Abstract
Context:
Platelet concentrates are rich in growth factors that assist in regenerative medicine to promote healing and tissue regeneration. Similarly, partially demineralized tooth is a storehouse of many growth factors, assisting in bone regeneration. Hence, the present study aimed to quantify the release of growth factors from different platelet concentrates individually and when mixed with a partially demineralized tooth matrix.
Method:
Human growth factors such as bFGF, EGF, PDGF-AB, TGF-beta-1, and VEGF-A present in platelet-rich fibrin and injectable platelet-rich fibrin from young and old male and female donors were quantified separately. Then these platelet concentrates were then mixed with a partially demineralized tooth matrix, which was powdered using a Smart Dentin Grinder. An enzyme-linked immunosorbent assay was used for the quantification of growth factors.
Results:
The release of growth factors, such as platelet-rich fibrin and injectable platelet-rich fibrin, was not statistically significant; however, it was significantly greater when i-PRF was mixed with a partially demineralized tooth matrix.
Conclusion:
The study revealed that the expression of growth factors was significantly greater when a partially demineralized tooth matrix was mixed with injectable platelet-rich fibrin than when combined with platelet-rich fibrin.
Keywords: Growth factors, I-PRF, partially demineralized tooth matrix, platelet concentrates, platelet rich fibrin
INTRODUCTION
Angiogenesis, the process of new capillary formation from the existing vasculature and “neovascularization,” plays very important role in wound healing and tissue regeneration along with polypeptide growth factors.[1] These growth factors are biological mediators that regulate the proliferation, differentiation, and formation of the extracellular matrix,[2] thereby acting as scaffolds along with autologous cells. In recent decades, blood-based biomaterials such as platelet-rich plasma (PRP), platelet-rich fibrin (PRF), fibrin sealant, platelet fibrin glue, and platelet gel have been extensively researched in regenerative medicine.[3] PRP was the first-generation platelet concentrate studied, mainly in periodontology for wound healing and bone formation; currently, second-generation PRF is used extensively, as it enhances healing and regeneration. The newer generation of platelet concentrates releases sustainable growth factors for longer durations, which may be attributed to variations in centrifugation techniques, including rotations per minute (rpm), time of exposure, and pressure applied.[4]
Platelet concentrates contain many growth factors, such as platelet-derived growth factor (PDGF), transforming growth factors beta 1 and 2 (TGF-β1 and TGF-β2), insulin-like growth factor (IGF), basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), and human vascular endothelial growth factor (VEGF).[5] The use of PRP is limited due to presence of anticoagulants, which may affect the release of growth factors and healing. Therefore, Choukroun’s platelet-rich fibrin membrane (PRF), which has no anticoagulants,[6] was more promising for its osteoblastic potential because of the increased release of growth factors; thus, used to treat gingival recession, socket preservation, and wound closure.[7,8,9]
These growth factors have different functions: PDGF regulates the migration, proliferation, and survival of mesenchymal cells; TGF-β assists in tissue repair and activates the synthesis of macrophages and endothelial cells; and VEGF assists in angiogenesis. The process of wound healing is multifactorial, and these platelet concentrates enhance healing by acting as a scaffold rich in leukocytes.[10] Although PRP contains 6–8-fold more blood-derived growth factors, Choukroun’s PRF membrane has shown sustained and prolonged release of growth factors due to its complex three-dimensional fibrin network. Injectable platelet-rich fibrin (i-PRF) obtained by low centrifugation speed and relative centrifugal force (RCF) is currently used in treating periodontal defects; however, more research needs to be done to understand its benefits and risk in comparison with other platelet concentrates.[11]
Studies have shown that by decreasing the relative centrifugal force (RCF), centrifugation speed, and time, the resulting PRF increases the release of leukocytes, platelets and growth factors, but its clinical relevance needs to be further researched, especially in the extracellular matrix.[12,13,14,15] In the past decade, the use of demineralized teeth as a graft material has been studied extensively, as many growth factors are present in demineralized teeth, and, their composition is similar to that of alveolar bone.[16]
Hence, the present study was undertaken to estimate and compare human growth factors bFGF, EGF, PDGF-AB, TGF-beta1, and VEGF-A expressed in PRF and i-PRF individually and, when combined with a partially demineralized tooth matrix (PDTM), on the basis of the age and sex of the donor. The null hypothesis states that there is no difference in the expression of growth factors from PRF or i-PRF individually or when PDTM is combined with both PRF and i-PRF, irrespective of age or sex.
