faecalis clones from intestinal colonisation and invasive disease. Clin Microbiol Infect 12:1193–1198 10.1111/j.1469-0691.2006.01533.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 344.Raven KE, Reuter S, Gouliouris T, Reynolds R, Russell JE, Brown NM, Török ME, Parkhill J, Peacock SJ. 2016. Genome-based characterization of hospital-adapted Enterococcus faecalis lineages. Nat Microbiol 1:15033 10.1038/nmicrobiol.2015.33. [DOI] [PubMed] [Google Scholar]
- 345.Palmer KL, Godfrey P, Griggs A, Kos VN, Zucker J, Desjardins C, Cerqueira G, Gevers D, Walker S, Wortman J, Feldgarden M, Haas B, Birren B, Gilmore MS. 2012. Comparative genomics of enterococci: variation in Enterococcus faecalis, clade structure in E. faecium, and defining characteristics of E. gallinarum and E. casseliflavus. MBio 3:e00318-11. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Clewell DB, Weaver KE, Dunny GM, Coque TM, Francia MV, Hayes F. 2014. Extrachromosomal and mobile elements in enterococci: transmission, maintenance, and epidemiology. In Gilmore MS, Clewell DB, Ike Y, Shankar N (ed), Enterococci: from Commensals to Leading Causes of Drug Resistant Infection. Eye and Ear Infirmary, Boston, MA. [PubMed] [Google Scholar]
- 347.Jensen LB, Garcia-Migura L, Valenzuela AJS, Løhr M, Hasman H, Aarestrup FM. 2010. A classification system for plasmids from enterococci and other Gram-positive bacteria. J Microbiol Methods 80:25–43 10.1016/j.mimet.2009.10.012. [PubMed] [DOI] [PubMed] [Google Scholar]
- 348.Wardal E, Gawryszewska I, Hryniewicz W, Sadowy E. 2013. Abundance and diversity of plasmid-associated genes among clinical isolates of Enterococcus faecalis. Plasmid 70:329–342 10.1016/j.plasmid.2013.07.003. [PubMed] [DOI] [PubMed] [Google Scholar]
- 349.Wattam AR, Abraham D, Dalay O, Disz TL, Driscoll T, Gabbard JL, Gillespie JJ, Gough R, Hix D, Kenyon R, Machi D, Mao C, Nordberg EK, Olson R, Overbeek R, Pusch GD, Shukla M, Schulman J, Stevens RL, Sullivan DE, Vonstein V, Warren A, Will R, Wilson MJC, Yoo HS, Zhang C, Zhang Y, Sobral BW. 2014. PATRIC, the bacterial bioinformatics database and analysis resource. Nucleic Acids Res 42(D1):D581–D591 10.1093/nar/gkt1099. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Carattoli A, Zankari E, García-Fernández A, Voldby Larsen M, Lund O, Villa L, Møller Aarestrup F, Hasman H. 2014. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 58:3895–3903 10.1128/AAC.02412-14. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Johnsen PJ, Townsend JP, Bøhn T, Simonsen GS, Sundsfjord A, Nielsen KM. 2011. Retrospective evidence for a biological cost of vancomycin resistance determinants in the absence of glycopeptide selective pressures. J Antimicrob Chemother 66:608–610 10.1093/jac/dkq512. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Freitas AR, Tedim AP, Francia MV, Jensen LB, Novais C, Peixe L, Sánchez-Valenzuela A, Sundsfjord A, Hegstad K, Werner G, Sadowy E, Hammerum AM, Garcia-Migura L, Willems RJ, Baquero F, Coque TM. 2016. Multilevel population genetic analysis of vanA and vanB Enterococcus faecium causing nosocomial outbreaks in 27 countries (1986–2012). J Antimicrob Chemother 71:3351–3366 10.1093/jac/dkw312. [PubMed] [DOI] [PubMed] [Google Scholar]
- 353.Lanza VF, Tedim AP, Martínez JL, Baquero F, Coque TM. 2015. The plasmidome of Firmicutes: impact on the emergence and the spread of resistance to antimicrobials. Microbiol Spectr 3:PLAS-0039-2014. [DOI] [PubMed] [Google Scholar]
