Abstract
Introduction
Aneurysmal subarachnoid hemorrhage (SAH) is more common in women than in men, contrary to most cardiovascular diseases. However, it is unclear whether the case fatality rate (CFR) of SAH also differs by sex. Thus, we performed a systematic review to address the relationship between sex and SAH CFRs.
Methods
We conducted a systematic literature search in PubMed, Scopus, and Cochrane library databases. We focused on population-based studies that included both nonhospitalized and hospitalized SAHs and had either reported 1-month (28–31 day) SAH CFRs separately for men and women or calculated risk estimates for SAH CFR by sex. For quality classification, we used the Cochrane Collaboration Handbook and Critical Appraisal Skills Program guidelines. We pooled the study cohorts and calculated relative risk ratios (RRs) with 95% confidence intervals (CIs) for SAH death between women and men using a random-effects meta-analysis model.
Results
The literature search yielded 5,592 initial publications, of which 33 study cohorts were included in the final review. Of the 33 study cohorts, only three reported significant sex differences, although the findings were contradictory. In the pooled analysis of all 53,141 SAH cases (60.3% women) from 26 countries, the 1-month CFR did not differ (RR = 0.99 [95% CI: 0.93–1.05]) between women (35.5%) and men (35.0%). According to our risk-of-bias evaluation, all 33 study cohorts were categorized as low quality. The most important sources of bias risks were related to the absence of proper confounding control (all 33 study cohorts), insufficient sample size (27 of 33 study cohorts), and poor/unclear diagnostic accuracy (27 of 33 study cohorts).
Conclusion
Contrary to SAH incidence rates, the SAH CFRs do not seem to differ between men and women. However, since none of the studies were specifically designed to examine the sex differences in SAH CFRs, future studies on the topic are warranted.
Keywords: Subarachnoid hemorrhage, Epidemiology, Sex differences, Case fatality, Systematic review
Introduction
Compared to other common cardiovascular diseases [1] and stroke types [2] with male predominance, aneurysmal subarachnoid hemorrhage (SAH) causes an exceptionally high burden among women due to increased rates of premature deaths [3] and disability-adjusted life-years [4]. Based on the consistent results of several worldwide systematic reviews [2, 5, 6], this disproportional burden may be attributed, at least partly, to the 30% higher SAH incidence among postmenopausal women as compared to age-matched men. However, less is known about the sex differences in the case fatality rates (CFRs) of SAH since previous studies have reported conflicting findings with varying study methods [7–12]. Moreover, we are not aware of previous systematic reviews on the topic. Therefore, we filled this gap by systematically reviewing previous studies with a focus on the sex differences in SAH CFRs. Specifically, we highlight the methodological challenges and differences of previous population-based studies including not only hospitalized cases but also sudden nonhospitalized SAH deaths that may contribute to as much as half of all fatal SAHs [13, 14]. We hypothesized that women have higher SAH CFRs than men, which could also explain their disproportional burden of SAH.
Methods
We performed this systematic review according to the checklist of Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [15].
Search Strategy
In line with general recommendations, we utilized the four-step PICO principle (Patient [aneurysmal SAH], Intervention/Risk factor [female sex], Comparison [male sex], Outcome [1-month CFR]) to formulate our study question, initial search term, and eligibility criteria. We conducted the systematic literature search using both index terms and keywords in three different databases: PubMed, Scopus, and Cochrane Library. To identify relevant keywords and index terms, we first performed a preliminary search in the PubMed database (exact search term presented in the online suppl. Material; for all online suppl. material, see https://doi.org/10.1159/000538562). Second, we screened the reference lists of the publications selected for full article review to reveal new studies along with additional keywords and index terms that were not included in our preliminary search. Third, an updated search was repeated by including the new keywords and index terms. This cross-checking method was continued until no further studies were identified, after which we conducted final searches in all three databases with a search term: (subarachnoid hemorrhage) AND (outcome OR survival OR mortality OR case-fatality OR “case fatality”) AND (sex OR gender). No language or time restrictions were imposed in the search. The first author (A.A.) reviewed all publications utilizing Rayyan QCRI [16] (a web application for study screening), after which two authors (A.A. and I.R.) independently reviewed all publications included in the full-text review of the final searches. In the event of disagreement, all authors reviewed these publications together to reach an agreement. The online supplementary Material presents a more detailed description of the search strategy.
Eligibility Criteria
Primary eligibility criteria included studies that reported 1-month (between 28 and 31 days) SAH CFRs for men and women separately, provided the number of SAH deaths and cases by sex, or calculated risk estimates (relative risk ratio [RR], hazard ratio, odds ratio [OR]) for SAH case fatality between women and men. Given that over 50% of fatal SAHs may occur before hospitalization [13, 14] and hospital-based studies do not take these deaths into account [17], we focused on studies comprising both hospitalized and nonhospitalized SAH cases. Moreover, if an included study did not provide a comprehensive description of study characteristics, we supplemented the data using other publications based on the same cohorts. In addition to excluding studies based only on hospitalized patients, we excluded all studies focusing on non-aneurysmal and secondary SAHs, SAHs occurring only in specific locations (e.g., cerebral convexity, fissures, or cisterns), as well as studies focusing only on selected patient subgroups such as children (<18 years old) or the elderly (>65 years old). The online supplementary material describes the study eligibility criteria in more detail.
Assessment of Study Quality
We assessed the risk of bias in the final included studies using the Cochrane Collaboration Handbook [18] and Critical Appraisal Skills Program [19]. Based on these guidelines, we formed four different domains with specific requirements to classify the included studies into low, unclear, or high-risk-of-bias categories by covering the most critical sources of biases: (1) sufficient sample size (type II error), (2) accuracy of case diagnostics (selection bias), (3) accuracy of outcome identification (detection bias), and (4) proper control of possible confounders (confounding). Our requirements for low-risk-of-bias classification in the four domains were respectively: (1) sufficient sample size to detect significant findings based on power analysis; (2) at least 80% of aneurysmal SAH cases verified via brain imaging (computed tomography angiogram or magnetic resonance angiography), positive lumbar puncture finding confirmed with angiography, surgery, autopsy or externally validated healthcare register (positive predictive value ≥ 80% for aneurysmal SAHs); (3) the proportion of sudden-death SAHs reported; and (4) risk estimates adjusted for age, smoking, and hypertension (well-known risk factors for overall SAH case fatality [20, 21] that differ between men and women [12, 22–28]). Finally, we categorized the included studies as low or high quality, based on the number of domains reaching the low-risk-of-bias classification. To be classified as high quality, all four domains had to fulfil the criteria for low risk of bias. A more detailed assessment of study quality and bases for the risk-of-bias classifications are described in the online supplementary material.
Statistical Analysis
To estimate the sample size sufficient for significant results, we performed a directional power analysis using standard values for statistical power (p = 0.80) and significance (p < 0.05). As an optimistic assumption, we estimated that women’s CFR is 1.25 times higher than men’s based on the SAH incidence difference by sex [29]. The reference CFR of men was set to 35% for power analysis calculations including the sudden-death SAHs [7, 13]. In addition, we used a random-effects meta-analysis model to calculate pooled RR estimates with 95% confidence intervals (CIs) for 1-month CFR between women and men. If a study did not report RRs, we computed them based on sex-specific CFRs. Moreover, we used I2-statistics to evaluate the level of heterogeneity (defined as low [I2 < 40%], considerable [I2 = 40–70%], or high [I2 > 70%]) [18], and a funnel plot to evaluate the risk of publication bias in previous findings. Additionally, we stratified the CFRs by region (primarily by countries and continents) and date (study midyear in 1960–1969, 1980–1989, 1990–1999, 2000–2009, or 2010–2019). We conducted all statistical analyses with Stata statistical software version 17.0 (Stata Corp, College Station, TX, USA).