METHODOLOGY
The in vitro observational study was conducted in Sterlix Laboratory, Peenya, Bangalore for a period of 1 month, and a total of 12 extracted teeth (3 young males and 3 young females and 3 each old males and females) were used in the study. The blood samples for preparation of the platelet concentrates were procured from 12 volunteers, of which 6 were aged between 40 and 50 years (3 males, 3 females) and 6 were aged 18 to 25 years (3 males, 3 females). The inclusion criterion for young patients was teeth extracted for orthodontic purposes only, and for older patients, extraction indicated for periodontal issues only. The exclusion criterion was tooth extracted due to trauma, root canal treatment, or caries. Blood drawn from healthy individuals with no underlying medical conditions was included in the study, and volunteers who were alcoholic, smokers, pregnant, or lactating were excluded from the study. Institutional ethical approval was obtained for the study (Ref: UECHT/2016-18/PhD_16DSDP732002), the results were tabulated, and statistical analysis was performed via an independent sample “t” test.
Preparation of PRF and i-PRF: PRF and i-PRF were prepared via a standard protocol of drawing 10 ml of blood for each preparation into sterile vials without anticoagulant from 12 volunteers (6 males, 6 females). The volunteers were further divided into 4 groups: 3 young male volunteers and 3 young female volunteers and old male and female volunteers aged 40--55 years. A total of 24 vials of blood were collected, 12 each for the PRF and i-PRF formulations. The blood was centrifuged (REMI; R-4C) at 3300 rpm for 10 minutes for PRF and at 700 rpm for 3 minutes for i-PRF. The middle fibrin layer from the PRF vials and the liquid from the i-PRF vials were carefully collected for the quantification of growth factors.
Preparation of Partially Demineralized Tooth Matrix Powder: The PDTM was prepared by using teeth extracted from each group. The teeth were cleaned, washed under running water, dried, and powdered using a Smart Dentin Grinder. This was followed by partial demineralization for 10 minutes with 0.6 M hydrochloric acid, and washed with phosphate-buffered saline (PBS, 1 mg/ml) and dried.
ELISA: The levels of growth factors expressed in PRF, i-PRF, and when both platelet concentrates were combined with PDTM were assessed via a 96-well plate ELISA Kit (Ray Biotech, Suite 100 Norcross, GA). The standards and samples were prepared according to the manufacturer’s instructions and pipetted into the wells containing the target and, the growth factors in the samples bounded in the wells by the immobilized antibody. The wells were then washed, and biotinylated anti-human antibody was added. The unbound biotinylated antibody was washed away, and horseradish peroxidase (HRP)-conjugated streptavidin was pipetted into the wells. The wells were again washed, and TMB (3,3’,5,5’, tetramethylbenzidine) substrate solution was added, which developed color in proportion to the amount of growth factor. A stop solution of 0.16 M sulfuric acid was added to the wells, which changed the color of the bonded substrate from blue to yellow, and the intensity of the color obtained was measured. The unknown concentrations of all growth factors present in the samples were evaluated. The ratio of the mixture of platelet concentrates with PDTM was 1 mg/ml (1:1 ratio). The data were analyzed for growth factors present PRF and i-PRF samples and when they were combined separately with PDTM. The enzyme activity was measured via a spectrophotometer at a specific wavelength of 450 nm. The study was performed and triplicate for accuracy.
Statistical analysis
Independent t test was done to compare the difference between the means of two groups to determine the difference from each other.
RESULTS
The independent samples t test revealed statistical significance in VEGF (P < 0.033) and TGF beta (P < 0.001) in the i-PRF group YM and for VEGF (P < 0.001) and bFGF (P < 0.002) in the PRF group of the OM samples. In YF, TGF beta (P < 0.003) and PDGF-AB (P < 0.001) was significantly higher in the PRF groups, and in OF, VEGF (P < 0.001), TGF beta (P < 0.001), and PDGF-AB (P < 0.001) were significantly higher in the i-PRF groups [Table 1].
Table 1.
