Abstract
Bovine leukemia virus (BLV) is a complex B-lymphotrophic retrovirus of cattle and the causative agent of enzootic bovine leukosis. Serum antibody in infected animals does not correlate with protection from disease, yet only some animals develop severe disease. While a cytotoxic T-lymphocyte response may be responsible for directing BLV pathogenesis, this possibility has been left largely unexplored, in part since the lack of readily established cytotoxic target cells in cattle has hampered such studies. Using long-term naturally infected alymphocytic (AL) cattle, we have established the existence of cytotoxic T-lymphocyte response against BLV envelope proteins (Env; gp51/gp30). In vitro-expanded peripheral blood mononuclear (PBM) cell effector populations consisted mainly of γδ+ (>40%), CD4+ (>35%), and CD8+ (>10%) T lymphocytes. Specific lysis of autologous fibroblasts infected with recombinant vaccinia virus (rVV) delivering the BLV env gene ranged from 30 to 65%. Depletion studies indicated that γδ+ and not CD8+ T cells were responsible for the cytotoxicity against autologous rVVenv-expressing fibroblasts. Additionally, cultured effector cells lysed rVVenv-expressing autologous fibroblasts and rVVenv-expressing xenogeneic targets similarly, suggesting a lack of genetic restricted killing. Restimulation of effector populations increased the proportion of γδ+ T cells and concomitantly Env-specific cytolysis. Interestingly, culture of cells from BLV-negative or persistently lymphocytic cattle failed to elicit such cytotoxic responses or increase in γδ+ T-cell numbers. These results imply that cytotoxic γδ+ T lymphocytes from only AL cattle recognize BLV Env without a requirement for classical major histocompatibility complex interactions. It is known that γδ+ T lymphocytes are diverse and numerous in cattle, and here we show that they may serve a surveillance role during natural BLV infection.
Bovine leukemia virus (BLV) is among the most widespread livestock pathogens in the United States. A recent comprehensive survey revealed that 89% of dairy operations in the United States, and 41 to 47% of all dairy cattle, are infected with BLV (25). Despite a continued effort to link immune responses against BLV and development of the nonbenign states of enzootic bovine leukosis (EBL) (i.e., persistent lymphocytic [PL] state and tumor development), the role of cell-mediated immunity remains enigmatic. Yet, the host must keep the number of BLV-positive (BLV+) cells from accumulating, as two-thirds of infected cattle remain in the benign alymphocytic (AL) state of infection. Due to the protracted pathology of EBL in cattle, most investigations of BLV pathogenesis have been done in sheep. In contrast, we chose to pursue the role of cellular cytotoxicity in the natural host, cattle. Consequently, the extended latency of BLV dictated the use of a cross-sectional study, comparing groups of cattle that had remained in their state of infection for an extended period of time. This study was designed to address whether the state of infection correlated with a specific immune response.
In BLV investigations using sheep, proviral integration and an increase in circulating CD8+ lymphocytes precede seroconversion (60). In the sheep model, identification of specific CD4 and CD8 T-cell epitopes (19) and protection against BLV challenge (38, 39, 42) have been demonstrated after rVVenv inoculation. Furthermore, peptide immunizations have also been shown protective against BLV infection in sheep (24). The picture is less defined in cattle. While several immunization studies in cattle have induced (2, 31) or failed to produce (6, 48) protection, none of these studies addressed the role of cellular cytotoxicity. Also, in cattle, class I and class II BoLA haplotypes show some correlation with state of infection (12, 32, 62, 64). However, an actual effector population of cellular cytotoxicity against components of BLV has not been identified in cattle.
A functional role of γδ+ T cells in response to pathogens in cattle is still poorly defined. γδ+ T lymphocytes in ruminants express a diverse repertoire of the T-cell receptor (TcR) (22, 23), and ruminants have an unusually high number of γδ+ cells in circulation as well as in certain tissues (9). The possible connection between a γδ+ T-cell response and the ability of most BLV-infected animals to avoid severe disease has not been addressed. γδ+ T cells have been shown to mount cytotoxic, cytokine, and proliferative responses in several other viral infections. Most relevant to the present study is herpes simplex virus infection, where γδ+ T cells have been shown to directly recognize the gI protein (51) and also correlate with protection (30, 51, 52). In addition, γδ+ T cells are notably activated in cytomegalovirus (14), influenza virus (26), and Sendai virus (37) infections and, importantly, in several bovine viral infections such as those caused by bovine respiratory syncytial virus (50), bovine herpesvirus 1 (47), and foot-and-mouth disease virus (3). Activated γδ+ T cells are also evident during simian and human immunodeficiency virus (SIV and HIV) infections, although their presence does not necessarily correlate with protection (63; reviewed in reference 41), and activated γδ+ T cells in SIV and HIV infections also react to certain cells lines (17, 57; reviewed in references 8 and 28).
Human T-lymphotropic virus type 1 (HTLV-1) is genetically and structurally closely related to BLV. However, the immune responses to these viruses may require separate consideration, as no report links (HTLV-1) and γδ+ T-cell responses. First, HTLV-1 infects T cells in humans, while BLV infects B cells in cattle. Inherently, the potential for affecting the immune system varies when a different lymphocyte population is the major target for infection. Second, the γδ-TcR repertoire in cattle is much greater than in humans (23), allowing for a more diversified γδ+ T-cell response in cattle.
Here, we test the hypothesis that AL cattle possess lymphocytes capable of lysing cells expressing BLV antigen. The results demonstrate that cytotoxic γδ+ T lymphocytes of the natural host, cattle, recognize both autologous and xenogeneic target cells expressing BLV env but not irrelevant viral antigen (wild-type vaccinia virus). Additionally, this response is not seen in cattle that are BLV negative (BLV−) or PL, suggesting that these γδ+ cytotoxic T lymphocytes (CTLs) are intimately connected to BLV pathogenesis.
MATERIALS AND METHODS
Classification of BLV− and AL animals.
Delineation between infectious states of naturally BLV-infected cattle used previously established criteria (1) of total white blood cell (WBC) counts and agar gel immunodiffusion (AGID) analysis. Four BLV−, five BLV+ AL, and five BLV+ PL adult cattle used in this investigation are listed in Table 1. Briefly, BLV− cattle were free of serum antibody to BLV and had no integrated provirus as seen by PCR of the pol gene (4). AL cattle were seropositive and carried BLV provirus. In contrast to AL animals, which had WBC and B-cell counts similar to those of BLV− animals, PL animals had elevated numbers of WBC and circulating B cells. All BLV+ cattle had remained unchanged in status for 5 to 8 years.
TABLE 1.
Classification of BLV-infected cattlea
| Classification | Animal no. | WBC/ml | BLV AGID | % B lymphocytesb |
|---|---|---|---|---|
| BLV− | 602, 617, 920, 4205 | 4,000–6,500 | Negative | 25–30 |
| AL | 1, 4, 17, 201, 234 | 4,000–7,000 | Positive | 20–35 |
| PL | 182,c 612, 2, 19, 191 | >8,000 | Positive | 40–70 |
Animals were tested throughout study, and none changed status.
Determined by flow cytometric analysis.
Animal died during the time of the study. Cause of death was not related to BLV status.
Flow cytometry for surface markers.
Briefly, mouse monoclonal antibodies (MAbs) specific to bovine surface markers were incubated with 106 cells for 90 min at room temperature. Cells were washed three times with phosphate-buffered saline (PBS) and incubated with fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G (IgG; heavy plus light chain) for 90 min at room temperature. Cells were washed three times with PBS, fixed in 1% paraformaldehyde in PBS, and analyzed on an EPICS Profile II (Coulter Corp., Miami, Fla.) within 5 days. Antibodies to CD2 (ILA42), CD4 (ILA11), CD8 (ILA51), and IgM (ILA30) (27), from the American Type Culture Collection (Manassas, Va.), and γδ-TcR1 subset (86D) (33), from the European Collection of Animal Cell Culture (Salisbury, United Kingdom), were produced from purchased hybridomas, while the pan-γδ-TcR MAb GD3.8 (61) was kindly provided by the laboratory of Mark Jutila (Bozeman, Mont.). Both 86D, which recognizes an external epitope, and GD3.8 γδ-specific antibodies immunoprecipitate 38- and 40-kDa peptides, supporting TcR1 specificity. Another CD8 MAb (38-65) from the First International Antibody Workshop (27) was available in our lab. Two-color flow cytometry was performed similarly, with the addition of a 20-min preincubation with Fc Block (PharMingen, San Diego, Calif.) to eliminate interference from Fc receptor expression. Biotinylated MAbs were added at preoptimized concentrations followed by strepavidin-phycoerythrin conjugate. Cells were analyzed the same day or fixed in 1% paraformaldehyde and analyzed within 5 days.
