Abstract
CD8+ T-cell responses can be induced by DNA immunization, but little is known about the kinetics of these responses in vivo in the absence of restimulation or how soon protective immunity is conferred by a DNA vaccine. It is also unclear if CD8+ T cells primed by DNA vaccines express the vigorous effector functions characteristic of cells primed by natural infection or by immunization with a recombinant live virus vaccine. To address these issues, we have used the sensitive technique of intracellular cytokine staining to carry out direct ex vivo kinetic and phenotypic analyses of antigen-specific CD8+ T cells present in the spleens of mice at various times after (i) a single intramuscular administration of a plasmid expressing the nucleoprotein (NP) gene from lymphocytic choriomeningitis virus (LCMV), (ii) infection by a recombinant vaccinia virus carrying the same protein (vvNP), or (iii) LCMV infection. In addition, we have evaluated the rapidity with which protective immunity against both lethal and sublethal LCMV infections is achieved following DNA vaccination. The CD8+ T-cell response in DNA-vaccinated mice was slightly delayed compared to LCMV or vvNP vaccinees, peaking at 15 days postimmunization. Interestingly, the percentage of antigen-specific CD8+ T cells present in the spleen at day 15 and later time points was similar to that observed following vvNP infection. T cells primed by DNA vaccination or by infection exhibited similar cytokine expression profiles and had similar avidities for an immunodominant cytotoxic T lymphocyte epitope peptide, implying that the responses induced by DNA vaccination differ quantitatively but not qualitatively from those induced by live virus infection. Surprisingly, protection from both lethal and sublethal LCMV infections was conferred within 1 week of DNA vaccination, well before the peak of the CD8+ T-cell response.
In many natural infections and experimental models, virus-specific CD8+ T cells are critical for clearance of primary virus infection and for subsequent protective immunity. In addition, these cells are an important component of the immunity conferred by attenuated and recombinant viruses and by plasmid DNA vaccines. Although the kinetics of the antiviral CD8+ T-cell response during the inductive and memory phases of certain systemic viral infections have been well characterized (8, 16, 19), there is little detailed knowledge about the kinetics of cellular immunity induced by conventional vaccines or by DNA immunization. Of particular interest is how rapidly CD8+ T-cell responses develop after vaccination, as these cells play a critical role in limiting virus replication.
Although a number of groups have independently demonstrated DNA vaccine-induced antigen-specific CD8+ T cells in a variety of animal models (1, 6, 12, 14, 18, 24, 26, 31) as well as in humans (29), the kinetics and functional attributes of these responses have not been fully characterized. In particular, most DNA vaccine studies to date have employed in vitro cytotoxicity assays to detect CD8+ T-cell responses, but these assays are not sufficiently sensitive to permit the direct ex vivo detection of DNA-induced cytotoxic T lymphocytes (CTLs); consequently, DNA-induced CTLs have been extensively restimulated in vivo or in vitro to expand their numbers to a detectable level. It has therefore been difficult to confidently enumerate the CD8+ T cells in a DNA-vaccinated host. Here we employ a more sensitive technique, intracellular cytokine staining (ICCS), to detect CD8+ T-cell responses, thereby avoiding the need for lengthy restimulation. In this way, we have been able to measure DNA-induced CD8+ T-cell numbers directly ex vivo at various times after a single inoculation of plasmid DNA. Using a well-defined murine model of viral pathogenesis and immunity, infection with the arenavirus lymphocytic choriomeningitis virus (LCMV), we have examined the temporal kinetics and functional attributes of CD8+ T cells induced by DNA vaccination and compared them to the responses induced by LCMV infection and by immunization with a recombinant vaccinia virus vaccine. We have also determined how rapidly a DNA vaccine can induce protective antiviral immunity.
MATERIALS AND METHODS
Mice.
BALB/c mice were purchased from the Scripps Research Institute animal facility and housed in specific-pathogen-free conditions. Mice were used between 4 and 16 weeks of age.
Viruses and viral infections.
Stocks of the Armstrong strain of LCMV were grown on BHK cells in RPMI containing 10% fetal bovine serum (FBS), penicillin G (50 U/liter), streptomycin (50 μg/liter), and 20 mM l-glutamine (all from Gibco-BRL, Rockville, Md.). LCMV titers were determined by plaque assay on Vero cells grown in medium 199 (Gibco-BRL) containing 5% FBS, penicillin G (50 U/liter), streptomycin (50 μg/liter), 20 mM l-glutamine, and 0.5% agarose as previously described (11). Mice were infected by the intraperitoneal route with 2 × 105 PFU of LCMV for all studies except intracranial challenge experiments, in which mice were infected intracranially with 30 50% lethal doses (LD50) (6 PFU) of LCMV. Stocks of a recombinant vaccinia virus expressing LCMV nucleoprotein (vvNP, the construction of which has been described previously [30]) were grown and counted on BSC40 cells grown in Dulbecco's modified Eagle's medium (Gibco-BRL) supplemented with 10% FBS, penicillin G (50 U/liter), streptomycin (50 μg/liter), and 20 mM l-glutamine. To analyze the kinetics of the T-cell response to recombinant vaccinia virus, mice were infected intraperitoneally with 2 × 107 PFU of vvNP.