Results
Literature Search
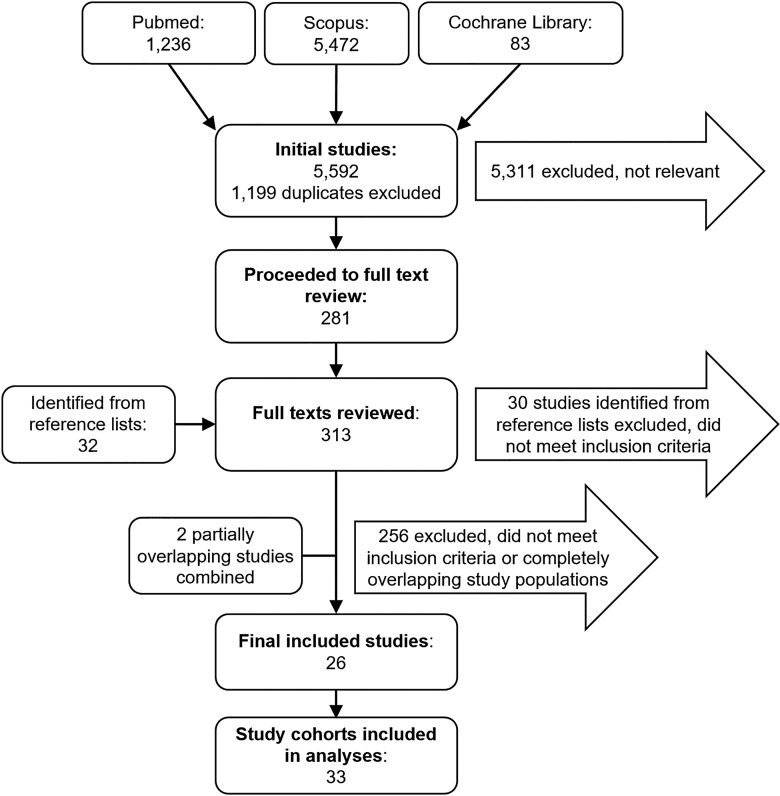
After excluding duplicate publications, our final search in three different databases yielded 5,592 articles, of which 281 (5%) were included in the full-text review. After screening the reference lists of the 281 publications, we found 32 additional articles for further review. Of the 313 fully reviewed articles, 35 (three of which were detected through reference lists screening) met the inclusion criteria. However, we excluded nine completely overlapping articles, and merged the data of two partially overlapping articles [30, 31], leaving a total of 26 articles [7, 9, 10, 12, 13, 22–24, 28, 30–46] with 33 study cohorts for further analyses (shown in Fig. 1). Detailed reasons for exclusions of the fully reviewed articles are presented in online supplementary Table 1.
Fig. 1.
Flowchart of the literature review.
Study Characteristics
The included studies were conducted in 26 countries across five continents (Table 1). The study periods ranged from 1949 to 2017, with 10 [13, 23, 24, 28, 30, 31, 40, 42, 43, 45, 46] out of 33 study cohorts having a study midyear in the 21st century (Table 1). Altogether, the 33 study cohorts included 53,141 SAH cases (median 244 cases per cohort), of which 39,942 (75.2%) came from three large register-based cohorts from Sweden [7], Finland [13], and Scotland [22]. Of the 24 study cohorts that reported sex-specific number of SAH cases, 30,779 out of 51,041 cases (60.3%) were women, and in 22 [7, 10, 12, 13, 22–24, 28, 30–32, 34–45] of these cohorts, women represented the majority of the SAH cases (Table 2). On average, women (mean age of 59.2 years old) were older than men (mean age of 54.1 years old) according to the seven study cohorts [10, 12, 13, 22, 28, 32, 35] that reported separate mean values of age for men and women (Table 1). In the four study cohorts that reported sex-specific prevalence of smoking [12, 23, 24, 28] or hypertension [12, 22, 23, 28] among SAH cases, smoking was more common in men, whereas hypertension was more common in women (Table 1).
Table 1.
Characteristics of the included study cohorts according to their publication year
| Author, year | Country | Midyear | Study period | SAH cases, n | Mean age, y, overall, (w/m) | Diagnosis confirmed*, % | Current smoking, % | Hypertension, % | ||
|---|---|---|---|---|---|---|---|---|---|---|
| women | men | women | men | |||||||
| Sacco et al., 1982 | USA | 1962 | 1949–1975 | 39 | 62.4 | . . . | . . . | . . . | . . . | . . . |
| Jakovljević et al., 1996 | Finland | 1987 | 1983–1992 | 1,105 | . . . | 80–97a | . . . | . . . | . . . | . . . |
| Truelsen et al., 1998 | New Zealand | 1987 | 1981–1993 | 342 | (54.1/48.8) | 92 | . . . | . . . | . . . | . . . |
| Kimura et al., 1998 | Japan | 1989 | 1989 | 339 | (60.7/55.0) | 99b | . . . | . . . | . . . | . . . |
| Ingall et al., 2000 | Multinationalc | |||||||||
| Denmark | 1986 | 1982–1991 | 198 | . . . | 95–99a,d | . . . | . . . | . . . | . . . | |
| Germany | 1986 | 1984–1989 | 125 | . . . | 16–68a,d | . . . | . . . | . . . | . . . | |
| China | 1988 | 1984–1993 | 85 | 51.4 | 86–100a,d | . . . | . . . | . . . | . . . | |
| Italy | 1988 | 1984–1993 | 423 | . . . | 95–99a,d | . . . | . . . | . . . | . . . | |
| Sweden | 1989 | 1984–1994 | 244 | . . . | 91–98a,d | . . . | . . . | . . . | . . . | |
| Poland | 1989 | 1984–1994 | 306 | 48.6 | 25–43a,d | . . . | . . . | . . . | . . . | |
| Russia | 1989 | 1985–1993 | 276 | . . . | 2–30a,d | . . . | . . . | . . . | . . . | |
| Serbia | 1989 | 1983–1995 | 128 | . . . | 58–80a,d | . . . | . . . | . . . | . . . | |
| Lithuania | 1990 | 1986–1995 | 215 | . . . | 33–83a,d | . . . | . . . | . . . | . . . | |
| Pobereskin et al., 2001 | UK | 1994 | 1992–1996 | 800 | (63/57) | 99 | . . . | . . . | . . . | . . . |
| Zhang et al., 2003 | China | 1998 | 1996–2000 | 147 | . . . | 92b | . . . | . . . | . . . | . . . |
| Stegmayr et al., 2004 | Sweden | 1992 | 1985–2000 | 984 | . . . | 98a | . . . | . . . | . . . | . . . |
| Khan et al., 2005 | Sweden | 1993 | 1989–1998 | 197 | . . . | 99a | . . . | . . . | . . . | . . . |
| Labovitz et al., 2006 | USA | 1995 | 1993–1997 | 54 | 51 | 85a | . . . | . . . | . . . | . . . |
| Kozák et al., 2007 | Japan | 1993 | 1989–1998 | 3,257 | (64.4/58.2) | ≥85 | 11.8 | 70.7 | 51.1 | 44.5 |
| Koffijberg et al., 2008 | Sweden | 1994 | 1987–2002 | 18,443 | 58.9 | 96PPV,b | . . . | . . . | . . . | . . . |
| Vadikolias et al., 2009 | Greece | 2003 | 2001–2005 | 51 | 59 | 100a | . . . | . . . | . . . | . . . |
| Alvarez et al., 2010 | Chile | 2001 | 2000–2003 | 33 | 50.7 | 97 | . . . | . . . | . . . | . . . |
| Macpherson et al., 2011 | Scotland | 1995 | 1986–2005 | 12,056 | (56.7/52.0) | 95PPV,a | . . . | . . . | 9.1d | 8.1d |
| Sandvei et al., 2011 | Norway | 1995 | 1984–2007 | 214 | 58.6 | 100 | . . . | . . . | . . . | . . . |
| González-Pérez et al., 2013 | UK | 2004 | 2000–2008 | 1,303 | 57.7 | 91a | . . . | . . . | . . . | . . . |
| Olindo et al., 2014 | France (Caribbean) | 2005 | 1999, 2012 | 37 | . . . | 97b | . . . | . . . | . . . | . . . |
| Madsen et al., 2017 and 2020 | USA | 2004 | 1993–2015 | 443 | . . . | 95# | . . . | . . . | . . . | . . . |
| Guéniat et al., 2018 | France | 1999 | 1987–2012 | 161 | . . . | 90–98b | . . . | . . . | . . . | . . . |
| Takashima et al., 2018 | Japan | 2011 | 2011 | 201 | 65.6 | 97a | 16.9 | 49.0 | 57.9 | 50.9 |
| Chen et al., 2020 | China | 2010 | 2004–2017 | 702 | . . . | 92b | 5.3 | 60.4 | . . . | . . . |
| Lavados et al., 2021 | Chile | 2015 | 2015–2016 | 47 | 57.4 | ≥64b | . . . | . . . | . . . | . . . |
| Rehman et al., 2022 | Multinational | 2005 | 1994–2017 | 643 | (60.2/51.8) | 100a | 26.2 | 31.3 | 52.3 | 50.0 |
| Asikainen et al., 2023 | Finland | 2007 | 1998–2017 | 9,443 | (60.4/55.4) | 97–100PPV,** | . . . | . . . | . . . | . . . |
aOf all SAHs (i.e., not specifically aneurysmal SAHs confirmed).