Cell culture of hybridomas, target cell lines, PBM cells, and autologous fibroblast lines.
All cells were maintained in complete RPMI 1640 (cRPMI) supplemented with either 5% (cRPMI-5) or 10% (cRPMI-10) fetal calf serum (FBS; Sigma, St. Louis, Mo.). Before use, each lot of serum was verified free of detectable anti-BLV antibody by the AGID assay. Hybridomas were grown in cRPMI-5. Peripheral blood mononuclear (PBM) cells were isolated and cultured as described below (“Effector cell expansion protocol”). Primary autologous fibroblast cultures used for target cells were established from skin biopsies of each AL and BLV− animal studied and were either used directly or frozen at −70°C in 10% dimethyl sulfoxide in FBS. Briefly, minced skin sections were cultured for 10 to 14 days, and outwardly migrating fibroblasts were collected for an initial expansion of three to four passages, at which point aliquots were frozen in liquid nitrogen to allow for retrieval of early passages. In general, fibroblasts could be passaged more than 30 times before senescence (approximately 100 generations). D17 cells (canine osteosarcoma; obtained from the lab of Howard Temin) were maintained in cRPMI-5.
Recombinant and wild-type vaccinia virus preparation.
Wild-type vaccinia virus and recombinant vaccinia virus expressing the BLV env gene (rVVenv) were kindly provided by Misao Onuma (39) and Virogenetics, Inc. (Troy, N.Y.). New viral working stocks were produced by infecting a monolayer of Vero cells with the original virus preparations at a multiplicity of infection of 2 and culturing the cells in cRPMI-10 for 5 days. The infected cells were pelleted for 10 min at 250 × g in a preparative centrifuge, and the pellet was freeze-thawed three times. Prior to use, viral stocks were trypsinized for 30 min at 37°C. Viral titer was determined using a standard plaque assay. Tenfold dilutions of vaccinia virus were added to monolayers of either Vero cells or autologous fibroblasts in cRPMI without FBS for 2 h. An equal volume of cRPMI-10 was then added to each well, and the plates were incubated for 3 to 4 days; then the culture medium was removed, and the cells were stained with 1% crystal violet (Roboz Surgical Instrument Co., Washington, D.C.). Similar PFU for a given virus preparation were observed with Vero cells or autologous fibroblasts (data not shown). Viral stocks were kept at 4°C or frozen at −70°C, depending on length of storage before use.
Effector cell expansion protocol.
Heparinized blood was obtained by jugular venipuncture from normal and infected adult Holstein females. PBM cells were isolated over IsoPrep (Robbins Scientific, Bloomington, N.J.) at 1,400 × g for 30 min, washed three times with PBS, resuspended in 25 ml of cRPMI-10, and incubated at 1 × 106 to 2 × 106/ml for 2 h in a 162-cm2 tissue culture flask. Adherent cells were then discarded; nonadherent cells were transferred to a standing 75-cm2 tissue culture flask and incubated for 3 days, after which 25 ml of cRPMI-10 with 50 U of recombinant human interleukin-2 (rhIL-2) per ml was added. Expansion was continued for 7 to 9 days; then viable cells were isolated over IsoPrep and used in cytotoxicity assays. Short-term cell lines were restimulated on a similar culture cycle. Briefly, viable cells were isolated from the expansion culture and combined with autologous irradiated PBM cells (3,500 rad) for 2 to 3 days; then rhIL-2 (25 U/ml; half the concentration used in the initial expansion) was added for another 7 to 9 days. Lines remained viable in sufficient numbers for use in assays for four to six cycles of restimulation. Although IL-12 can elicit γδ+ T cells, we have shown that macrophages from AL animals produce prostaglandin E2, which can inhibit in vitro expansion of γδ+ T cells (46).
Cytotoxicity assays.
Autologous fibroblasts, uninfected or infected with vaccinia virus vectors (multiplicity of infection of 5) for 12 to 18 h, were trypsinized, rinsed, and labeled with 1 μCi of Na51CrO4 per 2,000 cells in cRPMI for 45 min. As target cells, autologous fibroblasts and D17 cells were >95 and 99% viable (by trypan blue exclusion), respectively, at 36 h of infection (data not shown). Using immunofluorescence, rVVenv-infected cells were approximately 80% positive for vaccinia virus antigen 12 h postinfection (data not shown). After labeling, targets were washed three times with PBS and resuspended in cRPMI-10; then 5,000 cells were added per well in 96-well plates for the assay. Gradient-purified effector cells were added at various effector-to-target (E:T) ratios, with a final volume of 200 μl. Plates were spun for 3 min at 150 × g before and after the 6-h assay. Supernatants were transferred to tubes and counted using a gamma counter.
Depletion of CD8+ and γδ+ T cells from the effector population.
Using MAbs to CD8, CD4, and γδ-TcR, specific subpopulations were depleted by complement lysis or two rounds of panning as adapted from previously published protocols (16, 34). Briefly, complement lysis was accomplished by incubating the population with a preoptimized amount of MAb on ice for 1 h in cRPMI. A final volume of 33% rabbit complement (Cedarlane, Hornby, Ontario, Canada) was added, and incubation continued with rotation for 1 h at 37°C; then cell debris was allowed to settle for 5 min on ice. Suspended cells were washed three times in cRPMI and adjusted to desired concentrations in cRPMI-10. Alternatively, plastic culture dishes were coated with MAb diluted in PBS with 1% bovine serum albumin at 4°C overnight and were washed five times with PBS before use. Cells were allowed to adhere to the plate for 1 h at room temperature. Nonadherent cells were transferred to a second plate, and the incubation was repeated. CD8 and CD4 depletions were done by complement lysis (MAb 38-65 [IgG2a] and ILA11 [IgG2a], respectively) and depletions by two rounds of panning (MAb 86D [IgG1]). Following depletions, the remaining cells were washed three times and resuspended to the original volume with cRPMI-10.
RESULTS
γδ+ T-lymphocyte expansion from PBM cells is unique to AL animals.
Because the majority of BLV-infected cattle remain in the benign AL state of infection, BLV-specific T cells may limit viral infection. To address the hypothesis that antigen-reactive effector cells could be expanded from BLV+ but not BLV− animals, PBM cells were cultured in vitro. T-cell proliferation is composed of two phases, antigen-specific activation resulting in up-regulated IL-2 receptor expression followed by IL-2-dependent expansion of activated cells (5). The distribution of phenotypes of freshly isolated and cultured populations from BLV−, AL, and PL cattle is shown in Table 2. Preexpansion cell populations were similar in both AL and BLV− animals, although AL animals had more CD4+ and CD2+ cells than either BLV− or PL animals. Further analysis of these CD markers was not done. PL cattle showed the symptomatic increase in B cells. However, following the 10- to 12-day expansion in the presence of endogenously expressed BLV and rhIL-2, AL animals had a significantly higher proportion of γδ+ T lymphocytes than either BLV− (P < 0.001) or PL (P < 0.001) animals, suggesting that activation of T cells by BLV followed by IL-2 expansion of antigen-specific T cells occurs preferentially in the cells from AL animals. The postexpansion cultures from BLV− and PL cattle did not contain statistically different numbers of γδ+ T cells (P > 0.025). While the relative numbers of CD4+ cells also increased slightly in all groups, the proportion of CD8+ cells in AL animals decreased in contrast to both BLV− (P < 0.001) and PL animals (P < 0.001). Typically, expansion of cells from BLV-free cattle yielded few viable cells, presumably due to the lack of antigen-specific stimulation during the first 3 days of in vitro culture.