DNA vaccinations and plasmid DNA.
Plasmid pCMVNP encodes the full-length LCMV nucleoprotein (Armstrong strain); pCMV, the vector control, contains no LCMV sequences. The construction of both of these plasmids has been described previously (31). Plasmids were propagated in Escherichia coli using standard techniques and purified using a Qiagen Endofree plasmid purification kit (Qiagen, Chatsworth, Calif.) according to the manufacturer's instructions. For the kinetic analysis of CD8+ T-cell responses and for survival studies, mice received bilateral 50-μl injections of plasmid DNA dissolved in saline (2 μg/μl; total, 200 μg/mouse) into the anterior tibial muscles. For analyses of CTL activity and of the acquisition of protective immunity following systemic or intracranial viral challenge, each mouse received a 50-μl injection of 50 μg of DNA dissolved in saline into the anterior tibial muscle.
CTL assays.
CTL assays were carried out as previously described (10).
ICCS and flow cytometric analysis of antigen-specific T-cell responses.
Splenocytes (2 × 106) were incubated for 5 h in 200 μl of RPMI containing 10% FBS, 20 mM HEPES, and brefeldin A (2 μg/ml) in the presence of a peptide corresponding to the immunodominant H-2d-restricted CD8+ T-cell epitope in the LCMV nucleoprotein (RPQASGVYM, NP residues 118 to 126; final concentration, 10−7 M). Background cytokine staining was determined by incubating cells in the same medium in the absence of peptide. After the stimulation, the cells were washed with phosphate-buffered saline (PBS)–5% FBS, stained overnight with an anti-mouse CD8 cychrome-conjugated antibody (clone 53-6.7), fixed in cold PBS–2% formaldehyde, and permeabilized with Cytofix/Cytoperm (Pharmingen, San Diego, Calif.). Intracellular cytokines were stained with anti-mouse gamma interferon (IFN-γ) conjugated to fluorescein isothiocyanate (clone XMG1.2) and anti-mouse tumor necrosis factor alpha (TNF-α) conjugated to phycoerythrin (clone MP6-XT22) antibodies (Pharmingen). From 2 × 105 to 4 × 105 events were acquired on a FACScan flow cytometer (Becton Dickinson, Oxnard, Calif.), and live cells were analyzed for expression of CD8 and cytokines using CellQuest software. The percentage of peptide-specific CD8+ T cells presented in the figures was calculated by subtracting the background percentage of cytokine-positive CD8+ T cells detected in the absence of peptide (invariably <0.2%) from the percentage of cytokine-positive CD8+ T cells detected in the presence of peptide.
RESULTS
Kinetics of CD8+ responses following DNA vaccination or virus infection.
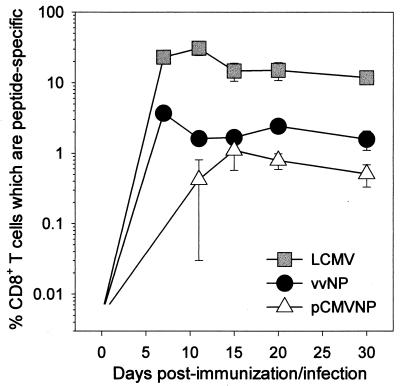
Both primary and memory antigen-experienced CD8+ T cells rapidly upregulate cytokine production after encountering a cell displaying their cognate peptide ligand in association with a major histocompatibility complex class I molecule (2, 19, 21, 22). We have previously used ICCS to identify cytokine-producing LCMV-specific CD8+ memory T cells directly ex vivo 1 year after vaccination (10), but it was unclear how quickly these responses developed after DNA inoculation and how they compared quantitatively to CD8+ responses developing in response to virus infection or to immunization with a live recombinant virus vaccine. Therefore, we used ICCS to directly enumerate antigen-specific effector CD8+ T cells in the spleens of mice at various times after DNA immunization (pCMVNP), LCMV infection, and vvNP infection. The average percentage of peptide-specific CD8+ T cells detected in three mice analyzed at each time point for each mode of immunization is shown in Fig. 1.
FIG. 1.