bOf all strokes.
cFinland and Northern Sweden removed due to completely overlapping study populations with studies by Jakovljević et al. [9] and Stegmayr et al. [37], respectively.
dOnly for hospitalized SAH patients.
#During the years 1993–2010.
*Aneurysmal SAH diagnosis confirmed by imaging (CT or MRI), autopsy, or positive lumbar puncture finding combined with angiography, surgery, or autopsy. Positive predictive value (PPV) (%) for register-based studies.
**Hospitalized aneurysmal SAHs and fatal SAHs validated separately with 99.8% and 97% PPVs, respectively.
Table 2.
Subarachnoid hemorrhage (SAH) case fatality rates (CFRs) and risk ratios (RRs) for death in women versus men (reference group) with 95% confidence intervals (CIs)
| Author, year | SAH cases, n | CFR-timepoint, d | CFR, % | Unadjusted RR (95% CI) | Adjusted RR (95% CI) | Sudden death, %, overall, (w/m) | ||
|---|---|---|---|---|---|---|---|---|
| women | men | women | men | |||||
| Sacco et al., 1982 | 23 | 16 | 30 | 39.1 | 56.3 | 0.70 (0.36–1.36) | . . . | . . . |
| Jakovljević et al., 1996 | 537 | 568 | 28 | 44.3 | 46.7 | 0.95 (0.83–1.08) | . . . | . . . |
| Truelsen et al., 1998 | 220 | 122 | 28 | 50.9 | 47.5 | 1.07 (0.85–1.34) | . . . | 15.2 |
| Kimura et al., 1998 | 232 | 107 | 28 | 28.0 | 35.5 | 0.79 (0.57–1.10) | . . . | 2.1 |
| Ingall et al., 2000 | ||||||||
| Denmark | 114* | 84* | 28 | 41a | 41a | . . . | 1.02 (0.73–1.43)b | 0.0 |
| East Germany | 112* | 113* | 28 | 42a | 44a | . . . | 0.93 (0.68–1.26)b | 5.8 |
| China | 33* | 52* | 28 | 13a | 28a | . . . | 0.42 (0.15–1.16)b | 0.0 |
| Italy | 212* | 211* | 28 | 37a | 37a | . . . | 1.01 (0.79–1.28)b | 2.8 |
| Sweden | 146* | 98* | 28 | 31a | 34a | . . . | 0.91 (0.63–1.30)b | 8.6 |
| Poland | 138* | 168* | 28 | 44a | 39a | . . . | 1.14 (0.87–1.49)b | 0.7 |
| Russia | 117* | 159* | 28 | 57a | 60a | . . . | 0.97 (0.79–1.18)b | 15.9 |
| Yugoslavia | 71* | 57* | 28 | 56a | 64a | . . . | 0.86 (0.67–1.12)b | 31.3 |
| Lithuania | 116* | 99* | 28 | 44a | 60a | . . . | 0.74 (0.57–0.96)b | 10.7 |
| Pobereskin et al., 2001 | 516 | 284 | 30 | 46.5 | 41.6 | 1.12 (0.95–1.32) | 1.15 (0.84–1.57)c | 16 |
| Zhang et al., 2003 | 84 | 63 | 28 | 32.1 | 34.9 | 0.92 (0.58–1.46) | . . . | . . . |
| Stegmayr et al., 2004 | 592 | 392 | 28 | 35.1 | 36.5 | 0.96 (0.81–1.14) | . . . | 10.8 |
| Khan et al., 2005 | 122 | 75 | 28 | 25.4 | 13.3 | 1.91 (0.99–3.66) | . . . | . . . |
| Labovitz et al., 2006 | 33 | 21 | 30 | 36.4 | 14.3 | 2.55 (0.81–7.96) | . . . | 1.9 |
| Kozák et al., 2007 | 2,111 | 1,146 | 28 | 26.8 | 26.5 | 1.01 (0.90–1.14) | . . . | . . . |
| Koffijberg et al., 2008 | 10,858 | 7,585 | 28 | 32.5 | 30.5 | 1.07 (1.02–1.11) | 1.06 (1.02–1.11)d | 11.5 |
| Vadikolias et al., 2009 | 23 | 28 | 28 | 34.8 | 35.7 | 0.97 (0.46–2.06) | . . . | 5.9 |
| Alvarez et al., 2010 | 19 | 14 | 30 | 42.1 | 71.4 | 0.59 (0.32–1.10) | . . . | (5.3/42.9) |
| Macpherson et al., 2011 | 7,637 | 4,419 | 30 | 41.2 | 39.6 | 1.04 (0.99–1.09) | . . . | (15.7/16.8) |
| Sandvei et al., 2011 | 143 | 71 | 30 | . . . | . . . | . . . | 1.00 (0.60–1.90)c,# | . . . |
| González-Pérez et al., 2013 | 816 | 487 | 30 | 28.6 | 29.0 | 0.99 (0.83–1.18) | . . . | . . . |
| Olindo et al., 2014 | 25 | 12 | 30 | 16.0 | 33.3 | 0.48 (0.14–1.60) | . . . | . . . |
| Madsen et al., 2017 and 2020 | 303 | 140 | 30 | 31.0 | 24.3 | 1.28 (0.91–1.79) | . . . | 4.5** |
| Guéniat et al., 2018 | 93 | 68 | 30 | 22.6 | 16.2 | 1.40 (0.72–2.70) | . . . | . . . |
| Takashima et al., 2018 | 140 | 61 | 28 | 30.7 | 36.1 | 0.85 (0.56–1.29) | . . . | . . . |
| Chen et al., 2020 | 437 | 265 | 28 | 22 | 16 | 1.39 (1.00–1.93) | . . . | . . . |
| Lavados et al., 2021 | 35 | 12 | 30 | 51.4 | 41.7 | 1.23 (0.59–2.59) | . . . | 13.6e |
| Rehman et al., 2022 | 346 | 297 | 28–30 | 36.4 | 30.0 | 1.28 (0.97–1.70) | . . . | . . . |
| Asikainen et al., 2023 | 5,434 | 4,009 | 30 | 37.7 | 41.6 | 0.91 (0.86–0.95) | 0.79 (0.75–0.83)f | (22.6/25.4) |
aAge-adjusted to Segi’s world population.
bRisk ratios calculated with approximated sex-specific number of SAHs and age-adjusted CFRs.
cAdjusted for age.
dAdjusted for age, geographical region, and time period.
eOf all strokes.
fAdjusted for age and time period.
#Odds ratio.
*Sex-specific number of SAHs was approximated using sex-specific weights from a random-effects meta-analysis model.