- 354.Sletvold H, Johnsen PJ, Hamre I, Simonsen GS, Sundsfjord A, Nielsen KM. 2008. Complete sequence of Enterococcus faecium pVEF3 and the detection of an omega-epsilon-zeta toxin-antitoxin module and an ABC transporter. Plasmid 60:75–85 10.1016/j.plasmid.2008.04.002. [PubMed] [DOI] [PubMed] [Google Scholar]
- 355.Sletvold H, Johnsen PJ, Wikmark OG, Simonsen GS, Sundsfjord A, Nielsen KM. 2010. Tn1546 is part of a larger plasmid-encoded genetic unit horizontally disseminated among clonal Enterococcus faecium lineages. J Antimicrob Chemother 65:1894–1906 10.1093/jac/dkq219. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Garcia-Migura L, Hasman H, Svendsen C, Jensen LB. 2008. Relevance of hot spots in the evolution and transmission of Tn1546 in glycopeptide-resistant Enterococcus faecium (GREF) from broiler origin. J Antimicrob Chemother 62:681–687 10.1093/jac/dkn265. [PubMed] [DOI] [PubMed] [Google Scholar]
- 357.Moura TM, Cassenego AP, Campos FS, Ribeiro AM, Franco AC, d’Azevedo PA, Frazzon J, Frazzon AP. 2013. Detection of vanC1 gene transcription in vancomycin-susceptible Enterococcus faecalis. Mem Inst Oswaldo Cruz 108:453–456 10.1590/S0074-0276108042013009. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Schwaiger K, Bauer J, Hörmansdorfer S, Mölle G, Preikschat P, Kämpf P, Bauer-Unkauf I, Bischoff M, Hölzel C. 2012. Presence of the resistance genes vanC1 and pbp5 in phenotypically vancomycin and ampicillin susceptible Enterococcus faecalis. Microb Drug Resist 18:434–439 10.1089/mdr.2011.0227. [PubMed] [DOI] [PubMed] [Google Scholar]
- 359.Batista Xavier D, Moreno Bernal FE, Titze-de-Almeida R. 2006. Absence of VanA- and VanB-containing enterococci in poultry raised on nonintensive production farms in Brazil. Appl Environ Microbiol 72:3072–3073 10.1128/AEM.72.4.3072-3073.2006. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Mammina C, Di Noto AM, Costa A, Nastasi A. 2005. VanB-VanC1 Enterococcus gallinarum, Italy. Emerg Infect Dis 11:1491–1492 10.3201/eid1109.050282. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Mikalsen T, Pedersen T, Willems R, Coque TM, Werner G, Sadowy E, van Schaik W, Jensen LB, Sundsfjord A, Hegstad K. 2015. Investigating the mobilome in clinically important lineages of Enterococcus faecium and Enterococcus faecalis. BMC Genomics 16:282 10.1186/s12864-015-1407-6. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Teuber M, Schwarz F, Perreten V. 2003. Molecular structure and evolution of the conjugative multiresistance plasmid pRE25 of Enterococcus faecalis isolated from a raw-fermented sausage. Int J Food Microbiol 88:325–329 10.1016/S0168-1605(03)00195-8. [DOI] [PubMed] [Google Scholar]
- 363.Schwarz FV, Perreten V, Teuber M. 2001. Sequence of the 50-kb conjugative multiresistance plasmid pRE25 from Enterococcus faecalis RE25. Plasmid 46:170–187 10.1006/plas.2001.1544. [PubMed] [DOI] [PubMed] [Google Scholar]
- 364.Werner G, Hildebrandt B, Witte W. 2003. Linkage of erm(B) and aadE-sat4-aphA-3 in multiple-resistant Enterococcus faecium isolates of different ecological origins. Microb Drug Resist 9(Suppl 1):S9–S16 10.1089/107662903322541847. [PubMed] [DOI] [PubMed] [Google Scholar]
- 365.Khan AA, Nawaz MS, Khan SA, Steele R. 2002. Detection and characterization of erythromycin-resistant methylase genes in Gram-positive bacteria isolated from poultry litter. Appl Microbiol Biotechnol 59:377–381 10.1007/s00253-002-1013-9. [PubMed] [DOI] [PubMed] [Google Scholar]