Comparison of growth factor expression between the i-PRF and PRF groups among young males (YMs), old males (OMs), young females (YFs), and old females (OFs)
| Group | N | Mean | SD | T | P | |
|---|---|---|---|---|---|---|
| b-FGF | YM-i-PRF | 3 | 0.22 | 0.01 | -0.472 | 0.661 |
| YM-PRF | 3 | 0.23 | 0.02 | |||
| VEGF | YM-i-PRF | 3 | 0.65 | 0.02 | 3.195 | 0.033* |
| YM-PRF | 3 | 0.58 | 0.03 | |||
| EGF | YM-i-PRF | 3 | 0.41 | 0.01 | -1.909 | 0.129 |
| YM-PRF | 3 | 0.42 | 0.01 | |||
| TGF Beta | YM-i-PRF | 3 | 0.38 | 0.01 | 23.992 | 0.001* |
| YM-PRF | 3 | 0.24 | 0.01 | |||
| PDGF-AB | YM-i-PRF | 3 | 0.99 | 0.02 | 2.345 | 0.079 |
| YM-PRF | 3 | 0.86 | 0.09 | |||
| b-FGF | OM-i-PRF | 3 | 0.14 | 0.01 | -7.238 | 0.002* |
| OM-PRF | 3 | 0.2 | 0.01 | |||
| VEGF | OM-i-PRF | 3 | 0.34 | 0.01 | -8.615 | 0.001* |
| OM-PRF | 3 | 0.42 | 0.01 | |||
| EGF | OM-i-PRF | 3 | 0.28 | 0.05 | 0.479 | 0.657 |
| OM-PRF | 3 | 0.27 | 0.01 | |||
| TGF Beta | OM-i-PRF | 3 | 0.27 | 0.01 | -1.851 | 0.138 |
| OM-PRF | 3 | 0.3 | 0.02 | |||
| PDGF-AB | OM-i-PRF | 3 | 0.59 | 0.15 | 0.189 | 0.859 |
| OM-PRF | 3 | 0.58 | 0.04 | |||
| b-FGF | YF-i-PRF | 3 | 0.17 | 0.01 | -1.502 | 0.207 |
| YF-PRF | 3 | 0.19 | 0.03 | |||
| VEGF | YF-i-PRF | 3 | 0.55 | 0.01 | -1.488 | 0.211 |
| YF-PRF | 3 | 0.57 | 0.03 | |||
| EGF | YF-i-PRF | 3 | 0.32 | 0.02 | -1.917 | 0.128 |
| YF-PRF | 3 | 0.34 | 0.01 | |||
| TGF Beta | YF-i-PRF | 3 | 0.23 | 0.02 | -6.404 | 0.003* |
| YF-PRF | 3 | 0.32 | 0.01 | |||
| PDGF-AB | YF-i-PRF | 3 | 0.43 | 0.03 | -20.744 | 0.001* |
| YF-PRF | 3 | 0.8 | 0.01 | |||
| b-FGF | OF-i-PRF | 3 | 0.22 | 0.02 | 0.804 | 0.467 |
| OF-PRF | 3 | 0.21 | 0 | |||
| VEGF | OF-i-PRF | 3 | 0.64 | 0.01 | 8.326 | 0.001* |
| OF-PRF | 3 | 0.45 | 0.04 | |||
| EGF | OF-i-PRF | 3 | 0.35 | 0.02 | 2.252 | 0.087 |
| OF-PRF | 3 | 0.3 | 0.04 | |||
| TGF Beta | OF-i-PRF | 3 | 0.41 | 0.01 | 13.535 | 0.001* |
| OF-PRF | 3 | 0.31 | 0.01 | |||
| PDGF-AB | OF-i-PRF | 3 | 0.94 | 0.03 | 7.886 | 0.001* |
| OF-PRF | 3 | 0.58 | 0.08 |
*Statistical significance set at 0.05; N: Number of samples; and SD: Standard deviation
The expression of growth factors associated with the combination of PDTM and i-PRF samples revealed that bFGF among YF (P < 0.009) and OF (P < 0.028) was significant, and VEGF expression was highest in all groups, YM, OM YF, and OF (P < 0.014), P < 0.001, P < 0.001, and P < 0.001, respectively. EGF and TGF beta expression was also similar to VEGF expression when PDTM was combined with i-PRF. EGF and TGF beta were significantly greater (P < 0.001), (P < 0.015), and (P < 0.041) (P < 0.001) in the YM, OM, and OF groups i-PRF mixed with PDTM group. PDGF-AB also had similar effects on the PDTM samples of YM, OM, YF, and OF mixed with i-PRF (P < 0.007), (P < 0.002), (P < 0.002), and (P < 0.007), respectively [Table 2].
Table 2.