TABLE 2.
Surface phenotypes of PBM cells from BLV−, AL, and PL cattle
| Marker | Cell population (% ± SD)a
|
|||||
|---|---|---|---|---|---|---|
| BLV−
|
AL
|
PL
|
||||
| Ex vivob | Culturedc | Ex vivo | Cultured | Ex vivo | Cultured | |
| CD2 | 38 ± 8 | 72 ± 13 | 57 ± 11 | 72 ± 7 | 18 ± 1 | 48 ± 10 |
| CD4 | 17 ± 4 | 19 ± 1 | 37 ± 8 | 38 ± 5 | 13 ± 4 | 24 ± 7 |
| CD8 | 13 ± 7 | 47 ± 5 | 16 ± 4 | 11 ± 2de | 7 ± 2 | 27 ± 8f |
| Surface IgM | 30 ± 3 | NDg | 36 ± 5 | 15 ± 4 | 56 ± 8 | 28 ± 11 |
| γδ-TcR | 14 ± 2 | 21 ± 14 | 15 ± 3 | 41 ± 3de | 9 ± 4 | 8 ± 2h |
For ex vivo cells, n = 10 for all groups. For cultured cells: BLV−, n = 3 (of 8); AL, n = 10 (of 10); PL, n = 10 (of 10).
Ex vivo cells are nonexpanded PBM cells freshly isolated from blood.
Nonadherent cells were cultured with endogenously expressed BLV antigen for 2 to 3 days and then rhIL-2 (50 U/ml) for an additional 7 to 9 days.
Statistically different from BLV− (P < 0.001).
Statistically different from PL (P < 0.001).
Statistically different from BLV− (P < 0.005).
ND, not determined.
Not statistically different from BLV− (P > 0.025).
In vitro expansion of AL PBM cells yields an Env-reactive cytotoxic population.
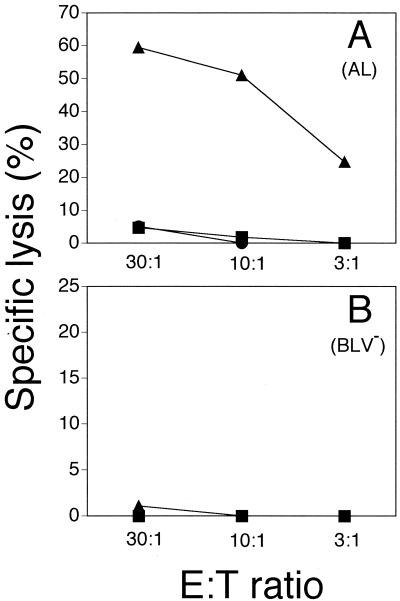
To examine the function of the expanded cell populations, autologous fibroblasts were used as targets to assess cytotoxic ability. Expression of rVVenv in target cells was confirmed by intracellular immunofluorescence and Western blot analysis using polyclonal anti-vaccinia virus serum and anti-gp51 MAbs, respectively (data not shown). Cytolytic activity against autologous fibroblasts infected with rVVenv but not against wild-type vaccinia virus was detected in expanded populations from AL animals (Fig. 1A). In seven different experiments with five different AL animals and three BLV− animals, the Env-specific lysis by cells from AL animals ranged from 30 to 65%, while cells from uninfected animals failed to lyse the target cells (less than 5%). Despite the expansion of mainly CD8+ T cells from BLV− animals on three occasions (Table 2), those effectors were not able to lyse rVVenv-expressing autologous fibroblasts (Fig. 1B).
FIG. 1.
Cytotolysis of rVVenv-expressing autologous fibroblasts by expanded PBM cells from AL animal 17 (A) and BLV− animal 4205 (B). Targets were either infected with rVVenv (triangles) or wild-type vaccinia virus (circles) or uninfected (squares) in a 6-h 51Cr release assay. Spontaneous release was less than 30%, and all determinations were performed in triplicate. The data are representative of seven experiments with three different AL animals and two different BLV− animals. Standard deviations were less than 5% for all data points.
Depletion of γδ+, but not CD8+ and CD4+, T lymphocytes reduces Env-specific lysis in AL animals.
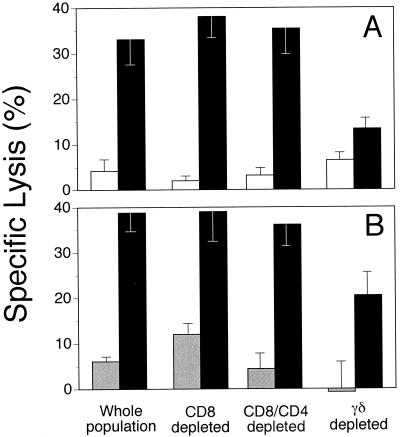
To determine the T-lymphocyte population responsible for the observed lysis, CD8+ and/or γδ+ T lymphocytes were depleted in the postexpansion effector populations. A reduction of cytotoxicity resulted when γδ+ cells were removed (Fig. 2). Treatment with MAb 86D, which recognizes 50 to 70% of γδ+ T cells in the expanded populations, caused an approximate 50% reduction in lysis of env-expressing target cells, suggesting the involvement of γδ+ T cells. Depletion of CD8+ T cells or CD4+ and CD8+ T cells, to remove the αβ-TcR population and address the role of αβ-TcR cells in BLV cytotoxicity, did not alter Env-specific lysis (Fig. 2). Thus, BLV-infected animals in the AL state of infection possess γδ+ T cells that lyse env-expressing target cells, while BLV− animals did not have detectable cytotoxic γδ+ T cells.
FIG. 2.
Cytotolysis of rVVenv-expressing autologous fibroblasts by expanded PBM cell subsets from two AL animals, 17 (A) and 201 (B). The assay was performed as for Fig. 1, at E:T ratios of 50:1 (A) and 30:1 (B). Target cells were either infected with rVVenv (filled) or wild-type vaccinia virus (hatched) or uninfected (clear). Spontaneous release was less than 30%, and all determinations were performed in triplicate. The data are representative of two experiments with two different AL animals. See Materials and Methods for depletion protocols.
Expanded AL effectors are not classically MHC restricted.
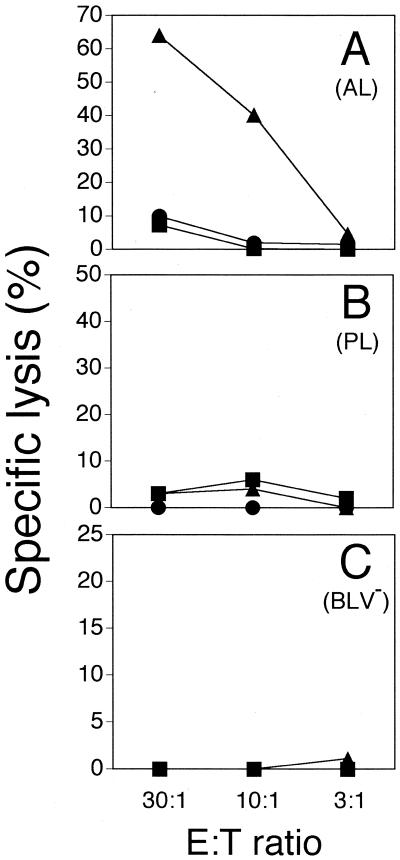
Previously, NK-like cell lines from cattle (40) were shown to lyse bovine herpesvirus 1-infected D17 cells (a canine osteosarcoma cell line). The vast majority of cells in that study were γδ+ T cells, which prompted us to test the cytolytic activity of the AL-derived effectors against several common target cell lines. Effectors expanded from AL animals would not kill K562, YAC-1, Daudi, Vero, NMU, or COLO cells (data not shown). However, similar to autologous fibroblasts (Fig. 1), D17 cells were also lysed by AL-derived effector cells when infected with rVVenv (Fig. 3). Minimal lysis of wild-type vaccinia virus-infected and uninfected D17 cells was noted at the highest E:T ratio (<10% specific lysis). The phenotypic distribution of the effector populations used in Fig. 3 are shown in Table 3. The use of D17 cells consequently allows comparison of animals in different states of infection using a single target population, while focusing the examination on major histocompatibility complex (MHC)-nonrestricted cytotoxicity. Thus, as expected, cytolytic γδ+ T cells from BLV-infected animals in the AL state are not classically MHC restricted. Interestingly, PL animals possess higher numbers of BLV-producing cells in vivo (18), and in vitro demonstration of BLV antigens is much enhanced using cells from PL animals compared to AL animals (15). Therefore, based on BLV antigen availability in culture, γδ+ T-cell expansion and cytotoxicity should be greater in cells from PL animals. This outcome was not observed in Table 2 and Fig. 3, suggesting only cytotoxic γδ+ T cells from AL animals recognize BLV Env.
