Abstract
Background:
Modified Cajal’s trichrome stain (MCTS) is a good differential stain that allows one to visibly distinguish between connective tissue and epithelial elements with different tonalities of colour.
Aim:
Our study aims to evaluate and analyse the effectiveness of oral squamous cell carcinoma (OSCC) using MCTS.
Materials and Methods:
A study was conducted retrospectively with 30 tissue blocks embedded in paraffin from cases of OSCC that have been confirmed by histopathology. Both standard haematoxylin and eosin (H&E) and MCTS were applied to each section. Then all the sections were analysed by two observers for nucleus cytoplasmic intensity, break in the basement membrane, and advancing front of the tumour, muscle, and surrounding stroma. The efficacy of the stain was assessed and was graded as 1, poor; 2, fair; and 3, good based on the staining intensity.
Statistical Analysis:
The parameters were graded for H&E and modified Cajal’s stain. The results were subjected to the Chi-square test.
Result:
The above-mentioned parameters analysed showed a uniformly significant P value of 0.001 for comparing modified Cajal’s trichrome stain to H&E stain. Measurement of the agreement was done based on Kappa statistics between two observers, and the values for each expression show that there was good agreement between the two for all the parameters.
Conclusion:
MCTS can also be used as a diagnostic aid to pathologists for better distinction of cellular components and easier identification, thereby solving difficulties in diagnosis at earlier stages.
Keywords: H&E, MCTS, oral squamous cell carcinoma (OSCC)
INTRODUCTION
Oral squamous cell carcinoma (OSCC) is the most prevalent oral cancer, making up approximately 94% of all malignant neoplasms in the oral cavity.[1] Head and neck cancer is one of the main causes of morbidity and death.[2] An accurate diagnosis and early detection of epithelial pathologies are essential for appropriate treatment planning and the determination of the prognosis of OSCC.[3] The search for reliable parameters as prognostic predictors has increased due to the unpredictable behaviour of squamous cell carcinoma.[1]
The histo-morphological parameters include cytoplasm and nuclear intensity, break in the basement membrane, and advancing front of the tumour in tumour cells along with surrounding stromal cells. Though research on OSCC is increasingly using molecular biology, in routine practice, haematoxylin and eosin (H&E)-stained formalin-fixed paraffin-embedded tissues are the gold standard for pathological staging and cancer diagnosis.[4] In many circumstances, H&E stain may not be able to accurately identify or recognise the initial epithelial pathology that pathologists require, particularly in cases of early, micro-invasive squamous cell carcinoma (SCC), carcinoma in situ, and atypical epithelial malignancies.[5,6,7,8,9] Hence, diagnosing initial micro-invasiveness in OSCC using H&E stain alone will be challenging. In modern histology, the staining process is made more effective by combining various stains, which are then utilised to identify specific cells and structures.[10]
A special or differential staining procedure that differentiates different tissue elements in a histology section can prove to be an easy and affordable fix facilitating an easy and accurate diagnosis of some challenging cases.[9,11] One such good differential stain capable of identifying epithelial cells in connective tissue stroma is the modified Cajal’s trichrome stain (MCTS), which was developed by Gallego in 1919 after being first presented by Ramon Y. Cajal in 1897. It provides distinct hues in SCC according to the degree of cellular differentiation and keratinisation.[12,13]
This study aimed to evaluate and analyse the effectiveness of OSCC using MCTS.
MATERIALS AND METHODS
A study was conducted retrospectively with 30 tissue blocks embedded in paraffin from cases of OSCC that have been confirmed by histopathology. Both standard H&E and MCTS were applied to each section. All sections were then analysed by two observers and were graded as 1, poor; 2, fair; and 3, good based on staining intensity. Ethical committee approved by VMSDC/IEC/Approval No. 338.
Staining procedure for modified Cajal’s trichrome staining
Four micron-thick sections were taken from selected tissue blocks.
To deparaffinise the sections, they were heated for an hour at 70°C on a slide heater and then rehydrated with isopropyl alcohol.