CD8+ T-cell kinetics following DNA immunization or virus infection. Mice were infected with LCMV or vvNP or immunized with pCMVNP 7, 11, 15, 20, or 30 days prior to the assay. Splenocytes were harvested, stimulated for 5 h with peptide antigen, and then stained for IFN-γ and CD8. A total of 5 × 105 events were acquired on a flow cytometer, and live cells were analyzed for expression of CD8 and IFN-γ. The values shown (plotted on a log10 scale) reflect the average number of peptide-specific CD8+ T cells expressed as a percentage of total CD8+ T cells present in the spleens ± standard deviation (three mice/group at each time point).
Following infection with either LCMV or vvNP, there was a rapid amplification of antigen-specific T cells evident by 7 days postinfection and peaking at 7 to 11 days after infection. In contrast, cells were barely detectable at day 7 in DNA-immunized mice but were easily identified by day 11 and peaked at 15 days postimmunization. Notably, from day 15 onward, the frequency of antigen-specific CD8+ T cells in DNA-immunized mice was only two- to threefold lower than that seen after live recombinant vaccinia virus vaccination (vvNP). Thus, CD8+ responses can be primed by a DNA vaccine, and memory CD8+ T cells remain detectable directly ex vivo for 30 days postinjection (∼0.7% of all splenic CD8+ T cells) (Fig. 1); this proportion of antigen-specific memory cells is maintained and can confer protective immunity, for at least a year after pCMVNP immunization (10).
Cytokine expression profiles of antigen-specific T cells at the peak of the DNA immunization response.
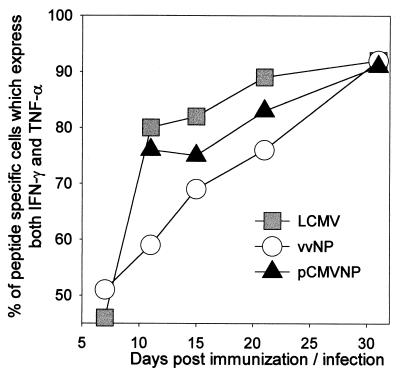
It has recently been shown that the population of LCMV-specific CD8+ T cells undergoes a temporally regulated transition in the production of IFN-γ and TNF-α (22). At the early stages of the infection, ∼50% of the antigen-specific CD8+ T cells produce only IFN-γ, and the remainder produce both IFN-γ and TNF-α. Over time, the fraction of IFN-γ+ TNF-α+ cells increases until, by day 30, >90% of LCMV-specific CD8+ cells produce both cytokines in response to peptide stimulation. To determine if the CD8+ T cells induced by DNA vaccination or recombinant viral infection undergo a similar transition in cytokine production, the splenocytes from the same mice shown in Fig. 1 were evaluated for IFN-γ and TNF-α expression, and the percentages of antigen-specific (IFN-γ+) CD8+ T cells which also express TNF-α are shown at each time point (Fig. 2). At the peak of the acute response to LCMV, at 7 days postinfection, two discrete populations of antigen-specific CD8+ T cells were observed, IFN-γ+ TNF-α− (54%) and IFN-γ+ TNF-α+ (46%). By day 11, 81% of the LCMV-specific CD8+ T cells were secreting both cytokines, and at 30 days postinfection, 93% of the antigen-specific cells were double positive. A similar transition was observed in CD8+ T cells primed by DNA vaccination or by recombinant vaccinia virus infection. At day 7, 52% of vvNP-induced cells are double positive. At this time point in DNA-vaccinated mice, antigen-specific CD8+ T cells were present at a frequency of less than 1 in 1,000 (Fig. 1), and therefore accurate analysis of IFN-γ and TNF-α production was not possible (Fig. 2). By 11 days postinfection, 56% of the vvNP-induced antigen-specific CD8+ T cells were secreting both cytokines, while DNA-induced cells were readily detectable and 76% were double positive. These proportions increased steadily until, by day 30, >90% of antigen-specific cells in both groups were double positive. Thus, the cytokine expression patterns of LCMV-specific T cells are similar regardless of the mode of immunization.
FIG. 2.
Similar cytokine expression patterns in virus-induced and DNA-induced CD8+ T cells. Splenocytes from the mice shown in Fig. 1 were assayed by flow cytometry for expression of IFN-γ, TNF-α, and CD8 after 5 h of peptide stimulation. The values shown for each time point reflect the percentage of antigen-specific (IFN-γ+) CD8+ cells also producing TNF-α (see text). For the DNA vaccinees, data are not shown for day 7, at which time CD8+ T-cell responses were too close to the limit of detection to permit confident analysis of cytokine expression patterns.
Avidity of antigen-specific T cells at the peak of the DNA immunization response.