FIG. 3.
Cytotolysis of rVVenv-expressing D17 cells by short-term cell lines (culture and one restimulation) from AL animal 17 (A), PL animal 2 (B), and BLV− animal 602 (C). Targets were either uninfected (squares) or infected with wild-type vaccinia virus (circles) or rVVenv (triangles) and used in a 6-h 51Cr release assay. Spontaneous release was less than 30%, and all determinations were performed in triplicate. The data are representative of two experiments with two different AL animals, two different BLV− animals, and three different PL animals. Standard deviations were less than 5% for all data points.
TABLE 3.
Phenotypes of short-term cell linesa
| Markerb | % Expressing marker
|
||
|---|---|---|---|
| 602-1x (BLV−) | 17-1x (AL) | 2-1x (PL) | |
| CD2 | 76 | 78 | 69 |
| CD4 | 61 | 52 | 26 |
| CD8 | 13 | 13 | 32 |
| γδ-TcR | 8 | 24 | 7 |
Cell lines used for Fig. 3 (one restimulation after initial expansion [−1x]).
Gate set to nonpermeable cells as determined by propidium iodide exclusion.
Restimulated AL cell lines retain their ability to lyse rVVenv-expressing targets.
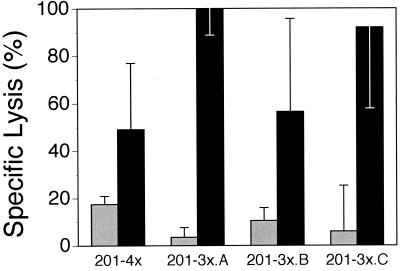
Since culture of PBM cells from AL animals evoked a cytotoxic effector population, the cultured cells were restimulated to assess continued antigen specificity and enrichment for cytotoxicity. Indeed, Fig. 4 shows that short-term cell lines were capable of substantial (50 to 100%) lysis after three to four rounds of restimulation. Although individual cultures varied in their relative lysis of rVVenv and wild-type vaccinia virus-infected targets, specific cytotoxicity remained high with continued in vitro culture (Fig. 4).
FIG. 4.
Env-specific lysis of rVVenv-infected D17 cells by short-term T-cell lines from animal 201. Lines were restimulated three to four times with irradiated PBM cells and 25 U of rhIL-2 per ml after initial expansion culture (−nx). Independently derived 3-week cultures are given the letters A, B, and C. D17 cells were infected with either rVVenv (solid) or wild-type vaccinia virus (hatched). Spontaneous release was less than 35%, and determinations were done in triplicate at an E:T ratio of 20:1. The data are representative of two restimulation experiments for this animal.
Cell lines from AL animals are unique in their lysis of rVVenv-expressing targets.
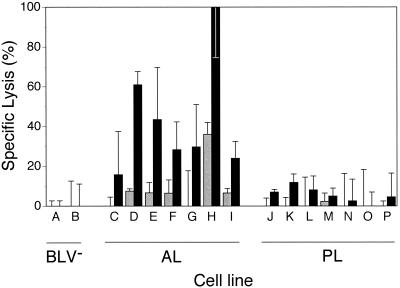
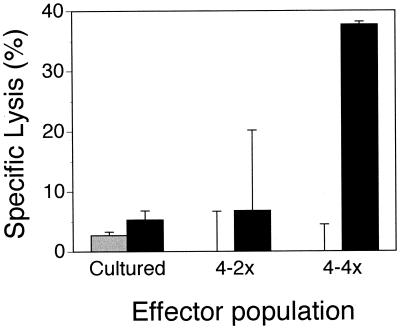
To test whether in vitro restimulation results in cytotoxic γδ+ T cells regardless of state of infection, cell lines from animals in all three categories were tested for cytotoxic ability. Figure 5 shows that restimulated AL-derived cell lines lysed rVVenv-expressing D17 cells, while cell lines from BLV− and PL animals did not (Fig. 5). Cells from one AL animal (animal 4) rarely lysed rVVenv-expressing targets after initial PBM cell expansion. However, following repeated restimulation, cells from this AL animal also showed high env-specific lysis (Fig. 6). Therefore, cattle in the AL state of infection possess cells capable of lysing cells that express a BLV antigen; in contrast, animals in the PL state of infection, as well as uninfected animals, do not have cytotoxic cells specific for BLV antigens.
FIG. 5.
rVVenv-specific lysis of D17 target cells by restimulated lines from AL but not from BLV− or PL animals. Lines were restimulated one to four times with irradiated PBM cells and 25 U of rhIL-2 per ml after initial expansion culture. D17 cells were infected with either rVVenv (solid) or wild-type vaccinia virus (hatched). Two BLV− (A, 920-3x; B, 920-2x), seven AL (C, 4-4x; D, 17-4x; E, 17-3x; F, 17-2x; G, 201-2x; H, 201-1x; I, 234-1x), and seven PL (J, 019-3x; K, 019-2x; L, 019-1x; M, 2-3x; N, 2-1x; O, 191-3x; P, 191-1x) cell lines were used. The cell lines are representative of a larger pool tested, and sequential numbers do not necessarily refer to a continuous restimulation culture. Spontaneous release was less than 30%, and determinations were done in triplicate at an E:T ratio of 30:1. The data are representative of two restimulation experiments.
FIG. 6.
rVVenv-specific lysis of D17 target cells by restimulated lines from AL animal 4 despite insignificant lysis after first culture. Effector populations were either cultured once from PBM cells (cultured) or subsequently restimulated two (4-2x) or four (4-4x) times with irradiated PBM cells and 25 U of rhIL-2 per ml after initial culture. D17 cells were infected with either rVVenv (solid) or wild-type vaccinia virus (hatched). Spontaneous release was less than 25%, and determinations were done in triplicate at an E:T ratio of 20:1.
γδ+ T cells in restimulated AL cell lines increase in relative number and correlate with cytotoxicity.
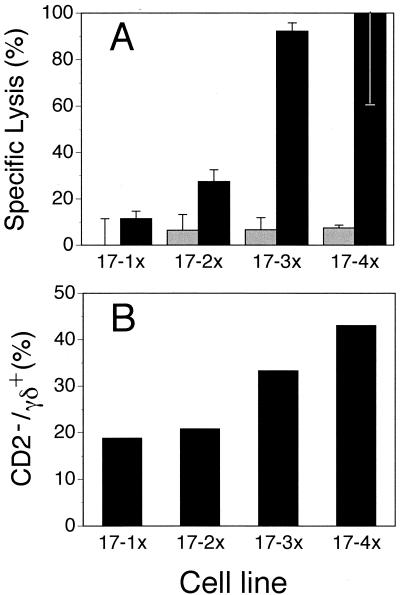
Because others have observed an increase in CD2− γδ+ T cells in BLV+ cattle (54), and the expanded γδ+ cells from AL animals may be CD2− (Table 2), we determined if cells of this phenotype from AL animals were expanded. The phenotypes of cell lines restimulated one to five times (Table 4) were compared for their cytotoxic potential (Fig. 7A). Increasing numbers of γδ+ T cells correlated with the level of cytotoxicity. Conversely, the number of CD8+ T cells declined more than 6-fold (Table 4), while env cytotoxicity increased nearly 10-fold (Fig. 7A). Additionally, when we determined the coexpression of CD2 and the γδ-TcR, a correlation between increased cytotoxicity and the number of γδ+ T cells that were CD2− was equally striking (Fig. 7B), suggesting the phenotype of the cytotoxic cell is γδ+ CD2−. Similar findings between CD2− γδ+ T cells and increasing cytotoxicity were evident for long-term-cultured cell lines from two other AL animals. The findings from this experiment further support the role of γδ+ T cells from the AL state of infection are responsible for in vitro lysis of cells expressing a BLV antigen.