- 366.Thumu SC, Halami PM. 2014. Phenotypic expression, molecular characterization and transferability of erythromycin resistance genes in Enterococcus spp. isolated from naturally fermented food. J Appl Microbiol 116:689–699 10.1111/jam.12386. [PubMed] [DOI] [PubMed] [Google Scholar]
- 367.Schwaiger K, Bauer J. 2008. Detection of the erythromycin rRNA methylase gene erm(A) in Enterococcus faecalis. Antimicrob Agents Chemother 52:2994–2995 10.1128/AAC.00230-08. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Werner G, Hildebrandt B, Klare I, Witte W. 2000. Linkage of determinants for streptogramin A, macrolide-lincosamide-streptogramin B, and chloramphenicol resistance on a conjugative plasmid in Enterococcus faecium and dissemination of this cluster among streptogramin-resistant enterococci. Int J Med Microbiol 290:543–548 10.1016/S1438-4221(00)80020-X. [DOI] [PubMed] [Google Scholar]
- 369.Werner G, Klare I, Heier H, Hinz KH, Böhme G, Wendt M, Witte W. 2000. Quinupristin/dalfopristin-resistant enterococci of the satA (vatD) and satG (vatE) genotypes from different ecological origins in Germany. Microb Drug Resist 6:37–47 10.1089/mdr.2000.6.37. [PubMed] [DOI] [PubMed] [Google Scholar]
- 370.Hammerum AM, Flannagan SE, Clewell DB, Jensen LB. 2001. Indication of transposition of a mobile DNA element containing the vat(D) and erm(B) genes in Enterococcus faecium. Antimicrob Agents Chemother 45:3223–3225 10.1128/AAC.45.11.3223-3225.2001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Jackson CR, Fedorka-Cray PJ, Barrett JB, Hiott LM, Woodley TA. 2007. Prevalence of streptogramin resistance in enterococci from animals: identification of vatD from animal sources in the USA. Int J Antimicrob Agents 30:60–66 10.1016/j.ijantimicag.2007.03.010. [PubMed] [DOI] [PubMed] [Google Scholar]
- 372.Jensen LB, Hammerum AM, Aarestrup FM. 2000. Linkage of vat(E) and erm(B) in streptogamin-resistant Enterococcus faecium isolates from Europe. Antimicrob Agents Chemother 44:2231–2232 10.1128/AAC.44.8.2231-2232.2000. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Jensen LB, Hammerum AM, Bager F, Aarestrup FM. 2002. Streptogramin resistance among Enterococcus faecium isolated from production animals in Denmark in 1997. Microb Drug Resist 8:369–374 10.1089/10766290260469642. [PubMed] [DOI] [PubMed] [Google Scholar]
- 374.Sørensen TL, Blom M, Monnet DL, Frimodt-Møller N, Poulsen RL, Espersen F. 2001. Transient intestinal carriage after ingestion of antibiotic-resistant Enterococcus faecium from chicken and pork. N Engl J Med 345:1161–1166 10.1056/NEJMoa010692. [PubMed] [DOI] [PubMed] [Google Scholar]
- 375.Smith DL, Johnson JA, Harris AD, Furuno JP, Perencevich EN, Morris JG Jr. 2003. Assessing risks for a pre-emergent pathogen: virginiamycin use and the emergence of streptogramin resistance in Enterococcus faecium. Lancet Infect Dis 3:241–249 10.1016/S1473-3099(03)00581-4. [DOI] [PubMed] [Google Scholar]
- 376.Li XS, Dong WC, Wang XM, Hu GZ, Wang YB, Cai BY, Wu CM, Wang Y, Du XD. 2014. Presence and genetic environment of pleuromutilin-lincosamide-streptogramin A resistance gene lsa(E) in enterococci of human and swine origin. J Antimicrob Chemother 69:1424–1426 10.1093/jac/dkt502. [PubMed] [DOI] [PubMed] [Google Scholar]