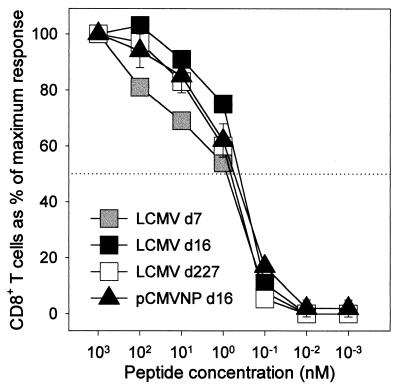
The avidity of antigen-specific CD8+ T cells is important in determining their biological function (27), and the avidity of DNA vaccine-induced CD8+ T cells has not been determined directly ex vivo. Therefore, we used ICCS to compare, directly ex vivo, the avidities of the antigen-specific T cells in mice which had received pCMVNP or LCMV. Three BALB/c mice were immunized with 200 μg of pCMVNP, and 16 days later, at the peak of the DNA-induced CD8+ T-cell response, lymphocytes from individual mice were analyzed in triplicate for IFN-γ production in the presence of different concentrations of stimulatory peptide. The responses in these three DNA-immunized mice were compared to those in individual mice infected with LCMV 7, 16, or 227 days previously (Fig. 3). The percentage of cytokine-producing CD8+ T cells observed at the highest concentration of peptide used (10−6 M) was defined as 100% response, and responses observed at lower concentrations of peptide were expressed as a percentage of this maximal response. The avidity profiles of the DNA-immunized mice were similar to the avidity profiles observed in acutely infected (LCMV day 7 and 16) and long-term immune (LCMV day 227) mice, with a half-maximal response being observed at a peptide concentration of 1 nM. Together, our data show that the antigen-specific CD8+ T cells present 16 days after DNA immunization differ quantitatively but not qualitatively (cytokine patterns or avidities) from CD8+ cells induced by virus infection.
FIG. 3.
CD8+ T cells primed by virus infection or by DNA immunization have similar avidities. Splenocytes were obtained from individual mice infected with LCMV 7, 16, or 227 days prior to the assay (d7, d16, and d227, respectively) and from three mice immunized with pCMVNP 16 days before the assay. Splenocytes were incubated for 5 h with an immunodominant CD8+ T-cell epitope peptide (NP118–126) over a 106-fold range of peptide concentrations, as shown. Cells were then stained for CD8 and IFN-γ expression. A total of 2 × 105 events were acquired on a flow cytometer, and live cells were analyzed for expression of CD8 and IFN-γ. For each of the four groups, the response at each peptide concentration is expressed as a percentage of the maximum response (measured at 10−6 M peptide) in that group. The half-maximal response is indicated by a horizontal dotted line.
Lytic CTL responses are primed within 7 days after DNA vaccination.
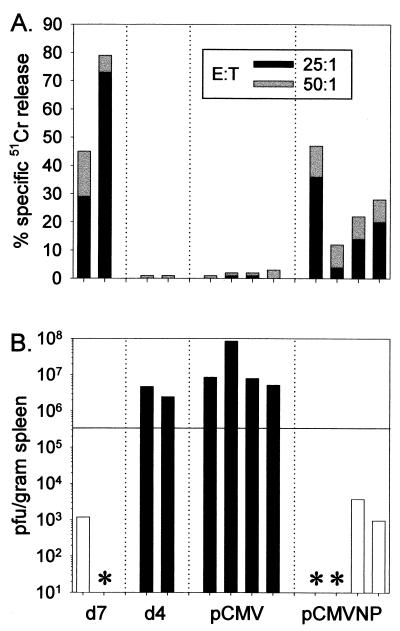
The above data indicate that within 7 days of DNA vaccination, the number of primed T cells remained at or below the level of detection by ICCS. We next attempted to determine whether this small number of cells was sufficient to permit a rapid and protective cytolytic response to viral challenge. Therefore, groups of four BALB/c mice were immunized intramuscularly with pCMVNP or with the control vector pCMV and 7 days later infected intraperitoneally with a sublethal dose of LCMV. Four days postinfection, splenocytes were assayed in vitro for lytic activity against target cells pulsed with a peptide (NP118–126) corresponding to the H-2d-restricted immunodominant CD8+ T-cell epitope contained with the nucleoprotein. Naive mice infected with LCMV 7 days before the assay were included as positive controls for CTL-mediated lysis, and naive mice infected 4 days previously were included as negative controls for lysis and viral clearance. As shown in Fig. 4A, by 7 days postinfection the two nonimmunized infected mice had mounted strong primary anti-LCMV CTL responses. In contrast, at 4 days postinfection, LCMV-specific lytic responses in nonimmunized mice had not yet expanded to detectable levels, and, as expected, all mice previously inoculated with pCMV failed to demonstrate lytic responses at 4 days postinfection. However, peptide-specific lytic responses were detected 4 days postinfection in all pCMVNP vaccinees, indicating that the DNA vaccine had successfully primed CTL responses in these mice; in all cases the lysis observed was at least fourfold higher than the background lysis observed using spleen cells from mice which had received the vector plasmid pCMV (Fig. 4A). Therefore, within 1 week of DNA vaccination, a sufficient number of CD8+ T cells had been primed to permit their rapid amplification to a level detectable 4 days after virus infection.