TABLE 4.
Phenotypes of multiple restimulated cell linesa
| Cell line | % Expressing surface marker
|
|||
|---|---|---|---|---|
| CD2+ | CD4+ | CD8+ | γδ+ | |
| 17-1xb | 72 | 74 | 26 | 25 |
| 17-2x | 83 | 71 | 21 | 23 |
| 17-3x | 77 | 73 | 14 | 31 |
| 17-4x | 54 | 35 | 9 | 59 |
| 17-5xc | 53 | 68 | 4 | 62 |
Cell lines from animal 17 used for Fig. 7.
−nx indicates number of restimulations for
Not enough cells for CTL assay; phenotypic distribution determined only to demonstrate continued decrease of CD8+ and increase of γδ+ T cells.
FIG. 7.
Cytotoxic activity against rVVenv-infected D17 cells correlated with CD2− γδ+ T cells in cell lines. Short-term cell lines from animal 17 (17-1x, 17-2x, 17-3x, and 17-4x) were restimulated with irradiated PBM cells one, two, three, and four times, respectively. For flow cytometry data on these lines, see Table 4. These are the aggregate data on sequential restimulations of the same culture. (A) Increase in cytolytic activity against rVVenv-infected (filled) but not wild-type vaccinia virus-infected (hatched) D17 cells with each restimulation. Spontaneous release was less than 30%, and determinations were done in triplicate at an E:T ratio of 20:1. (B) Increase in percent CD2− γδ+ T cells of total γδ+ T cells with each restimulation.
DISCUSSION
BLV is among the most persistent pathogens in cattle, whose immune system, like that of all ruminants, is unique in its γδ+ T-cell prevalence and diversity. Despite these two distinct phenomena, the relevance of γδ+ T cells to BLV pathogenesis has not been thoroughly explored. To address this lack of understanding, we determined if γδ+ T cells from BLV-infected animals could recognize cells expressing components of BLV. A large proportion of the expanded effector cell population from adult AL animals were γδ-TcR positive. These γδ+ T cells were cytotoxic to rVVenv-expressing target cells. In contrast to the AL animals, PBM cells from BLV− animals typically failed to survive in parallel cultures. Only approximately 1/10 of the number of the cells survived in 3 of 8 attempted BLV− cultures, compared to the constant recovery of cells from each of 10 AL and 10 PL cultures (Table 2). Furthermore, cultures from PL animals contained approximately equal numbers of B cells, CD4+ T cells, and CD8+ T cells, all being threefold more prevalent than γδ+ T cells, and these PL-derived effectors failed to lyse rVVenv-expressing target cells.
The role of γδ+ T cells in ruminants may extend beyond the proposed role of complementing γδ+ T cells suggested for primates (58). The prominence of γδ+ T cells in our in vitro studies and the high proficiency of BLV-specific CTL activity suggests that γδ+ CTL play a significant role in controlling BLV infection in the AL state. The lack of this reactive γδ+ CTL population in PL animals implies that γδ+ T cells are intimately connected to BLV pathogenesis. To our knowledge, this is the first time that γδ+ T cells have been shown to respond specifically to retroviral proteins in ruminants.
Depletion of CD8+, CD4+, and γδ+ cells indicated that γδ+ cells expanded from AL animals were largely responsible for the target cell lysis. Additionally, the cytotoxicity to Env was not MHC restricted, as evidenced by the lysis of rVVenv-infected D17 cells (Fig. 3). However, expanded PBM cells from AL cattle typically showed no significant cytotoxicity against noninfected and wild-type vaccinia virus-infected autologous target cells, indicating that the cytotoxicity was not the result of NK cells. Cultured cells from AL animals also contained a large proportion of CD4+ T lymphocytes, while CD8+ T lymphocytes comprised less than a third of the γδ+ T-lymphocyte population. While the 10 to 20% CD8+ T lymphocytes may be responsible for the high levels of cytotoxicity observed, depletion of CD8+ effector cells did not alter cytotoxicity (Fig. 2), and reduction in CD8+ cells occurred while cytotoxicity increased in the short-term cell lines (Table 4 and Fig. 7). Also, the substantially higher levels of CD8+ T cells in BLV− and PL cattle following culture was not accompanied by increased cytotoxicity (Fig. 3, Fig. 5, and data not shown). The relatively large proportion (>35%) of CD4+ T lymphocytes in expanded cultures from AL animals would be additional candidates for cytotoxic effector cells. However, CD4+ CTLs are MHC class II restricted (reviewed in reference 21), while autologous fibroblast targets are MHC class II negative and D17 cells are xenogeneic to cattle. Recently, γδ+ T cells were reported to present antigen and stimulate CD4+ cells (10). This possiblity would explain the continued presence of CD4+ cells in our cultures (Tables 2 to 4). Alternatively, there may be a reciprocal relationship between CD4+ and γδ+ cells that is required for γδ+ cell expansion and/or cytotoxicity. Also, CD4+ cells may be stimulated nonspecifically in culture through the presence of endogenous IL-2 or other cytokines. Although investigation regarding the continued CD4+ T-cell presence in culture was beyond the scope of this study, depletion of CD4+ T cells did not affect cytotoxicity in our system (Fig. 2); moreover, despite large proportions (26 to 61%) of CD4+ cells in cell lines from BLV− and PL animals, these effector populations were not cytotoxic (Table 3, Fig. 3, and data not shown).
Since CD4+ and CD8+ cells are primarily CD2+, the single surface marker staining in Table 2 suggests that cultured cells from AL animals increase in γδ+ cells but not in CD2+ cells. Additionally, CD2− γδ+ cells increase in BLV+ cattle (54), and we show here that loss of CD2 expression on γδ+ T cells correlated with restimulation of effector cells concomitantly with increased cytotoxicity (Table 4 and Fig. 7). However, decreasing CD2+ cells over time may be a consequence of CD2-mediated apoptosis (55). Future experiments should address the relevance of this γδ+ subpopulation in the rVVenv-specific cytotoxic response. In all cytotoxicity assays, the relative numbers of γδ cell subsets, TcR-N3, TcR-N4, TcR-N6, TcR-N7, TcR-N12 (13), and 86D, were determined, but none of these populations demonstrated a correlation with BLV infection status or cytotoxicity in cultured cell populations (data not shown). The exclusive role for γδ+ CTLs is corroborated by the lysis of D17 targets expressing BLV proteins, where a conventional coreceptor model cannot explain the data. While an apparent lack of MHC restriction was observed, nonclassical presentation molecules (such as CD1) cannot be excluded. However, recognition of nonclassical MHC molecules by bovine γδ+-TcR has not been shown to date and may be an unlikely explanation.
Occasionally, AL animals in this study had slightly elevated levels of circulating γδ+ cells relative to noninfected control animals, but the increase was not statistically significant (Table 2). However, the BLV− animals used in this study had a slightly higher number of circulating γδ+ cells than previously reported (18), possibly due to the lack of a specific pan-γδ-TcR MAb in the earlier study.
Considering that BLV mRNA can be detected ex vivo in sheep (43), the immune system of infected cattle may encounter BLV antigen with relative frequency despite the extended latency of BLV in cattle. Indeed, individual cells of cattle have been shown to express BLV mRNA in vivo (20). In the sheep model, periodic appearance of Rex-specific antibodies and sustained antibodies against other BLV components was detected (43), implying opportunity for repeated contact with BLV antigen and repeated stimulation of BLV-specific γδ+ T cells. Although disease development in cattle is not precisely mimicked by sheep, infected cattle similarly remain seropositive and proviral DNA can be found throughout life. Furthermore, endogenous BLV antigen is produced by B cells from cattle during in vitro culture (references 1, 15, and 29 and data not shown), providing a source of BLV antigen for γδ+ T cells.