**During the years 1993–2010.
Case Fatality Rates of SAH
Overall, 15 study cohorts [7, 12, 22, 24, 28, 30–33, 35, 38, 39, 44, 45] reported higher 1-month CFRs for women, 17 study cohorts [9, 10, 13, 23, 33, 34, 36, 37, 40, 42, 43, 46] reported higher 1-month CFRs for men, and one study cohort [41] reported identical 1-month CFRs for women and men (Table 2). However, only three study cohorts found significant CFR differences by sex; a Swedish cohort [7] reported an increased risk of death in women (unadjusted RR of women = 1.07 [95% CI: 1.02–1.11]), whereas the Finnish [13] and Lithuanian [33] cohorts found an increased risk of death in men (unadjusted and adjusted RR of women = 0.91 [0.86–0.95] and 0.74 [0.57–0.96], respectively) (Table 2). In total, the CFRs ranged from 13.0% [33] to 51.4% [45] among women and from 13.3% [38] to 71.4% [40] among men (Table 2). All three [13, 22, 40] study cohorts that reported sex-specific proportions of sudden deaths observed higher sudden-death rates in men than in women, even though the difference was only significant in cohorts from Finland [13] (men 25.4% vs. 22.6% women; age, sex, and study-year-adjusted RR = 1.30 [1.20–1.41]) and Chile [40] (men 42.9% vs. women 5.3%; unadjusted RR = 8.14 [1.10–60.25]), but not in a cohort from Scotland [22] (men 16.7% vs. 15.8%; unadjusted RR = 1.07 [0.98–1.16]) (Table 2).
Study Quality and Power Analysis
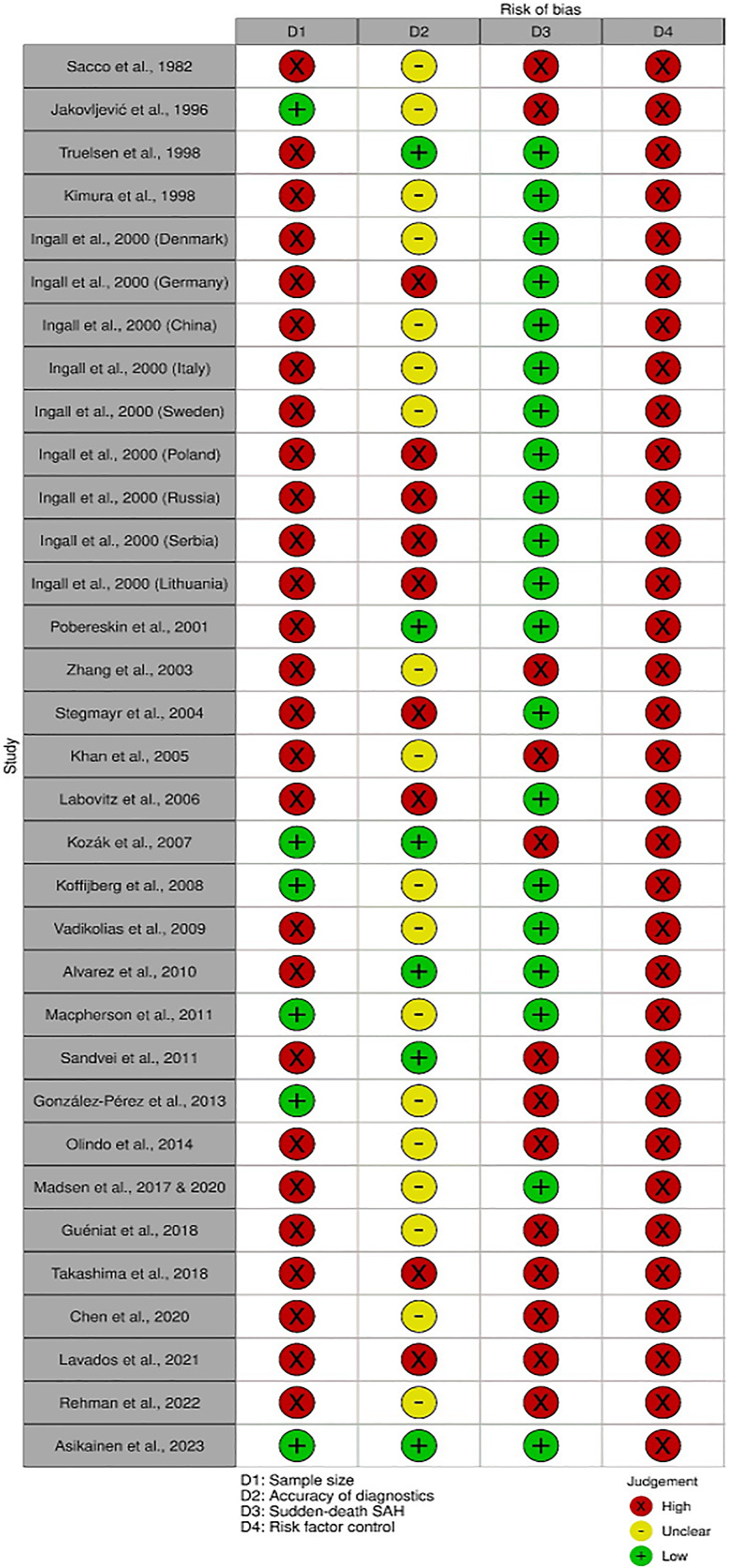
Of the 33 included study cohorts, none were specifically designed to examine the SAH CFR differences between men and women. Accordingly, none of the study cohorts reached the low-risk-of-bias classification in all four domains, thus all 33 included study cohorts were classified as low quality (shown in Fig. 2). The main reason for the low quality was the absence of sufficient confounder control for age, smoking, and hypertension (all 33 study cohorts) (shown in Fig. 2). In fact, only four studies reported any covariate-adjusted risk estimates for SAH death between women and men. Specifically, the Swedish cohort [7] adjusted the analyses for age, geographical region, and time period (RR of women = 1.06 [95% CI: 1.02–1.11]), the Finnish cohort [13] adjusted for age and time period (RR of women = 0.79 [0.75–0.83]), and the UK [35] and Norwegian [41] cohorts adjusted for patients’ age (RR and OR of women = 1.15 [0.84–1.57] and 1.00 [0.60–1.90], respectively). Regarding our power analysis, we calculated that the minimum number of SAH cases needed to detect a significant difference between men and women with 80% power was 976 (488 cases per sex). Accordingly, only six [7, 9, 12, 13, 22, 42] of the 33 study cohorts met this criterion (shown in Fig. 2). Besides the low number of study cohorts with sufficient confounder control and sample size, other factors compromising study quality included not reporting sudden-death rates (13 [9, 12, 23, 24, 28, 34, 36, 38, 41–45] out of 33 study cohorts) and poor/unclear diagnostic accuracy (27 [7, 9, 10, 22–24, 28, 30, 31, 33, 34, 36–39, 42–46] out of 33 study cohorts) (shown in Fig. 2).
Fig. 2.
Risk-of-bias classification by domain.