FIG. 4.
Immunity to systemic viral challenge is conferred within 1 week of DNA immunization. Groups of four BALB/c mice were immunized intramuscularly with 50 μg of pCMV or pCMVNP and challenged 1 week later with LCMV (2 × 105 PFU intraperitoneally). Four days postchallenge, splenocytes were assayed for LCMV-specific lytic responses in a standard 5-h chromium release assay at effector-to-target cell (E:T) ratios of 50:1 and 25:1 (A). Spleen samples from the same mice were also analyzed for infectious virus by plaque assay (B). Naive mice infected with LCMV 7 days before (d7) were included as positive controls for CTL-mediated lysis, and mice infected 4 days previously (d4) were included as controls to evaluate viral clearance. Each bar in panels A and B reflects the results for an individual mouse. To display the correlation between cytolytic activity and accelerated viral clearance, the bars in panel B are color coded; white and black bars represent animals which were positive and negative for CTL activity, respectively. Asterisks denote animals which were positive for CTL activity (and which, therefore, had been successfully infected) but in which virus was below the limit of detection by plaque assay (≤200 PFU/g of tissue). The horizontal bar in panel B represents a 90% reduction in virus titers at 4 days postinfection compared to titers in unvaccinated (d4) mice.
Protection from acute systemic viral challenge is conferred within 7 days of plasmid administration.
We next determined whether or not the DNA-induced CD8+ T cells revealed by the above assay were capable of rapidly controlling LCMV replication in vivo. Infectious virus present in the spleens of the same animals shown in Fig. 4A was quantitated by plaque assay, and the results are presented in Fig. 4B. High levels of infectious LCMV were present in the spleens of the two unvaccinated, infected control mice at 4 days postinfection (mean titer = 3.6 × 106 PFU/g). In contrast, virus was low to undetectable in the unvaccinated control mice analyzed 7 days postinfection; both of these mice showed clear evidence of antiviral lytic activity at this time point. Thus, as has been observed previously, cytolytic activity is associated with LCMV clearance. All four mice inoculated with vector alone (pCMV) showed evidence of unrestricted viral growth in the spleen at 4 days postchallenge, confirming that vaccination with the vector DNA itself provides no protective benefit against acute LCMV infection. In contrast, all of the mice vaccinated with pCMVNP 7 days before LCMV challenge exhibited a substantial decrease in virus titers compared both to the unvaccinated day 4 controls and to the pCMV vaccinees. Virus was below the limit of detection in two of four mice, and the remaining two mice had LCMV titers >99% lower than those in nonimmune mice.
Protection from lethal viral challenge is conferred within 1 week of plasmid administration.
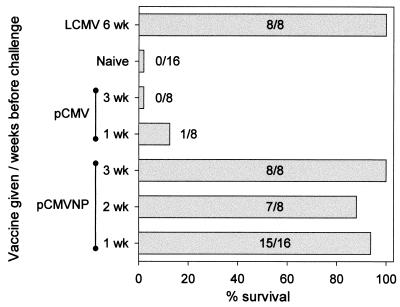
To determine how rapidly protective immunity is acquired against a normally lethal LCMV infection of the central nervous system, groups of mice were vaccinated intramuscularly with a single dose of pCM-VNP 1, 2, or 3 weeks prior to receiving a lethal intracranial dose of LCMV. As controls, groups of mice which were either unvaccinated (naive), inoculated 1 or 3 weeks previously with pCMV, or immunized 6 weeks previously with live LCMV (LCMV immune) were used. All mice were infected concurrently with 30 LD50 of LCMV intracranially, and mice were observed twice daily for 15 days. All deaths occurred between days 6 and 8. As shown in Fig. 5, immunization with live LCMV 6 weeks previously resulted in the acquisition of immunity which enabled all mice within this group to survive the lethal intracranial challenge. In contrast, none of the unvaccinated naive control mice survived beyond 8 days postinfection (0 of 16), and only one pCMV vaccinee survived (1 of 16), indicating that vaccination with the plasmid vector provides little or no benefit against intracranial LCMV challenge. In contrast, vaccination with pCMVNP as little as 1 week before challenge led to strong protection (15 of 16 mice) against a lethal dose of LCMV. Similar outcomes were observed in mice challenged 2 weeks (7 of 8) or 3 weeks (8 of 8) after pCMVNP immunization. Collectively, these data conclusively demonstrate that a single administration of a DNA vaccine can very rapidly prime biologically relevant immune responses which are capable of protecting against a lethal dose of virus.