In HIV, Vγ9/Vδ2 T-cell responses to HIV-infected targets exist in both uninfected and infected individuals (59), but these cells are diverse in the complementarity-determining region 3 of the TcR and also recognize other virus-infected cells. We have no evidence that the γδ+ T cells identified in this study are similarly limited in γδ chain expression, but they recognize neither wild-type vaccinia virus-infected targets nor any of several common cell lines in our cytotoxicity assay. In HIV-negative humans, Vγ9/Vδ2+ T cells recognize HIV- or SIV-infected cells and Daudi cells without a requirement for prior activation (7; reviewed in reference 57), suggesting that antigen-specific stimulation may not be necessary for antiretroviral activity. Additionally, Vγ9/Vδ2+ T cells from HIV-positive individuals are functionally defective in the ability to respond to stimulation by Daudi cells and ethyl pyrophosphate (59). Similarly, in SIV, γδ+ CTLs respond to infected target cells and Daudi cells (17, 56). The extent to which the γδ+ T cells in HIV- or SIV-positive primates parallel the PBM cells from BLV+ cattle remains to be determined. Additionally, γδ+ CTLs have not been identified in individuals infected with HTLV-1, and the bovine γδ+ CTLs in the present study were unique to AL animals and were not reactive to Daudi cells.
Often, CTL studies use a specific antigen derived from the pathogen of interest, many times in the form of peptide-pulsed antigen-presenting cells (APC). By necessity, peptide-pulsing studies select for a CTL population capable of engaging APC surface molecules carrying the administered peptide antigen. In contrast, the present study relied on endogenous BLV as a source of antigen, followed by expansion of antigen-responsive T lymphocytes in culture. Consequently, the antigen presentation pathway is determined by natural viral expression during effector stimulation. The presence of BLV antigen and reverse transcriptase activity in culture supernatants from BLV-infected animals in this study was confirmed but not quantified (data not shown). Addition of rhIL-2 at the start of the in vitro culture or omission of rhIL-2 during the 7- to 9-day expansion phase resulted in high nonspecific or no cytotoxicity, respectively (data not shown). The use of endogenous BLV as the antigen may more accurately represent the role that BLV plays in vivo. The minimal manipulation of the cultured PBM cells more likely representative than peptide-pulsed APC stimulation in modeling what occurs as the integrated BLV provirus breaks latency.
Aside from antigen availability during culture, correlation of the AL and PL states of infection with BoLA haplotypes (12, 32, 62, 64) and cytokine profiles (36, 44–46, 54) suggests that immune responses from BLV+ animals are linked to cytokine production. All PBM cell cultures from BLV+ animals produce IL-10 over time (D. Pyeon, personal communication), and macrophages from PL cattle also secrete significant amounts of IL-10 ex vivo (45) whereas macrophages from AL animals secrete IL-12 (44). The combination of IL-2 and IL-12 has been found to elicit highly reactive human γδ+ CTLs (49). Additionally, IL-12 and IL-1 are necessary for the development and activation of γδ+ responses (53). We know from our previous work (reference 11 and data not shown) that large quantities of IL-1 are present at early stages of in vitro culture. Consequently, the choice between IL-10 and IL-12 production and/or secretion may help determine, or result from, the infection state of EBL. Similarly, reactivity to Mycobacterium tuberculosis (35) and HIV (7) by γδ+ T cells is enhanced by IL-12 and abrogated by IL-10 (35). Interestingly, the PBM cells from the less γδ+ T-cell-reactive AL animal 4 contained higher baseline levels of IL-10 mRNA ex vivo than the more reactive AL animals (Pyeon, personal communication). As shown in Fig. 6, restimulation of PBM cells from this animal and removal of macrophages as the source of IL-10 in vitro (45) overcame an initial lack of a γδ+ T-cell response, indicating that unresponsiveness was not permanent. However, under identical culture conditions, cells from PL animals did not possess rVVenv-specific cytotoxicity (Fig. 5). In conclusion, this study shows that AL cattle, but not PL cattle, are capable of eliciting Env-specific γδ+ CTLs. These CTLs are not MHC restricted and may recognize the BLV protein directly. Defining the role of this unique cell population from AL animals could be crucial to understanding the pathology of EBL.
ACKNOWLEDGMENTS
We thank Virogenetics, Inc., and Misao Onuma for providing samples of recombinant vaccinia virus. Thanks go to Oto Orlik for providing expert advice as well as anti-BLV MAbs, to Jerome Harms, David Pauza, and Paul Lambert for helpful discussions, and to Cathryn Lundberg for assistance with the immunofluorescence. We are also grateful for the generous supply of GD3.8 antibody from Mark Jutila.
This work was supported by National Cancer Institute grant ROI CA59127, BARD 95-34339-2556, and the University of Wisconsin College of Agricultural and Life Sciences.
REFERENCES
- 1.Adomaitiene D, Tamosiunas V, Mauricas M, Surovas V, Markevicius A. Establishment of a cell line from leucocytes of a cow with chronic lymphocytic leukemia. Vet Immunol Immunopathol. 1983;4:565–577. doi: 10.1016/0165-2427(83)90065-x. [DOI] [PubMed] [Google Scholar]
- 2.Altaner C, Ban J, Altanerova V, Janik V. Protective vaccination against bovine leukaemia virus infection by means of cell-derived vaccine. Vaccine. 1991;9:889–895. doi: 10.1016/0264-410x(91)90009-u. [DOI] [PubMed] [Google Scholar]
- 3.Amadori M, Archetti I L, Verardi R, Berneri C. Role of a distinct population of bovine T cells in the immune response to viral agents. Viral Immunol. 1995;8:81–91. doi: 10.1089/vim.1995.8.81. [DOI] [PubMed] [Google Scholar]
- 4.Boris-Lawrie K, Altanerova V, Altaner C, Kucerova L, Temin H. In vivo study of genetically simplified bovine leukemia virus derivatives that lack tax and rex. J Virol. 1997;71:1514–1520. doi: 10.1128/jvi.71.2.1514-1520.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chain B, McCafferty I, McCafferty G, Wallace G, Askenase P W. Improvement of the in vitro T cell proliferation assay by a modified method that separates the antigen recognition and IL-2-dependent steps. J Immunol Methods. 1987;99:221–228. doi: 10.1016/0022-1759(87)90131-1. [DOI] [PubMed] [Google Scholar]
- 6.Cherney T M, Schultz R D. Viral status and antibody response in cattle inoculated with recombinant bovine leukemia virus-vaccinia virus vaccines after challenge exposure with bovine leukemia virus-infected lymphocytes. Am J Vet Res. 1996;57:812–818. [PubMed] [Google Scholar]
- 7.Chervenak K A, Lederman M M, Boom W H. Bacterial antigen activation of Vδ+1 and Vδ+2 γδ T cells of persons infected with human immunodeficiency virus type 1. J Infect Dis. 1997;175:429–433. doi: 10.1093/infdis/175.2.429. [DOI] [PubMed] [Google Scholar]
- 8.Chien Y H, Jores R, Crowley M P. Recognition by γ/δ+ T cells. Annu Rev Immunol. 1996;14:511–532. doi: 10.1146/annurev.immunol.14.1.511. [DOI] [PubMed] [Google Scholar]