The sections were then stained with Ziehl’s acetic fuchsin (Cajal fuchsin, 9 ml; acetic acid, 0.9 ml; distilled water, 15 ml) for 1 minute and then rinsed with water.
Differentiation was performed by treating the stained slide with a freshly prepared formalin–acetic acid solution for 5 minutes and then rinsing with water (equal proportions of acetic acid, 10% formalin, and distilled water)
Sections were treated with picroindigocarmine solution for 1 minute and rinsed with water (equal proportions of picric acid and indigocarmine).
The sections were then dehydrated in alcohol, cleaned, dried, and mounted.
RESULTS
MCTS is a suitable differential stain that is capable of recognising epithelial cells in connective tissue stroma. Nuclei colour red when stained with Cajal’s trichrome stain, epithelial cytoplasm stains pink green, connective tissue stains blue, muscle stains olive green, keratin stains green, and red blood cells colour grass green. The parameters that were evaluated are nucleus/cytoplasmic intensity, break in the basement membrane, advancing front of the tumour, muscle, and surrounding stroma. Using MCTS instead of H&E allowed for a clearer visualisation of all these [Table 1].
Table 1.
Percentage of the parameter graded for H&E and modified Cajal’s stain using the Chi-square test
| Parameters | H&E | Modified Cajal’s Stain | P | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Poor (N%) | Fair (N%) | Good (N%) | Poor (N%) | Fair (N%) | Good (N%) | ||
| Nucleus/cytoplasmic intensity | 54.84 | 45.16 | 0.00 | 16.13 | 45.16 | 38.71 | 0.001** |
| Break in basement membrane | 64.52 | 32.26 | 3.23 | 16.13 | 48.39 | 35.48 | 0.001** |
| Advancing front of tumour | 87.10 | 12.90 | 0.00 | 19.35 | 51.61 | 29.03 | 0.001** |
| Muscle | 70.97 | 29.03 | 0.00 | 16.13 | 51.61 | 32.26 | 0.001** |
| Surrounding stroma | 87.10 | 12.90 | 0.00 | 19.35 | 51.61 | 29.03 | 0.001** |
In modified Cajal’s trichrome stain, muscle damage is visible as under H&E, the tumour cells are masked in the infiltratory background. Fibroblasts, inflammatory cells, and other cellular structures stained with MCTS showed clear cellular details when compared to H&E [Figure 1a and b]. Measurement of agreement between the two observers for each parameter stained with MCTS and H&E is tabulated as follows [Table 2]. The different tonalities for the structure in the tumour in MCTS are seen in Figure 1b-d.
Figure 1.
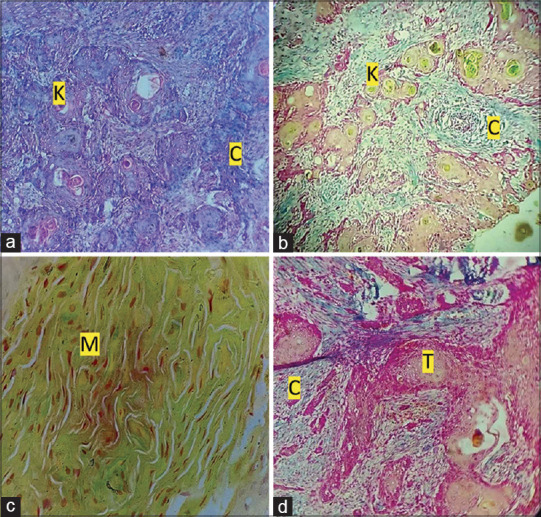
(a): Photomicro graph showing OSCC (H&E, 10×). (b): Photomicrograph showing evident keratin pearls (grass-green colour), connective tissue (blue colour), and tumour islands in OSCC (modified Cajal trichrome stain, 10×). (c): Photomicrograph showing muscle (olive green) in OSCC (modified Cajal trichrome stain, 40×). (d): Photomicrograph showing connective tissue (blue colour) and tumour islands in OSCC (modified Cajal trichrome stain, 10×). [K-Keratin pearl, C-Connective tissue, M-Muscle, T-Tumour island]
Table 2.