Pooled Analysis
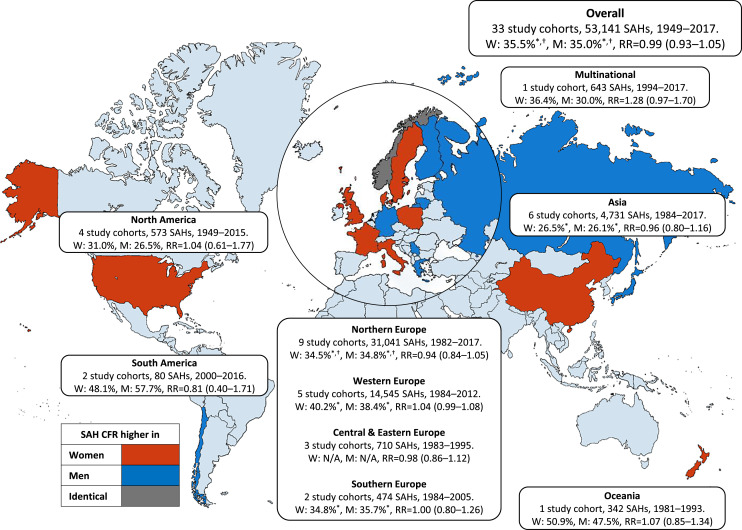
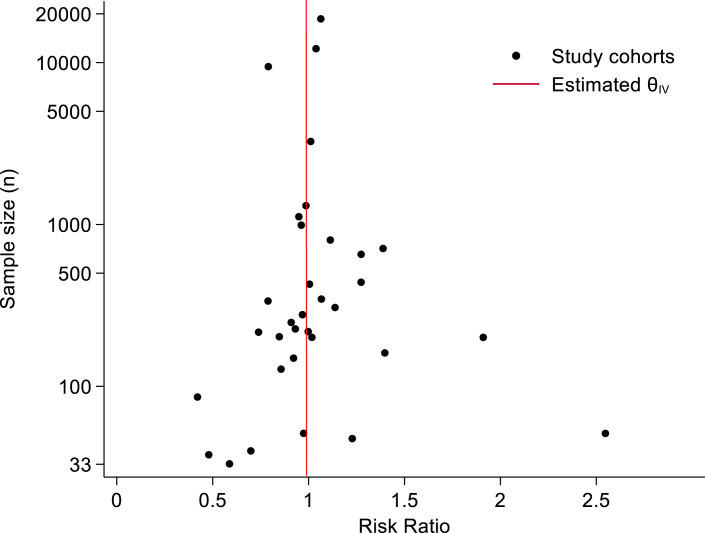
In the pooled analysis of the 33 low-quality study cohorts, there was no significant difference in 1-month CFRs between women (35.5%) and men (35.0%) (RR = 0.99 [0.93–1.05]) (shown in Fig. 3). Heterogeneity was considerable (I2 = 64.1%) between the individual study cohorts (online suppl. Fig. 1). The CFRs did not differ between women and men after calculating covariate-adjusted (RR = 0.94 [0.86–1.03]) and unadjusted (RR = 1.02 [0.97–1.07]) risk estimates separately (online suppl. Fig. 2–3). By region, point-estimate CFRs of women were slightly higher in cohorts from Europe (women 36.4% vs. men 35.9%) [7, 9, 13, 22, 35, 37, 38, 42, 44, 46], Asia (women 26.5% vs. men 26.1%) [10, 12, 23, 24, 36], North America (women 31.0% vs. men 26.5%) [30, 31, 34, 39, 43], and Oceania (women 50.9% vs. men 47.5%) [32], and slightly lower in South American cohorts (women 48.1% vs. men 57.7%) [40, 45]; none of these differences were statistically significant (shown in Fig. 3). When analyzed by study period, the point estimates of CFRs were lower in the more recent decades for both sexes, but with no significant trend in the CFR differences between women and men over time (online suppl. Table 2) (online suppl. Fig. 4). Lastly, based on the symmetrical funnel plot, there was no clear evidence of publication bias (shown in Fig. 4).
Fig. 3.
Pooled subarachnoid hemorrhage (SAH) case fatality rates (CFRs) and risk ratios (RRs) for death in women versus men (reference group) with 95% CIs by study region. Pooled risk ratios were calculated with a random-effects meta-analysis model. For the forest plot, see online supplementary Figure 1. Europe was further divided into subregions according to the Publications Office of the European Union (https://op.europa.eu/en/home. Accessed May 10, 2023) due to the large number of studies and differences in population structures. Continents, countries, and covered regions: (1) Northern Europe (Finland [Nationwide, Kuopio, North Karelia, and Turku/Loimaa], Sweden [Nationwide, Umeå, Göteborg, and Malmö], Norway [Nord-Trøndelag and Tromsø], Denmark [Glostrup], Lithuania [Kaunas]). (2) Western Europe (The UK [Nationwide and Devon/Cornwall], France [Dijon]). (3) Southern Europe (Italy [L’Aquila and Friuli], Greece [Evros]). (4) Central and Eastern Europe (Poland [Warsaw], Serbia [Novi Sad], and Russia [Moscow]). (5) North America (The US [Northern Manhattan, Greater Cincinnati/Northern Kentucky, and Framingham], French West [Martinique]). (6) South America (Chile [Aconcagua Valley and Ñuble]). (7) Asia (China [Middle/Eastern China and Beijing], Japan [Okinawa, Akita, and Shiga]). (8) Oceania (New Zealand [Auckland]). (9) Multinational (Argentina [Tandil], Brazil [Joinville and Matão], French West [Martinique], Sweden [Orebro], Estonia [Tartu], Portugal [Porto], Italy [L’Aquila], New Zealand [Auckland], Australia [Melbourne], India [Ludhiana], Iran [Mashhad], no individual CFRs for countries). * Ingall et al. [33] removed due to lack of sex-specific numbers of fatal and total SAHs. † Sandvei et al. [41] removed due to lack of sex-specific number of fatal SAHs.
Fig. 4.
Funnel plot of risk ratio for 1-month SAH case fatality in women versus men (reference group).
Discussion
The 1-month CFRs of SAH were similar between women and men, based on a pooled analysis of 33 study cohorts including over 50,000 SAH cases from 26 different countries. The 33 study cohorts were selected based on the systematic review of nearly 6,000 screened articles. In fact, the number of study cohorts reporting slightly but nonsignificantly higher CFRs in men (15/33) was almost the same as the number of study cohorts reporting opposite findings (14/33). Moreover, the three study cohorts [7, 13, 33] that reported significant CFR differences between women and men found contradicting results. As a possible cause of this heterogeneity, all included study cohorts were classified as low quality according to our risk-of-bias evaluation, and none of the studies were specifically designed to address the sex differences in SAH CFRs. In short, it seems unlikely that the CFRs of SAH would differ as much as the incidence rates [2, 5, 6] between women and men. Therefore, the effects of CFR differences on women’s exceptional SAH burden may be modest. Nevertheless, high-quality studies with an adequate SAH identification, sufficient sample size, inclusion of outside-hospital sudden deaths, and control of the most important confounders are still warranted.
According to our risk-of-bias evaluation, none of the studies adjusted their between-sex CFR comparisons for age, smoking and hypertension, even though all these factors have been reported to differ by sex and are associated with an increased risk of death after SAH. Specifically, the mean age [8, 10, 12, 13, 22, 32, 35] and the prevalence of hypertension [12, 22, 23, 25] are predominantly reported to be higher in women, while male SAH patients are more often smokers [12, 23, 24, 27]. Hence, the possible confounding of these three factors would be important to control in future studies investigating the independent sex differences of SAH CFRs. For example, the contradictory findings in the nationwide Swedish [7] (higher CFR in women) and Finnish [13] (higher CFR in men) cohorts may partly be attributed to Swedish women having higher smoking rates [28] and Finnish women having substantially lower rates [47] compared to male SAH patients. Furthermore, a comprehensive confounder control in future studies would likely require an even larger sample size than estimated in our optimistic power analysis. In addition, because less fatal non-aneurysmal SAHs (i.e., traumatic [48, 49], perimesencephalic [50, 51], and non-perimesencephalic SAHs [50, 51]) appear to be more common among men, future studies should aim for more accurate confirmation of aneurysmal SAHs to prevent reporting falsely low CFRs for men. Lastly, since men were reported to have higher sudden-death rates than women [13, 22, 40], studies without comprehensive case identification through routine post-mortem examinations [17] may underestimate the overall SAH CFRs of men. Indeed, this may further explain why in Finland – where the autopsy rate for sudden deaths is higher than in Sweden [52] – men had higher overall CFRs than women [13] and not the opposite [7].