FIG. 5.
Protection from a lethal virus challenge is conferred within 1 week of DNA vaccination. BALB/c mice were immunized intramuscularly with pCMV or pCMVNP at the indicated times before viral challenge. LCMV-nonimmune mice (naive) served as additional negative vaccine controls, and mice which had been infected systemically with LCMV 6 weeks prior to the assay (LCMV) constituted positive vaccine controls. On the day of challenge, all mice received an intracranial injection of a normally lethal dose of LCMV (30 LD50 = 6 PFU). Mice were observed for 15 days; all deaths occurred between days 6 and 8. Bars indicate the percentage of mice which survived, and for each group the number of mice surviving/total is shown.
DISCUSSION
Pathogenic viruses contribute significantly to human morbidity and mortality worldwide; HIV alone has caused >16 million deaths. Although available vaccines have allowed us to make significant inroads against viruses such as influenza virus, measles virus, and hepatitis B virus, these agents have stubbornly resisted our best efforts at control and cause millions of deaths each year. Recently, DNA vaccines have emerged as a possible candidate to augment or even supplant currently used vaccines. The benefits of this approach to vaccination are many (reviewed in references 5 and 9), and a number of clinical trials are being conducted to test their safety and effectiveness in humans (3, 4, 17, 23, 25, 29), despite the fact that much remains to be understood about their mechanisms of action. For example, the quantitative and qualitative assessments of CD8+ T-cell numbers and function published to date have employed in vivo or in vitro restimulation, which complicates data interpretation. Here, for the first time directly ex vivo, we establish the kinetics of a DNA-induced CD8+ T-cell response. We show that a single inoculation of plasmid DNA can induce CD8+ T cells which are detectable as early as days 7 to 11 postinoculation and which peak at day 15. By day 30, approximately 1 in every 150 splenic CD8+ T cells is antigen specific (Fig. 1), and this level is maintained for at least a year without boosting (10). Surprisingly, the number of antigen-specific T cells primed by DNA vaccination was not much lower than that primed by systemic infection with recombinant vaccinia virus, an extremely potent and effective means of eliciting protective anti-LCMV CTL responses. However, even at the peak of the T-cell response to the DNA vaccine, the number of antigen-specific T cells in the spleen was at least 30-fold lower than that seen at the peak of an LCMV infection (Fig. 1) (19), showing that standard DNA vaccination provides a much weaker stimulus to the immune system than that provided by a natural infection; thus, there remains considerable room for improvement.
Our use of a sensitive detection system allowed us to evaluate certain functional attributes of the DNA-induced CD8+ T cells. As we have recently noted (22), the antigen-specific T-cell population following viral infection comprises two discrete pools, defined by their cytokine expression profiles (IFN-γ+ TNF-α− and IFN-γ+ TNF-α+); but as the immune response progresses from the acute into the memory phase, cytokine expression patterns shift until the great majority of antigen-specific CD8+ T cells express both antiviral cytokines. Here we show that similar changes in cytokine expression profiles occur after DNA vaccination and after infection with a recombinant vaccinia virus (Fig. 2). The mechanism behind the regulation of this differential cytokine secretion pattern is under investigation, but it is clear that these changes do not require live virus infection.
As an additional criterion by which to compare DNA-induced and virus-induced cells, we evaluated functional avidity, replacing the traditional in vitro cytotoxicity readout with the more sensitive ICCS (Fig. 3). We found that CD8+ T cells induced by DNA had avidities identical to those observed in cells from virus-infected mice. Interestingly, the half-maximal ICCS response for day 7 LCMV cells was reached at a peptide concentration of 1 nM, precisely the concentration which provided half-maximal lysis when avidity was determined using an in vitro cytotoxicity assay (20). Thus, ICCS is a more sensitive approach which allows avidity assays to be carried out without secondary restimulation, and it provides a result consistent with earlier, less-sensitive methods. Taken together, the foregoing data clearly indicate that DNA immunization induces populations of protective CD8+ T cells which mimic, at least phenotypically, those cells induced by infection with the pathogen itself. These findings confirm and expand our previous analysis in which we found that, 1 year after immunization, DNA-induced and virus-induced CD8+ memory T cells showed similar IFN-γ production, intracellular perforin stores, and lytic potential (10).