Meameasurement of the agreement was done based on Kappa statistics between 2 observers. The below values for each expression also show there was a good agreement between the two observers for all the parameters
| Modified Cajal’s stain | H&E | |||
|---|---|---|---|---|
|
|
|
|||
| Kappa value | P value | Kappa value | P value | |
| Nucleus/cytoplasmic intensity | 0.603 | 0.001 | 0.535 | 0.002 |
| Break in basement membrane | 0.426 | 0.002 | 0.73 | 0.001 |
| Advancing front of tumour | 0.464 | 0.001 | 0.597 | 0.001 |
| Muscle | 0.299 | 0.031 | 0.469 | 0.009 |
| Surrounding stroma | 0.442 | 0.001 | 0.304 | 0.05 |
DISCUSSION
H&E staining remains the standard method for routine histopathological diagnosis. It is a histological stain that is regularly used for light microscopy. H&E staining is useful for a wide range of nuclear and cytoplasmic findings and is compatible with several fixatives; nevertheless, it is sometimes not precise enough to identify or detect some of the aberrant components that pathologists need.[5,14] In these cases, diagnosing disease based on H&E stains alone can be difficult. A special or differential staining procedure that distinguishes different tissue components in a histological section may prove to be a simple and inexpensive solution that enables easy and accurate diagnosis of some difficult cases.
MCTS is one of these differential stains that may visually distinguish the epithelium and connective tissue parts with different colour tones. The overall differentiation of tissue elements using this stain is striking and can be used in histology teaching.[15] The principle of the modified CTS is that Ziehl’s acetic fuchsin, a low-molecular-weight dye, colours all structural elements dark pink. Most tissue elements lose their fuchsin colour when exposed to formol acetic acid, a differentiating agent; only the acidic tissues keep their fuchsin colour. The core retains its fuchsin colour through a process of “viro fixation” with formalin–acetic acid. Acetic acid was added as the differentiation fluid, and formaldehyde as the “viro fixation fluid” in modified Cajal’s procedure. The counterstain picroindigocarmine stains vital tissue (e.g., collagen) blue and strongly acidic tissue green.
In our study, the parameters studied under H&E and MCTS were assessed by two observers. Measurement of the agreement was done based on Kappa statistics between the two observers. The values for each expression show that there was good agreement between the two observers for all the parameters.
The cytoplasmic intensity of cells stained with modified CTS was compared with that of H&E and was found to be more pronounced in modified CTS, with a highly significant P value of 0.001**. This result is in line with the research that Srinivasan et al.[5] previously presented.
Tumour depth is one of the significant parameters for predicting regional metastasis,[16] which is usually masked by stromal components like inflammatory cell infiltrate. In our study, In our study, the invasion of the epithelial cells into the connective tissue and the presence of keratin beads are remarkably visible with a clear and distinct basement membrane, which was consistent with the research from Sanjay et al.[3]
Muscle destruction is visible in the modified Cajal’s trichrome stain as in routine H&E stain mostly the inflammatory cells and infiltrating tumor cells in the background obscures the field. Similar results were observed in a study by Ganapathy N et al.[11]
The histopathological examination of surgically removed formalin-fixed tissue forms the basis for tumour diagnosis, pathological assessment, and staging.[17] MCTS is easily implemented in histopathology laboratories with minimal time and cost and can be used to screen large samples. In our study, in addition to other parameters, advancing front of tumour has been analysed with MCTS, which was not done in the previous studies. Use of MCTS serves as an essential laboratory tool to identify the early invasion of tumour cells by differentiating the tumour cells from the surrounding stroma and its invasion in deeper structures and thus aids in accurate diagnosis and thereby improves the prognosis of the patient.