In agreement with our findings of population-based cohorts, previous hospital-based studies have neither found significant sex differences in the short-term CFRs [11, 53, 54] nor functional outcomes [11, 53, 55] of hospitalized SAH patients. Furthermore, symptoms of SAH [56], neurological condition on admission [21, 56, 57], delay of admission/treatment [53, 55, 56], aneurysm size [55, 58], aneurysm location in anterior versus posterior circulation [53, 55, 59], and the amount of bleeding [11, 53, 60] have been reported to be similar in women and men. Likewise, the treatment approach (i.e., neurosurgical clipping, endovascular coiling, or conservative treatment [11, 27, 55, 61]) and the occurrences of important SAH complications, such as rebleeding [11, 62], delayed cerebral ischemia, [63] and hydrocephalus [11, 25, 55] do not appear to differ by sex. Our results are also in line with a recent meta-analysis [64] that reported no sex differences in unadjusted CFRs for ischemic stroke (IS) and intracerebral hemorrhage (ICH). However, after adjusting the analyses for possible confounders (e.g., age, hypertension, and smoking), the included high-quality studies revealed lower CFRs for IS in women [65, 66], which emphasizes the need for such investigations on SAH. In terms of incidence rates, studies on IS [2, 67, 68] and ICH [2, 68, 69] have largely reported male predominance, while females’ higher SAH incidence [2, 5, 6] (linked to greater risk of aneurysm formation [70] and rupture [60]) likely contributes to their disproportionate premature mortality [3] and morbidity [4]. Taken together, differences in incidence seem to account for most of the sex gap in the burden of SAH, highlighting the importance of primary prevention in attenuating these sex disparities in the future. Notably, the absence of high-quality primary analyses on the presence of sex differences in SAH CFRs warrants further research on this topic.
Based on our comprehensive literature search in three different databases, this is the first systematic review that investigates the quantity and quality of previous studies on the SAH CFR differences between men and women. However, this study also has some limitations. First, we used strict criteria in our quality assessment, which classified all study cohorts as low-quality despite the considerable differences in bias risks between studies. This conservative approach was taken because the absence of even one of the selected critical low-risk-of-bias criteria may compromise the quality necessary for robust conclusions. Second, the small number of study cohorts, especially from low- and middle-income countries [24, 33, 36, 40, 45], further limits the generalizability of our findings. However, given the large between [71] and within-country [72] variation in SAH CFRs, the main goal of our review was not to provide definitive worldwide sex-specific CFRs, but rather to recognize common methodological challenges and provide new considerations for future studies. Third, some of the missing data could have been retrieved by contacting the corresponding author of previous studies. However, most of the included and excluded studies were quite old, and retrospective data sharing for external researchers has become more difficult due to evolved data protection regulations. Therefore, we decided to only request missing data for a single recent study [28]. Even if we had received such missing data for other studies (e.g., sex-specific sudden-death rates from New Zealand [32] or Sweden [7]), it is unlikely that any included or excluded studies would have reached the high-quality classification or substantially changed our conclusions. Lastly, although we used three different publication databases and did not impose any language or time restrictions, our literature search may have missed some non-English and unpublished articles, or articles that have focused on strokes in general and only reported SAH-specific findings as supplementary without targeted keywords or index terms. On the other hand, we believe it is unlikely that such studies that fulfil our strict inclusion and high-quality criteria would have been left unpublished as a separate international publication.
Conclusion
Since women and men seem to have similar CFRs after SAH, women’s exceptional SAH burden may arise from their increased incidence rates. However, because high-quality studies on sex-specific CFR differences are lacking, future studies with proper confounder control, sufficient sample size, and accurate diagnostics of hospitalized and nonhospitalized SAH events are still needed.
Acknowledgments
We thank Jacquelin De Faveri for the language revision and Dr. Sabah Rehman from the INSTRUCT project for providing unpublished data on sex-specific SAH CFRs.
Statement of Ethics
Institutional ethics approval and participant consent were not required as this study was based on published data and therefore did not involve human participants.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
A.A. has been supported by the Juho Vainio Foundation, the Päivikki and Sakari Sohlberg Foundation, the Finnish Brain Foundation, the Finnish Medical Foundation, the Biomedicum Helsinki Foundation, the Maire Taponen Foundation, the Orion Research Foundation, the Paavo Nurmi Foundation, and the Paulo Foundation. I.R. has received personal research grants from the Sigrid Juselius Foundation, the Finnish Medical Foundation, the Sakari Alhopuro Foundation, the Finnish Foundation for Cardiovascular Research, and the Maud Kuistila Foundation. J.K. has been supported by the Academy of Finland (Grant # 352792). The funders had no role in the design and conduct of the study; in collection, management, analysis, and interpretation of the data; or in preparation, review, or approval of the manuscript.
Author Contributions
Study concept and design: A.A., M.K., J.K., and I.R.; acquisition, analysis, or interpretation of data: A.A. and I.R.; drafting the manuscript and statistical analysis: A.A. and I.R.; critical revision of the manuscript for important intellectual content and study supervision: M.K., J.K., and I.R.; and administrative, technical, or material support: n/a.
Funding Statement
A.A. has been supported by the Juho Vainio Foundation, the Päivikki and Sakari Sohlberg Foundation, the Finnish Brain Foundation, the Finnish Medical Foundation, the Biomedicum Helsinki Foundation, the Maire Taponen Foundation, the Orion Research Foundation, the Paavo Nurmi Foundation, and the Paulo Foundation. I.R. has received personal research grants from the Sigrid Juselius Foundation, the Finnish Medical Foundation, the Sakari Alhopuro Foundation, the Finnish Foundation for Cardiovascular Research, and the Maud Kuistila Foundation. J.K. has been supported by the Academy of Finland (Grant # 352792). The funders had no role in the design and conduct of the study; in collection, management, analysis, and interpretation of the data; or in preparation, review, or approval of the manuscript.
Data Availability Statement
The first author takes full responsibility for all data, analyses, and interpretations, as well as has access to all the data and has the right to publish any and all data separate and apart from any sponsor. Contact Aleksanteri Asikainen (aleksanteri.asikainen@helsinki.fi) for more details about the study protocol, our data, and analyses.
Supplementary Material.
Supplementary Material.
References
- 1. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation. 2019;139(10):e56–28. [DOI] [PubMed] [Google Scholar]
- 2. Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40(4):1082–90. [DOI] [PubMed] [Google Scholar]
- 3. Rautalin I, Kaprio J, Korja M. Burden of aneurysmal subarachnoid haemorrhage deaths in middle-aged people is relatively high. J Neurol Neurosurg Psychiatry. 2021;92(5):563–5. [DOI] [PubMed] [Google Scholar]