- 9.Ciccarese S, Lanave C, Saccone C. Evolution of T-cell receptor gamma and delta constant region and other T-cell-related proteins in the human-rodent-artiodactyl triplet. Genetics. 1997;145:409–419. doi: 10.1093/genetics/145.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins R A, Werling D, Duggan S E, Bland A P, Parsons K R, Howard C J. γδ+ T cells present antigen to CD4+ αβ T cells. J Leukoc Biol. 1998;63:707–714. doi: 10.1002/jlb.63.6.707. [DOI] [PubMed] [Google Scholar]
- 11.Covert J, Splitter G. Detection of cytokine transcriptional profiles from bovine peripheral blood mononuclear cells and CD4+ lymphocytes by reverse transcriptase polymerase chain reaction. Vet Immunol Immunopathol. 1995;49:39–50. doi: 10.1016/0165-2427(95)05451-b. [DOI] [PubMed] [Google Scholar]
- 12.Da Y, Shanks R D, Stewart J A, Lewin H A. Milk and fat yields decline in bovine leukemia virus-infected dairy cattle with persistent lymphocytosis. Proc Natl Acad Sci USA. 1993;94:6538–6541. doi: 10.1073/pnas.90.14.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis W C, Brown W C, Hamilton M J, Wyatt C R, Orden J A, Khalid A M, Naessens J. Analysis of monoclonal antibodies specific for the gamma delta TcR. Vet Immunol Immunopathol. 1996;52:275–283. doi: 10.1016/0165-2427(96)05578-x. [DOI] [PubMed] [Google Scholar]
- 14.Dechanet J, Merville P, Lim A, Retiere C, Pitard V, Lafarge X, Michelson S, Meric C, Hallet M M, Kourilsky P, Potaux L, Bonneville M, Moreau J F. Implication of γδ T cells in the human immune response to cytomegalovirus. J Clin Investig. 1999;103:1437–1449. doi: 10.1172/JCI5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Driscoll D M, Olson C. Bovine leukemia virus-associated antigens in lymphocyte cultures. Am J Vet Res. 1977;38:1897–1898. [PubMed] [Google Scholar]
- 16.Falkoff R M, Peters M, Fauci A S. T cell enrichment and depletion of human peripheral blood mononuclear cell preparations. Unexpected findings in the study of the separated populations. J Immunol Methods. 1982;50:39–49. doi: 10.1016/0022-1759(82)90302-7. [DOI] [PubMed] [Google Scholar]
- 17.Gan Y H, Malkovsky M. Mechanisms of simian gamma delta T cell cytotoxicity against tumor and immunodeficiency virus-infected cells. Immunol Lett. 1996;49:191–196. doi: 10.1016/0165-2478(96)02508-4. [DOI] [PubMed] [Google Scholar]
- 18.Gatei M H, Brandon R B, Naif H M, McLennan M W, Daniel R C, Lavin M F. Changes in B cell and T cell subsets in bovine leukaemia virus-infected cattle. Vet Immunol Immunopathol. 1989;23:139–147. doi: 10.1016/0165-2427(89)90116-5. [DOI] [PubMed] [Google Scholar]
- 19.Gatei M H, Good M F, Daniel R C, Lavin M F. T-cell responses to highly conserved CD4 and CD8 epitopes on the outer membrane protein of bovine leukemia virus: relevance to vaccine development. J Virol. 1993;67:1796–1802. doi: 10.1128/jvi.67.4.1796-1802.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaynor E M, Mirsky M L, Lewin H A. Use of flow cytometry and RT-PCR for detecting gene expression by single cells. BioTechniques. 1996;21:286–291. doi: 10.2144/96212rr02. [DOI] [PubMed] [Google Scholar]
- 21.Hahn S, Gehri R, Erb P. Mechanism and biological significance of CD4-mediated cytotoxicity. Immunol Rev. 1995;146:57–79. doi: 10.1111/j.1600-065x.1995.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 22.Hein W R, Dudler L. Divergent evolution of T cell repertoires: extensive diversity and developmentally regulated expression of the sheep γδ T cell receptor. EMBO J. 1993;12:715–724. doi: 10.1002/j.1460-2075.1993.tb05705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hein W R, Dudler L. TcR gamma delta+ cells are prominent in normal bovine skin and express a diverse repertoire of antigen receptors. Immunology. 1997;91:58–64. doi: 10.1046/j.1365-2567.1997.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hislop A D, Good M F, Mateo L, Gardner J, Gatei M H, Daniel R C, Meyers B V, Lavin M F, Suhrbier A. Vaccine-induced cytotoxic T lymphocytes protect against retroviral challenge. Nat Med. 1998;4:1193–1196. doi: 10.1038/2690. [DOI] [PubMed] [Google Scholar]
- 25.Hopkins S G, DiGiacomo R F. Natural transmission of bovine leukemia virus in dairy and beef cattle. Vet Clin North Am Food Anim Pract. 1997;13:107–128. doi: 10.1016/s0749-0720(15)30367-4. [DOI] [PubMed] [Google Scholar]
- 26.Hoq M M, Suzitani T, Toyoda T, Horiike G, Yoshida I, Azuma M. Role of γδ TcR+ lymphocytes in the augmented resistance of trehalose 6,6′-dimycolate-treated mice to influenza virus infection. J Gen Virol. 1997;78:1597–1603. doi: 10.1099/0022-1317-78-7-1597. [DOI] [PubMed] [Google Scholar]
- 27.Howard C J, Morrison W I, Hein W R, MacKay C R, Splitter G A. Leukocyte antigens in cattle, sheep and goats. Vet Immunol Immunopathol. 1991;27:1–276. [Google Scholar]
- 28.Kaufmann S H. γδ and other unconventional T lymphocytes: what do they see and what do they do? Proc Natl Acad Sci USA. 1996;93:2272–2279. doi: 10.1073/pnas.93.6.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerkhofs P, Adam E, Droogmans L, Portetelle D, Mammerickx M, Burny A, Kettman R, Willems L. Cellular pathways involved in the ex vivo expression of bovine leukemia virus. J Virol. 1996;70:2170–2177. doi: 10.1128/jvi.70.4.2170-2177.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kodukula P, Liu T, Rooijen N V, Jager M J, Hendricks R L. Macrophage control of herpes simplex virus type 1 replication in the peripheral nervous system. J Immunol. 1999;162:2895–2905. [PubMed] [Google Scholar]
- 31.Kukaine R A, Nagayeva L I, Chapenko S V, Iljinskaya T N, Bratsslvskaya O I, Vitolin L A, Yanchev I K, Bozhkov S, Alexandrov I I, Stefanova R N, Florova I S, Parvoulov B, Mateev M I, Sotirov N Z. Protection against bovine leukemia virus infection in two breeds of cattle. Comp Immunol Microbiol Infect Dis. 1993;16:63–71. doi: 10.1016/0147-9571(93)90062-a. [DOI] [PubMed] [Google Scholar]
- 32.Lewin H A, Wu M C, Stewart J A, Nolan T J. Association between BoLA and subclinical bovine leukemia virus infection in a herd of Holstein-Friesian cows. Immunogenetics. 1988;27:338–344. doi: 10.1007/BF00395129. [DOI] [PubMed] [Google Scholar]
- 33.Mackay C R, Beya M-F, Matzinger P. T cells express a unique surface molecule appearing late during thymic development. Eur J Immunol. 1989;19:1477–1488. doi: 10.1002/eji.1830190820. [DOI] [PubMed] [Google Scholar]
- 34.Mage M G. Fractionation of T cells and B cells. In: Coico R, editor. Current protocols in immunology. New York, N.Y: John Wiley & Sons, Inc.; 1994. pp. 3.5.1–3.5.6. [Google Scholar]
- 35.Marx S, Wesch D, Kabelitz D. Activation of human gamma delta T cells by Mycobacterium tuberculosis and Daudi lymphoma cells: differential regulatory effect of IL-10 and IL-12. J Immunol. 1997;158:2842–2848. [PubMed] [Google Scholar]
- 36.Meirom R, Moss S, Brenner J, Heller D, Trainin Z. Levels and role of cytokines in bovine leukemia virus (BLV) infection. Leukemia. 1997;11(Suppl. 3):219–220. [PubMed] [Google Scholar]