CONCLUSION
MCTS can also be used as a diagnostic aid to pathologists for better distinction of cellular components and easier identification, thereby solving difficulties in diagnosis at earlier stages. The inclusiveness of this stain to oral pathology can be beneficial due to its striking difference in tissue elements when compared to routine H&E staining.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ghazi N, Ghazi A, Shafiee S, Fayyazi M. Importance of depth of invasion in patients with oral squamous cell carcinoma: A review article. J Orofac Sci. 2018;10:3–6. [Google Scholar]
- 2.Bugshan A, Farooq I. Oral squamous cell carcinoma: Metastasis, potentially associated malignant disorders, etiology and recent advancements in diagnosis. F1000Res. 2020;9:29. doi: 10.12688/f1000research.22941.1. doi:10.12688/f1000research. 22941.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanjay K, Baker A, Reddy LP, Pandey B. Modified Cajal's trichrome stain as a diagnostic aid in the study of epithelial pathology. Indian J Pathol Microbiol. 2017;60:528. doi: 10.4103/IJPM.IJPM_202_16. [DOI] [PubMed] [Google Scholar]
- 4.Woolgar JA, Triantafyllou A. Pitfalls and procedures in the histopathological diagnosis of oral and oropharyngeal squamous cell carcinoma and a review of the role of pathology in prognosis. Oral Oncol. 2009;45:361–85. doi: 10.1016/j.oraloncology.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Srinivasan V, Kumar GK, Shyam ND, Narayen V, Konda P, Rani KS. Microinvasive oral squamous cell carcinoma redefined by using modified cajal trichrome differential stain - A histochemical study. J Orofac Sci. 2022;14:71–9. [Google Scholar]
- 6.Bolesina N, Femopase FL, Lópezde Blanc SA, Morelatto RA, Olmos MA. Ogbureke KUE, editor. Oral squamous cell carcinoma clinical aspects, oral cancer. InTech. [Last accessed on 2017 Aug 13]. doi:10.5772/32968. Available from:https://cdn.intechopen.com/pdfs-wm/31751.pdf .
- 7.Shankar AA, Gokul S. The dilemma of microinvasion. Head Neck Oncol. 2014;6:30. [Google Scholar]
- 8.Soyab T. Special stains used in histopathological techniques: A brief view. Indian J Forensic Med Toxicol. 2020;14:8632–6. [Google Scholar]
- 9.Musumeci G. Past, present and future: Overview on histology and histopathology. J Histol Histopathol. 2014;1:1–3. [Google Scholar]
- 10.Black JG, Black LJ. Microbiology:principles and explorations. John Wiley &Sons, Inc; United States of America: 2018. [Google Scholar]
- 11.Ganapathy N, Sudha JP, Thangadurai M, Shankar JD, Prabhunath TR. Evaluation of efficacy of modified cajal's trichrome stain in oral lesions in comparison with hematoxylin &eosin stain–a histopathological study. Int J Med Dent Pharm Allied Health Sci. 2021;1:8. [Google Scholar]
- 12.Castroveijo R. Modifications of differential stains with special reference to the trichromic stain of Cajal. Am J Clin Pathol. 1932;2:135–41. [Google Scholar]
- 13.Titford M. Progress in the development of microscopical techniques for diagnostic pathology. J Histotechnol. 2009;32:9–19. [Google Scholar]
- 14.Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harbor Protocols. 2008;3:pdb.prot4986. doi: 10.1101/pdb.prot4986. doi:10.1101/pdb.prot4986. [DOI] [PubMed] [Google Scholar]
- 15.OrtizHidalgo C. Abelardo Gallego (18791930) and his contributions to histotechnology: The Gallego stains. Acta Histochem. 2011;113:189–93. doi: 10.1016/j.acthis.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Jangir NK, Singh A, Jain P, Khemka S. The predictive value of depth of invasion and tumor size on risk of neck node metastasis in squamous cell carcinoma of the oral cavity: A prospective study. J Cancer Res Ther. 2022;18:977–83. doi: 10.4103/jcrt.JCRT_783_20. [DOI] [PubMed] [Google Scholar]
- 17.Chan JK. The wonderful colors of the hematoxylin-eosin stain in diagnostic surgical pathology. Int J Surg Pathol. 2014;22:12–32. doi: 10.1177/1066896913517939. [DOI] [PubMed] [Google Scholar]