Finally we show, using two different viral challenge models, that protective immunity is acquired within 7 days after a single administration of a DNA vaccine (Fig. 4 and 5). Protection correlated with the amplification of a population of antigen-specific CTL (Fig. 4), consistent with the requirement for CTL-mediated lysis in the clearance of LCMV (13, 28). Our observation that the virus-specific CD8+ T cells present at 7 days postinfection can protect against virus challenge despite being difficult to detect by ICCS is consistent with previous data (7, 15) and underscores the remarkable biological efficacy of these cells. These data raise the interesting possibility that DNA vaccines may be useful in combating rapidly evolving viral populations or in quickly conferring prophylactic immunity during viral epidemics with pandemic potential, such as influenza.
ACKNOWLEDGMENTS
We are grateful to Annette Lord for excellent secretarial support.
This work was supported by NIH grant AI-37186.
Footnotes
Manuscript no. 12958-NP from the Scripps Research Institute.
REFERENCES
- 1.Bot A, Shearer M, Bot S, Woods C, Limmer J, Kennedy R, Casares S, Bona C. Induction of antibody response by DNA immunization of newborn baboons against influenza virus. Viral Immunol. 1999;12:91–96. doi: 10.1089/vim.1999.12.91. [DOI] [PubMed] [Google Scholar]
- 2.Butz E A, Bevan M J. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calarota S, Bratt G, Nordlund S, Hinkula J, Leandersson A C, Sandstrom E, Wahren B. Cellular cytotoxic response induced by DNA vaccination in HIV-1-infected patients. Lancet. 1998;351:1320–1325. doi: 10.1016/S0140-6736(97)09440-3. [DOI] [PubMed] [Google Scholar]
- 4.Calarota S A, Leandersson A C, Bratt G, Hinkula J, Klinman D M, Weinhold K J, Sandstrom E, Wahren B. Immune responses in asymptomatic HIV-1-infected patients after HIV-DNA immunization followed by highly active antiretroviral treatment. J Immunol. 1999;163:2330–2338. [PubMed] [Google Scholar]
- 5.Donnelly J J, Ulmer J B, Liu M A. DNA vaccines. Life Sci. 1997;60:163–172. doi: 10.1016/s0024-3205(96)00502-4. [DOI] [PubMed] [Google Scholar]
- 6.Drunen Littel-van den Hurk S, Braun R P, Lewis P J, Karvonen B C, Babiuk L A, Griebel P J. Immunization of neonates with DNA encoding a bovine herpesvirus glycoprotein is effective in the presence of maternal antibodies. Viral Immunol. 1999;12:67–77. doi: 10.1089/vim.1999.12.67. [DOI] [PubMed] [Google Scholar]
- 7.Ehl S, Klenerman P, Zinkernagel R M, Bocharov G. The impact of variation in the number of CD8+ T-cell precursors on the outcome of virus infection. Cell Immunol. 1998;189:67–73. doi: 10.1006/cimm.1998.1344. [DOI] [PubMed] [Google Scholar]
- 8.Gallimore A, Glithero A, Godkin A, Tissot A C, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R M. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassett D E, Whitton J L. DNA immunization. Trends Microbiol. 1996;4:307–312. doi: 10.1016/0966-842x(96)10048-2. [DOI] [PubMed] [Google Scholar]
- 10.Hassett D E, Zhang J, Slifka M K, Whitton J L. Immune responses following neonatal DNA vaccination are long-lived, abundant, and qualitatively similar to those induced by conventional immunization. J Virol. 2000;74:2620–2627. doi: 10.1128/jvi.74.6.2620-2627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassett D E, Zhang J, Whitton J L. Plasmid DNA vaccines are effective in the absence of interferon-γ. Virology. 1999;263:175–183. doi: 10.1006/viro.1999.9957. [DOI] [PubMed] [Google Scholar]
- 12.Henke A, Wagner E, Whitton J L, Zell R, Stelzner A. Protection of mice against lethal coxsackievirus B3 infection by using DNA immunization. J Virol. 1998;72:8327–8331. doi: 10.1128/jvi.72.10.8327-8331.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen K J, Podack E R, Zinkernagel R M, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 14.Klavinskis L S, Barnfield C, Gao L, Parker S. Intranasal immunization with plasmid DNA-lipid complexes elicits mucosal immunity in the female genital and rectal tracts. J Immunol. 1999;162:254–262. [PubMed] [Google Scholar]
- 15.Klavinskis L S, Tishon A, Oldstone M B A. Efficiency and effectiveness of cloned virus-specific cytotoxic T lymphocytes in-vivo. J Immunol. 1989;143:2013–2016. [PubMed] [Google Scholar]