- 4. GBD 2019 Stroke Collaborators . Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Etminan N, Chang HS, Hackenberg K, De Rooij NK, Vergouwen MDI, Rinkel GJE, et al. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and meta-analysis. JAMA Neurol. 2019;76(5):588–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Rooij NK, Linn FH, van der Plas JA, Algra A, Rinkel GJ. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry. 2007;78(12):1365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koffijberg H, Buskens E, Granath F, Adami J, Ekbom A, Rinkel GJE, et al. Subarachnoid haemorrhage in Sweden 1987-2002: regional incidence and case fatality rates. J Neurol Neurosurg Psychiatry. 2008;79(3):294–9. [DOI] [PubMed] [Google Scholar]
- 8. Sacco S, Totaro R, Toni D, Marini C, Cerone D, Carolei A. Incidence, case-fatalities and 10-year survival of subarachnoid hemorrhage in a population-based registry. Eur Neurol. 2009;62(3):155–60. [DOI] [PubMed] [Google Scholar]
- 9. Jakovljević D, Salomaa V, Sivenius J, Tamminen M, Sarti C, Salmi K, et al. Seasonal variation in the occurrence of stroke in a Finnish adult population: the FINMONICA Stroke Register. Stroke. 1996;27(10):1774–9. [DOI] [PubMed] [Google Scholar]
- 10. Kimura Y, Takishita S, Muratani H, Kinjo K, Shinzato Y, Muratani A, et al. Demographic study of first-ever stroke and acute myocardial infarction in Okinawa, Japan. Intern Med. 1998;37(9):736–45. [DOI] [PubMed] [Google Scholar]
- 11. Duijghuisen JJ, Greebe P, Nieuwkamp DJ, Algra A, Rinkel GJ. Sex-related differences in outcome in patients with aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2016;25(8):2067–70. [DOI] [PubMed] [Google Scholar]
- 12. Kozák N, Hayashi M. Trends in the incidence of subarachnoid hemorrhage in Akita Prefecture, Japan. J Neurosurg. 2007;106(2):234–8. [DOI] [PubMed] [Google Scholar]
- 13. Asikainen A, Korja M, Kaprio J, Rautalin I. Case fatality in patients with aneurysmal subarachnoid hemorrhage in Finland: a nationwide register-based study. Neurology. 2023;100(3):e348–56. [DOI] [PubMed] [Google Scholar]
- 14. Korja M, Lehto H, Juvela S, Kaprio J. Incidence of subarachnoid hemorrhage is decreasing together with decreasing smoking rates. Neurology. 2016;87(11):1118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred Reporting Items for Systematic review and Meta-Analysis Protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- 16. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Korja M, Kaprio J. Controversies in epidemiology of intracranial aneurysms and SAH. Nat Rev Neurol. 2016;12(1):50–5. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane; 2022. [online]. www.training.cochrane.org/handbook. [Google Scholar]
- 19. Harrison JK, Reid J, Quinn TJ, Shenkin SD. Using quality assessment tools to critically appraise ageing research: a guide for clinicians. Age Ageing. 2017;46(3):359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lindbohm JV, Kaprio J, Korja M. Survival bias explains improved survival in smokers and hypertensive individuals after aSAH. Neurology. 2019;93(23):E2105–e2109. [DOI] [PubMed] [Google Scholar]
- 21. Rosengart AJ, Schultheiss KE, Tolentino J, Macdonald RL. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38(8):2315–21. [DOI] [PubMed] [Google Scholar]
- 22. Macpherson KJ, Lewsey JD, Jhund PS, Gillies M, Chalmers JWT, Redpath A, et al. Trends in incidence and in short term survival following a subarachnoid haemorrhage in Scotland, 1986–2005: a retrospective cohort study. BMC Neurol. 2011;11:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takashima N, Arima H, Kita Y, Fujii T, Miyamatsu N, Komori M, et al. Two-year survival after first-ever stroke in a general population of 1.4 million Japanese: Shiga stroke registry. Circ J. 2018;82(10):2549–56. [DOI] [PubMed] [Google Scholar]
- 24. Chen Y, Wright N, Guo Y, Turnbull I, Kartsonaki C, Yang L, et al. Mortality and recurrent vascular events after first incident stroke: a 9-year community-based study of 0·5 million Chinese adults. Lancet Glob Health. 2020;8(4):e580–e590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rehman S, Chandra RV, Zhou K, Tan D, Lai L, Asadi H, et al. Sex differences in aneurysmal Subarachnoid Haemorrhage (aSAH): aneurysm characteristics, neurological complications, and outcome. Acta Neurochir. 2020;162(9):2271–82. [DOI] [PubMed] [Google Scholar]
- 26. Lindekleiv H, Sandvei MS, Njølstad I, Løchen ML, Romundstad PR, Vatten L, et al. Sex differences in risk factors for aneurysmal subarachnoid hemorrhage: a cohort study. Neurology. 2011;76(7):637–43. [DOI] [PubMed] [Google Scholar]
- 27. Cai Y, Liu Z, Jia C, Zhao J, Chai S, Li Z, et al. Comparison of sex differences in outcomes of patients with aneurysmal subarachnoid hemorrhage: a single-center retrospective study. Front Neurol. 2022;13:853513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rehman S, Phan HT, Reeves MJ, Thrift AG, Cadilhac DA, Sturm J, et al. Case-fatality and functional outcome after Subarachnoid Hemorrhage (SAH) in INternational STRoke oUtComes sTudy (INSTRUCT). J Stroke Cerebrovasc Dis. 2022;31(1):106201. [DOI] [PubMed] [Google Scholar]
- 29. Rautalin I, Lindbohm JV, Kaprio J, Korja M. Substantial within-country variation in the incidence of subarachnoid hemorrhage: a nationwide Finnish study. Neurology. 2021;97(1):e52–e60. [DOI] [PubMed] [Google Scholar]
- 30. Madsen TE, Khoury JC, Leppert M, Alwell K, Moomaw CJ, Sucharew H, et al. Temporal trends in stroke incidence over time by sex and age in the GCNKSS. Stroke. 2020;51(4):1070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Madsen TE, Khoury J, Alwell K, Moomaw CJ, Rademacher E, Flaherty ML, et al. Sex-specific stroke incidence over time in the greater cincinnati/northern Kentucky stroke study. Neurology. 2017;89(10):990–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Truelsen T, Bonita R, Duncan J, Anderson NE, Mee E. Changes in subarachnoid hemorrhage mortality, incidence, and case fatality in New Zealand between 1981-1983 and 1991-1993. Stroke. 1998;29(11):2298–303. [DOI] [PubMed] [Google Scholar]
- 33. Ingall T, Asplund K, Mähönen M, Bonita R. A multinational comparison of subarachnoid hemorrhage epidemiology in the WHO MONICA stroke study. Stroke. 2000;31(5):1054–61. [DOI] [PubMed] [Google Scholar]
- 34. Sacco RL, Wolf PA, Kannel WB, McNamara PM. Survival and recurrence following stroke. The framingham study. Stroke. 1982;13(3):290–5. [DOI] [PubMed] [Google Scholar]
- 35. Pobereskin LH. Incidence and outcome of subarachnoid haemorrhage: a retrospective population based study. J Neurol Neurosurg Psychiatry. 2001;70(3):340–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang LF, Yang J, Hong Z, Yuan GG, Zhou BF, Zhao LC, et al. Proportion of different subtypes of stroke in China. Stroke. 2003;34(9):2091–6. [DOI] [PubMed] [Google Scholar]
- 37. Stegmayr B, Eriksson M, Asplund K. Declining mortality from subarachnoid hemorrhage: changes in incidence and case fatality from 1985 through 2000. Stroke. 2004;35(9):2059–63. [DOI] [PubMed] [Google Scholar]
- 38. Khan FA, Engstrom G, Jerntorp I, Pessah-Rasmussen H, Janzon L. Seasonal patterns of incidence and case fatality of stroke in Malmö, Sweden: the STROMA study. Neuroepidemiology. 2005;24(1–2):26–31. [DOI] [PubMed] [Google Scholar]
- 39. Labovitz DL, Halim AX, Brent B, Boden-Albala B, Hauser WA, Sacco RL. Subarachnoid hemorrhage incidence among whites, blacks and caribbean hispanics: the Northern Manhattan Study. Neuroepidemiology. 2006;26(3):147–50. [DOI] [PubMed] [Google Scholar]
- 40. Alvarez G, Cox P, Pairoa M, García M, Delgado I, Lavados PM. Incidence of subarachnoid haemorrhage in the Aconcagua Valley, Chile: a community-based, prospective surveillance project. J Neurol Neurosurg Psychiatry. 2010;81(7):778–82. [DOI] [PubMed] [Google Scholar]
- 41. Sandvei MS, Mathiesen EB, Vatten LJ, Müller TB, Lindekleiv H, Ingebrigtsen T, et al. Incidence and mortality of aneurysmal subarachnoid hemorrhage in two Norwegian cohorts, 1984-2007. Neurology. 2011;77(20):1833–9. [DOI] [PubMed] [Google Scholar]