- 37.Ogasawara T, Emoto M, Kiyotani K, Shimokata K, Yoshida T, Nagai Y, Yoshikai Y. Sendai virus pneumonia: evidence for the early recruitment of γδ T cells during the disease course. J Virol. 1994;68:4022–4027. doi: 10.1128/jvi.68.6.4022-4027.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohishi K, Suzuki H, Yamamoto T, Maruyama T, Miki K, Ikawa Y, Numakunai S, Okada K, Ohshima K-I, Sugimoto M. Protective immunity against bovine leukaemia virus (BLV) induced in carrier sheep by inoculation with a vaccinia virus-BLV env recombinant: association with cell-mediated immunity. J Gen Virol. 1991;72:1887–1892. doi: 10.1099/0022-1317-72-8-1887. [DOI] [PubMed] [Google Scholar]
- 39.Onuma M, Hodatsu T, Yamamoto S, Higashihara M, Masu S, Mikami T, Izawa H. Protection by vaccination against bovine leukemia virus infection in sheep. Am J Vet Res. 1984;45:1212–1215. [PubMed] [Google Scholar]
- 40.Palmer L, Leary T, Wilson K, Splitter G. Bovine natural killer-like cell responses against cell lines expressing recombinant bovine herpesvirus type 1 glycoproteins. J Immunol. 1990;145:1009–1014. [PubMed] [Google Scholar]
- 41.Poccia F, Wallace M, Colizzi V, Malkovsky M. Possible protective and pathogenic roles of γδ T lymphocytes in HIV-infections. Int J Mol Med. 1998;1:409–413. doi: 10.3892/ijmm.1.2.409. [DOI] [PubMed] [Google Scholar]
- 42.Portetelle D, Limbach K, Burny A, Mammerickx M, Desmettre P, Riviere M, Zavada J, Paoletti E. Recombinant vaccinia virus expression of the bovine leukaemia virus envelope gene and protection of immunized sheep against infection. Vaccine. 1991;9:194–200. doi: 10.1016/0264-410x(91)90153-w. [DOI] [PubMed] [Google Scholar]
- 43.Powers M A, Grossman D, Kidd L C, Radke K. Episodic occurrence of antibodies against the bovine leukemia virus Rex protein during the course of infection in sheep. J Virol. 1991;65:4959–4965. doi: 10.1128/jvi.65.9.4959-4965.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pyeon D, Splitter G A. Interleukin-12 p40 mRNA expression in bovine leukemia virus-infected animals: increased in alymphocytosis but decreased in persistent lymphocytosis. J Virol. 1998;72:6917–6921. doi: 10.1128/jvi.72.8.6917-6921.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pyeon D, O'Reilly K L, Splitter G A. Increased interleukin-10 mRNA expression in tumor-bearing or persistently lymphocytotic animals infected with bovine leukemia virus. J Virol. 1996;70:5706–5710. doi: 10.1128/jvi.70.8.5706-5710.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pyeon D, Diaz F J, Splitter G A. Prostaglandin E2 increases bovine leukemia virus tax and pol mRNA via cyclooxygenase-2: regulation by interleukin-1, interleukin-10, and bovine leukemia virus. J Virol. 2000;74:5740–5745. doi: 10.1128/jvi.74.12.5740-5745.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quade M J, Roth J A. Antigen-specific in vitro activation of T-lymphocyte subsets of cattle immunized with a modified live bovine herpesvirus 1 vaccine. Viral Immunol. 1999;12:9–21. doi: 10.1089/vim.1999.12.9. [DOI] [PubMed] [Google Scholar]
- 48.Ristau E, Wittman W, Starick E, Kluge K H. Bovine leucosis virus challenge infection of calves following application of BL-3 cells. Arch Exp Veterinaermed. 1989;43:155–158. [PubMed] [Google Scholar]
- 49.Satoh M, Seki S, Hashimoto W, Ogasawara K, Kobayashi T, Kumagai K, Matsuno S, Takeda K. Cytotoxic prostaglandin γδ- or αβ-T cells with a natural killer cell marker, CD56, induced from human peripheral blood lymphocytes by a combination of IL-12 and IL-2. J Immunol. 1996;157:3886–3892. [PubMed] [Google Scholar]
- 50.Schrijver R S, Langedijk J P, Keil G M, Middel W G, Maris-Veldhuis M, Van Oirschot J T, Rijsewijk F A. Immunization of cattle with a BHV1 vector vaccine or a DNA vaccine both coding for the G protein of BRSV. Vaccine. 1997;15:1908–1916. doi: 10.1016/s0264-410x(97)00129-1. [DOI] [PubMed] [Google Scholar]
- 51.Sciammas R, Bluestone J A. HSV-1 glycoprotein I-reactive TcR γδ cells directly recognize the peptide backbone in a conformationally dependent manner. J Immunol. 1998;161:5187–5192. [PubMed] [Google Scholar]
- 52.Sciammas R, Kodukula P, Tang Q, Hendricks R L, Bluestone J A. T cell receptor-γδ cells protect mice from herpes simplex virus type 1-induced lethal encephalitis. J Exp Med. 1997;185:1969–1975. doi: 10.1084/jem.185.11.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skeen M J, Ziegler H K. Activation of gamma delta T cells for production of IFN-gamma is mediated by bacteria via macrophage-derived cytokines IL-1 and IL-12. J Immunol. 1995;154:5832–5841. [PubMed] [Google Scholar]
- 54.Sordillo L M, Hicks C R, Pighetti G M. Altered interleukin-2 production by lymphocyte populations from bovine leukemia virus-infected cattle. Proc Soc Exp Biol Med. 1994;207:268–273. doi: 10.3181/00379727-207-43815. [DOI] [PubMed] [Google Scholar]
- 55.Spinozzi F, Nicoletti I, Agea E, Belia S, Moraca R, Migliorati G, Riccardi C, Grignani F, Bertotto A. IL-4 is able to reverse the CD2-mediated negative apoptotic signal to CD4-CD8- alpha beta and/or gamma delta T lymphocytes. Immunology. 1995;86:379–384. [PMC free article] [PubMed] [Google Scholar]
- 56.Wallace M, Gan Y H, Pauza C D, Malkovsky M. Antiviral activity of primate gamma delta T lymphocytes isolated by magnetic cell sorting. J Med Primatol. 1994;23:131–135. doi: 10.1111/j.1600-0684.1994.tb00113.x. [DOI] [PubMed] [Google Scholar]
- 57.Wallace M, Malkovsky M, Carding S R. Gamma/delta T lymphocytes in viral infections. J Leukoc Biol. 1995;58:277–283. doi: 10.1002/jlb.58.3.277. [DOI] [PubMed] [Google Scholar]
- 58.Wallace M, Bartz S R, Chang W L, Mackenzie D A, Pauza C D, Malkovsky M. Gamma delta T lymphocyte responses to HIV. Clin Exp Immunol. 1996;103:177–184. doi: 10.1046/j.1365-2249.1996.d01-625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wallace M, Scharko A M, Pauza C D, Fisch P, Imaoka K, Kawabata S, Fujihashi K, Kiyono H, Tanaka Y, Bloom B R, Malkovsky M. Functional gamma delta T-lymphocyte defect associated with human immunodeficiency virus infections. Mol Med. 1997;3:60–71. [PMC free article] [PubMed] [Google Scholar]
- 60.Ward W H, Dimmock C K, Eaves F W. T lymphocyte responses of sheep to bovine leukaemia virus infection. Immunol Cell Biol. 1992;70:329–336. doi: 10.1038/icb.1992.42. [DOI] [PubMed] [Google Scholar]
- 61.Wilson E, Walcheck B, Davis W C, Jutila M A. Preferential tissue localization of γδ T cell subsets defined by anti-T cell receptor antibodies. Immunol Lett. 1998;64:39–44. doi: 10.1016/s0165-2478(98)00077-7. [DOI] [PubMed] [Google Scholar]
- 62.Xu A, Van Eijk M J T, Park C, Lewin H A. Polymorphism of BoLA-DRB3 exon 2 correlates with resistance and susceptibility to persistent lymphocytosis caused by bovine leukemia virus. J Immunol. 1993;151:6977–6986. [PubMed] [Google Scholar]
- 63.Yin C, Wu M S, Pauza C D, Salvato M S. High major histocompatibility complex-unrestricted lysis of simian immunodeficiency virus envelope-expressing cells predispose macaques to rapid AIDS progression. J Virol. 1999;73:3692–3701. doi: 10.1128/jvi.73.5.3692-3701.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zanotti M, Poli G, Ponti W, Polli M, Rocchi M, Bolzani E, Longeri M, Russo S, Lewin H A, van Eijk M J. Association of BoLA class II haplotypes with subclinical progression of bovine leukaemia virus infection in Holstein-Friesian cattle. Anim Genet. 1996;27:337–341. [PubMed] [Google Scholar]