- 377.Wang XM, Li XS, Wang YB, Wei FS, Zhang SM, Shang YH, Du XD. 2015. Characterization of a multidrug resistance plasmid from Enterococcus faecium that harbours a mobilized bcrABDR locus. J Antimicrob Chemother 70:609–611 10.1093/jac/dku416. [PubMed] [DOI] [PubMed] [Google Scholar]
- 378.Zhu XQ, Wang XM, Li H, Shang YH, Pan YS, Wu CM, Wang Y, Du XD, Shen JZ. 2017. Novel lnu(G) gene conferring resistance to lincomycin by nucleotidylation, located on Tn6260 from Enterococcus faecalis E531. J Antimicrob Chemother 72:993–997. [PubMed] [DOI] [PubMed] [Google Scholar]
- 379.Liu H, Wang Y, Wu C, Schwarz S, Shen Z, Jeon B, Ding S, Zhang Q, Shen J. 2012. A novel phenicol exporter gene, fexB, found in enterococci of animal origin. J Antimicrob Chemother 67:322–325 10.1093/jac/dkr481. [PubMed] [DOI] [PubMed] [Google Scholar]
- 380.Novais C, Campos J, Freitas AR, Barros M, Silveira E, Coque TM, Antunes P, Peixe L. 2018. Water supply and feed as sources of antimicrobial-resistant Enterococcus spp. in aquacultures of rainbow trout (Oncorhyncus mykiss), Portugal. Sci Total Environ 625:1102–1112 10.1016/j.scitotenv.2017.12.265. [DOI] [PubMed] [Google Scholar]
- 381.Aarestrup FM, Hasman H, Jensen LB, Moreno M, Herrero IA, Domínguez L, Finn M, Franklin A. 2002. Antimicrobial resistance among enterococci from pigs in three European countries. Appl Environ Microbiol 68:4127–4129 10.1128/AEM.68.8.4127-4129.2002. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Amachawadi RG, Scott HM, Alvarado CA, Mainini TR, Vinasco J, Drouillard JS, Nagaraja TG. 2013. Occurrence of the transferable copper resistance gene tcrB among fecal enterococci of U.S. feedlot cattle fed copper-supplemented diets. Appl Environ Microbiol 79:4369–4375 10.1128/AEM.00503-13. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Kim J, Lee S, Choi S. 2012. Copper resistance and its relationship to erythromycin resistance in Enterococcus isolates from bovine milk samples in Korea. J Microbiol 50:540–543 10.1007/s12275-012-1579-6. [PubMed] [DOI] [PubMed] [Google Scholar]
- 384.Hasman H, Kempf I, Chidaine B, Cariolet R, Ersbøll AK, Houe H, Bruun Hansen HC, Aarestrup FM. 2006. Copper resistance in Enterococcus faecium, mediated by the tcrB gene, is selected by supplementation of pig feed with copper sulfate. Appl Environ Microbiol 72:5784–5789 10.1128/AEM.02979-05. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Amachawadi RG, Scott HM, Vinasco J, Tokach MD, Dritz SS, Nelssen JL, Nagaraja TG. 2015. Effects of in-feed copper, chlortetracycline, and tylosin on the prevalence of transferable copper resistance gene, tcrB, among fecal enterococci of weaned piglets. Foodborne Pathog Dis 12:670–678 10.1089/fpd.2015.1961. [PubMed] [DOI] [PubMed] [Google Scholar]
- 386.Pasquaroli S, Di Cesare A, Vignaroli C, Conti G, Citterio B, Biavasco F. 2014. Erythromycin- and copper-resistant Enterococcus hirae from marine sediment and co-transfer of erm(B) and tcrB to human Enterococcus faecalis. Diagn Microbiol Infect Dis 80:26–28 10.1016/j.diagmicrobio.2014.06.002. [PubMed] [DOI] [PubMed] [Google Scholar]
- 387.Zhang S, Wang D, Wang Y, Hasman H, Aarestrup FM, Alwathnani HA, Zhu YG, Rensing C. 2015. Genome sequences of copper resistant and sensitive Enterococcus faecalis strains isolated from copper-fed pigs in Denmark. Stand Genomic Sci 10:35 10.1186/s40793-015-0021-1. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]