- 42. González-Pérez A, Gaist D, Wallander MA, McFeat G, García-Rodríguez LA. Mortality after hemorrhagic stroke: data from general practice (the health improvement network). Neurology. 2013;81(6):559–65. [DOI] [PubMed] [Google Scholar]
- 43. Olindo S, Chausson N, Mejdoubi M, Jeannin S, Rosillette K, Saint-Vil M, et al. Trends in incidence and early outcomes in a Black Afro-Caribbean population from 1999 to 2012: Etude Réalisée en Martinique et Centrée sur l’Incidence des Accidents Vasculaires Cérébraux II Study. Stroke. 2014;45(11):3367–73. [DOI] [PubMed] [Google Scholar]
- 44. Guéniat J, Brenière C, Graber M, Garnier L, Mohr S, Giroud M, et al. Increasing burden of stroke: the dijon stroke registry (1987-2012). Neuroepidemiology. 2018;50(1–2):47–56. [DOI] [PubMed] [Google Scholar]
- 45. Lavados PM, Hoffmeister L, Moraga AM, Vejar A, Vidal C, Gajardo C, et al. Incidence, risk factors, prognosis, and health-related quality of life after stroke in a low-resource community in Chile (ÑANDU): a prospective population-based study. Lancet Glob Health. 2021;9(3):e340–e351. [DOI] [PubMed] [Google Scholar]
- 46. Vadikolias K, Tsivgoulis G, Heliopoulos I, Papaioakim M, Aggelopoulou C, Serdari A, et al. Incidence and case fatality of subarachnoid haemorrhage in northern Greece: the evros registry of subarachnoid haemorrhage. Int J Stroke. 2009;4(5):322–7. [DOI] [PubMed] [Google Scholar]
- 47. Lindbohm JV, Kaprio J, Jousilahti P, Salomaa V, Korja M. Sex, smoking, and risk for subarachnoid hemorrhage. Stroke. 2016;47(8):1975–81. [DOI] [PubMed] [Google Scholar]
- 48. Mattioli C, Beretta L, Gerevini S, Veglia F, Citerio G, Cormio M, et al. Traumatic subarachnoid hemorrhage on the computerized tomography scan obtained at admission: a multicenter assessment of the accuracy of diagnosis and the potential impact on patient outcome. J Neurosurg. 2003;98(1):37–42. [DOI] [PubMed] [Google Scholar]
- 49. Tian HL, Xu T, Hu J, Cui YH, Chen H, Zhou LF. Risk factors related to hydrocephalus after traumatic subarachnoid hemorrhage. Surg Neurol. 2008;69(3):241–6; discussion 6. [DOI] [PubMed] [Google Scholar]
- 50. Konczalla J, Platz J, Schuss P, Vatter H, Seifert V, Güresir E. Non-aneurysmal non-traumatic subarachnoid hemorrhage: patient characteristics, clinical outcome and prognostic factors based on a single-center experience in 125 patients. BMC Neurol. 2014;14:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Khan AA, Smith JD, Kirkman MA, Robertson FJ, Wong K, Dott C, et al. Angiogram negative subarachnoid haemorrhage: outcomes and the role of repeat angiography. Clin Neurol Neurosurg. 2013;115(8):1470–5. [DOI] [PubMed] [Google Scholar]
- 52. Penttilä A, Lahti RA, Lunetta P. [Autopsies as a guarantee of legal protection and quality of patient care]. Duodecim. 1999;115(14):1524–30. [PubMed] [Google Scholar]
- 53. Kongable GL, Lanzino G, Germanson TP, Truskowski LL, Alves WM, Torner JC, et al. Gender-related differences in aneurysmal subarachnoid hemorrhage. J Neurosurg. 1996;84(1):43–8. [DOI] [PubMed] [Google Scholar]
- 54. Shigematsu K, Watanabe Y, Nakano H; Kyoto Stroke Registry Committee . Lower hazard ratio for death in women with cerebral hemorrhage. Acta Neurol Scand. 2015;132(1):59–64. [DOI] [PubMed] [Google Scholar]
- 55. Hamdan A, Barnes J, Mitchell P. Subarachnoid hemorrhage and the female sex: analysis of risk factors, aneurysm characteristics, and outcomes. J Neurosurg. 2014;121(6):1367–73. [DOI] [PubMed] [Google Scholar]
- 56. Westphal LP, Bögli SY, Werner J, Casagrande F, Keller E, Brandi G. Sex-related differences in symptom presentation of patients with aneurysmal subarachnoid hemorrhage. F1000Res. 2022;11:1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Langham J, Reeves BC, Lindsay KW, van der Meulen JH, Kirkpatrick PJ, Gholkar AR, et al. Variation in outcome after subarachnoid hemorrhage: a study of neurosurgical units in UK and Ireland. Stroke. 2009;40(1):111–8. [DOI] [PubMed] [Google Scholar]
- 58. Attenello FJ, Wang K, Wen T, Cen SY, Kim-Tenser M, Amar AP, et al. Health disparities in time to aneurysm clipping/coiling among aneurysmal subarachnoid hemorrhage patients: a national study. World Neurosurg. 2014;82(6):1071–6. [DOI] [PubMed] [Google Scholar]
- 59. Zheng Y, Zhou B, Wang X, Chen H, Fang X, Jiang P, et al. Size, aspect ratio and anatomic location of ruptured intracranial aneurysms: consecutive series of 415 patients from a prospective, multicenter, observational study. Cell Transplant. 2019;28(6):739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zuurbier CCM, Molenberg R, Mensing LA, Wermer MJH, Juvela S, Lindgren AE, et al. Sex difference and rupture rate of intracranial aneurysms: an individual patient data meta-analysis. Stroke. 2022;53(2):362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bögli SY, Utebay D, Smits N, Westphal LP, Hirsbrunner L, Unseld S, et al. Sex-related differences of invasive therapy in patients with aneurysmal subarachnoid hemorrhage. Acta Neurochir. 2022;164(11):2899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. van Donkelaar CE, Bakker NA, Veeger NJ, Uyttenboogaart M, Metzemaekers JD, Luijckx GJ, et al. Predictive factors for rebleeding after aneurysmal subarachnoid hemorrhage: rebleeding aneurysmal subarachnoid hemorrhage study. Stroke. 2015;46(8):2100–6. [DOI] [PubMed] [Google Scholar]
- 63. Rautalin I, Juvela S, Martini ML, Macdonald RL, Korja M. Risk factors for delayed cerebral ischemia in good-grade patients with aneurysmal subarachnoid hemorrhage. J Am Heart Assoc. 2022;11(23):e027453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Abdel-Fattah AR, Pana TA, Smith TO, Pasdar Z, Aslam M, Mamas MA, et al. Gender differences in mortality of hospitalised stroke patients. Systematic review and meta-analysis. Clin Neurol Neurosurg. 2022;220:107359. [DOI] [PubMed] [Google Scholar]
- 65. Irie F, Matsuo R, Nakamura K, Wakisaka Y, Ago T, Kamouchi M, et al. Sex differences in the risk of 30-day death after acute ischemic stroke. Neurol Clin Pract. 2021;11(6):e809–e816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Denti L, Artoni A, Scoditti U, Caminiti C, Giambanco F, Casella M, et al. Impact of gender-age interaction on the outcome of ischemic stroke in an Italian cohort of patients treated according to a standardized clinical pathway. Eur J Intern Med. 2013;24(8):807–12. [DOI] [PubMed] [Google Scholar]
- 67. Zhang R, Wang Y, Fang J, Yu M, Wang Y, Liu G. Worldwide 1-month case fatality of ischaemic stroke and the temporal trend. Stroke Vasc Neurol. 2020;5(4):353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Koton S, Schneider AL, Rosamond WD, Shahar E, Sang Y, Gottesman RF, et al. Stroke incidence and mortality trends in US communities, 1987 to 2011. Jama. 2014;312(3):259–68. [DOI] [PubMed] [Google Scholar]
- 69. Gokhale S, Caplan LR, James ML. Sex differences in incidence, pathophysiology, and outcome of primary intracerebral hemorrhage. Stroke. 2015;46(3):886–92. [DOI] [PubMed] [Google Scholar]
- 70. Ding C, Toll V, Ouyang B, Chen M. Younger age of menopause in women with cerebral aneurysms. J Neurointerv Surg. 2013;5(4):327–31. [DOI] [PubMed] [Google Scholar]
- 71. Mahlamäki K, Rautalin I, Korja M. Case fatality rates of subarachnoid hemorrhage are decreasing with substantial between-country variation: a systematic review of population-based studies between 1980 and 2020. Neuroepidemiology. 2022;56(6):402–12. [DOI] [PubMed] [Google Scholar]
- 72. Asikainen A, Korja M, Kaprio J, Rautalin I. Case fatality of aneurysmal subarachnoid hemorrhage varies by geographic region within Finland: a nationwide register-based study. Neurology. 2023;101(20):e1950–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The first author takes full responsibility for all data, analyses, and interpretations, as well as has access to all the data and has the right to publish any and all data separate and apart from any sponsor. Contact Aleksanteri Asikainen (aleksanteri.asikainen@helsinki.fi) for more details about the study protocol, our data, and analyses.