- 16.Lin M Y, Welsh R M. Stability and diversity of T cell receptor repertoire usage during lymphocytic choriomeningitis virus infection of mice. J Exp Med. 1998;188:1993–2005. doi: 10.1084/jem.188.11.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacGregor R R, Boyer J D, Ugen K E, Lacy K E, Gluckman S J, Bagarazzi M L, Chattergoon M A, Baine Y, Higgins T J, Ciccarelli R B, Coney L R, Ginsberg R S, Weiner D B. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J Infect Dis. 1998;178:92–100. doi: 10.1086/515613. [DOI] [PubMed] [Google Scholar]
- 18.Manickan E, Kanangat S, Rouse R J, Yu Z, Rouse B T. Enhancement of immune response to naked DNA vaccine by immunization with transfected dendritic cells. J Leukocyte Biol. 1997;61:125–132. doi: 10.1002/jlb.61.2.125. [DOI] [PubMed] [Google Scholar]
- 19.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez F, An L L, Harkins S, Zhang J, Yokoyama M, Widera G, Fuller J T, Kincaid C, Campbell I L, Whitton J L. DNA immunization with minigenes: low frequency of memory cytotoxic lymphocytes and inefficient antiviral protection are rectified by ubiquitination. J Virol. 1998;72:5174–5181. doi: 10.1128/jvi.72.6.5174-5181.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slifka M K, Rodriguez F, Whitton J L. Rapid on/off cycling of cytokine production by virus-specific CD8+ T cells. Nature. 1999;401:76–79. doi: 10.1038/43454. [DOI] [PubMed] [Google Scholar]
- 22.Slifka M K, Whitton J L. Activated and memory CD8+ T cells can be distinguished by their cytokine profiles and phenotypic markers. J Immunol. 2000;164:208–216. doi: 10.4049/jimmunol.164.1.208. [DOI] [PubMed] [Google Scholar]
- 23.Tacket C O, Roy M J, Widera G, Swain W F, Broome S, Edelman R. Phase 1 safety and immune response studies of a DNA vaccine encoding hepatitis B surface antigen delivered by a gene delivery device. Vaccine. 1999;17:2826–2829. doi: 10.1016/s0264-410x(99)00094-8. [DOI] [PubMed] [Google Scholar]
- 24.Thomson S A, Sherritt M A, Medveczky J, Elliott S L, Moss D J, Fernando G J, Brown L E, Suhrbier A. Delivery of multiple CD8 cytotoxic T cell epitopes by DNA vaccination. J Immunol. 1998;160:1717–1723. [PubMed] [Google Scholar]
- 25.Ugen K E, Nyland S B, Boyer J D, Vidal C, Lera L, Rasheid S, Chattergoon M, Bagarazzi M L, Ciccarelli R, Higgins T, Baine Y, Ginsberg R, MacGregor R R, Weiner D B. DNA vaccination with HIV-1 expressing constructs elicits immune responses in humans. Vaccine. 1998;16:1818–1821. doi: 10.1016/s0264-410x(98)00180-7. [DOI] [PubMed] [Google Scholar]
- 26.Ulmer J B, Donnelly J J, Parker S E, Rhodes G H, Felgner P L, Dwarki V J, Gromkowski S H, Deck R R, DeWitt C M, Friedman A, Hawe L A, Leander K R, Martinez D, Perry H C, Shiver J W, Montgomery D L, Liu M A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 27.von Herrath M G, Coon B, Oldstone M B A. Low-affinity cytotoxic T-lymphocytes require IFN-γ to clear an acute viral infection. Virology. 1997;229:349–359. doi: 10.1006/viro.1997.8442. [DOI] [PubMed] [Google Scholar]
- 28.Walsh C M, Matloubian M, Liu C C, Ueda R, Kurahara C G, Christensen J L, Huang M T, Young J D, Ahmed R, Clark W R. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci USA. 1994;91:10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang R, Doolan D L, Le T P, Hedstrom R C, Coonan K M, Charoenvit Y, Jones T R, Hobart P, Margalith M, Ng J, Weiss W R, Sedegah M, de Taisne C, Norman J A, Hoffman S L. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science. 1998;282:476–480. doi: 10.1126/science.282.5388.476. [DOI] [PubMed] [Google Scholar]
- 30.Whitton J L, Tishon A, Lewicki H, Gebhard J R, Cook T, Salvato M S, Joly E, Oldstone M B A. Molecular analyses of a five-amino-acid cytotoxic T-lymphocyte (CTL) epitope: an immunodominant region which induces nonreciprocal CTL cross-reactivity. J Virol. 1989;63:4303–4310. doi: 10.1128/jvi.63.10.4303-4310.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yokoyama M, Zhang J, Whitton J L. DNA immunization confers protection against lethal lymphocytic choriomeningitis virus infection. J Virol. 1995;69:2684–2688. doi: 10.1128/jvi.69.4.2684-2688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]