Comparison of growth factor i-PRF and PRF groups among young males (YM), old males (OM), young females (YF), and old females (OF) when combined with a partially demineralized tooth matrix (PDTM)
| Growth factors | Groups | N | Mean | SD | T | P |
|---|---|---|---|---|---|---|
| b-FGF | YM-iPRF+PDTM | 3 | 0.26 | 0.04 | 2.417 | 0.073 |
| YM-PRF+PDTM | 3 | 0.19 | 0.03 | |||
| OM-i-PRF+PDTM | 3 | 0.21 | 0.01 | 1.076 | 0.342 | |
| OM-PRF+PDTM | 3 | 0.2 | 0.02 | |||
| YF-i-PRF+PDTM | 3 | 0.23 | 0.01 | 4.711 | 0.009* | |
| YF-PRF+PDTM | 3 | 0.19 | 0.01 | |||
| OF-i-PRF+PDTM | 3 | 0.27 | 0.01 | 3.375 | 0.028* | |
| OF-PRF+PDTM | 3 | 0.2 | 0.04 | |||
| VEGF | YM-iPRF+PDTM | 3 | 0.78 | 0.02 | 4.193 | 0.014* |
| YM-PRF+PDTM | 3 | 0.68 | 0.03 | |||
| OM-iPRF+PDTM | 3 | 0.77 | 0.01 | 11.538 | <.001* | |
| OM-PRF+PDTM | 3 | 0.58 | 0.02 | |||
| YF-iPRF+PDTM | 3 | 0.67 | 0.02 | 8.054 | 0.001* | |
| YF-PRF+PDTM | 3 | 0.53 | 0.02 | |||
| OF-iPRF+PDTM | 3 | 0.78 | 0.02 | 8.004 | 0.001* | |
| OF-PRF+PDTM | 3 | 0.58 | 0.03 | |||
| EGF | YM-iPRF+PDTM | 3 | 0.46 | 0.01 | 5.954 | 0.004* |
| YM-PRF+PDTM | 3 | 0.39 | 0.02 | |||
| OM-iPRF+PDTM | 3 | 0.43 | 0.02 | 5.789 | 0.004* | |
| OM-PRF+PDTM | 3 | 0.36 | 0.01 | |||
| YF-iPRF+PDTM | 3 | 0.41 | 0.02 | 5.747 | 0.005* | |
| YF-PRF+PDTM | 3 | 0.33 | 0.02 | |||
| OF-iPRF+PDTM | 3 | 0.44 | 0.01 | 4.466 | 0.011* | |
| OF-PRF+PDTM | 3 | 0.36 | 0.03 | |||
| TGF-β | YM-iPRF+PDTM | 3 | 0.45 | 0.01 | 10.215 | <.001* |
| YM-PRF+PDTM | 3 | 0.34 | 0.01 | |||
| OM-iPRF+PDTM | 3 | 0.35 | 0.03 | 4.125 | 0.015* | |
| OM-PRF+PDTM | 3 | 0.28 | 0.01 | |||
| YF-iPRF+PDTM | 3 | 0.28 | 0.01 | 2.962 | 0.041* | |
| YF-PRF+PDTM | 3 | 0.23 | 0.02 | |||
| OF-iPRF+PDTM | 3 | 0.47 | 0.01 | 7.811 | 0.001* | |
| OF-PRF+PDTM | 3 | 0.34 | 0.03 | |||
| PDGF-AB | YM-iPRF+PDTM | 3 | 1.05 | 0.03 | 5.179 | 0.007* |
| YM-PRF+PDTM | 3 | 0.91 | 0.03 | |||
| OM-iPRF+PDTM | 3 | 0.95 | 0.04 | 7.67 | 0.002* | |
| OM-PRF+PDTM | 3 | 0.76 | 0.02 | |||
| YF-iPRF+PDTM | 3 | 0.85 | 0.03 | 7.217 | 0.002* | |
| YF-PRF+PDTM | 3 | 0.67 | 0.03 | |||
| OF-iPRF+PDTM | 3 | 0.96 | 0.04 | 5.192 | 0.007* | |
| OF-PRF+PDTM | 3 | 0.7 | 0.08 |
*Statistical significance set at 0.05; N: Number of samples; and SD: Standard deviation
Independent sample t tests revealed significantly higher levels of all growth factors when PDTM was combined with i-PRF than when it was combined with PRF.
The observed concentrations of bFGF in PRF alone were 0.4 pg/ml, 0.31 pg/ml, 0.29 pg/ml, and 0.34 pg/ml in young and old male and female (YM, OM, YF, and OF) samples and 0.28 pg/ml, 0.3 pg/ml, 0.28 pg/ml, and 0.3 pg/ml when combined with PDTM, respectively. There was little variation in the bFGF content between PRF and PRF mixed with PDTM [Table 3; Figures 1 and 1.1].
Table 3.
Summarized concentrations of growth factors observed in PRF and i-PRF alone and when partially demineralized tooth matrix (PDTM) is combined with PRF and i-PRF
| Growth factor | bFGF | EGF | PDGF | TGF-beta | VEGF |
|---|---|---|---|---|---|
| YM-PRF | 0.41±0.01 | 1.22±0.07 | 4.73±0.09 | 1.27±0.007 | 1.43±0.03 |
| OM-PRF | 0.31±0.06 | 0.59±0.01 | 1.67±0.03 | 1.55±0.19 | 0.77±0.08 |
| YF-PRF | 0.29±0.03 | 0.89±0.08 | 3.62±0.01 | 1.66±0.09 | 1.37±0.02 |
| OF-PRF | 0.34±0.01 | 0.71±0.03 | 1.67±0.07 | 1.6±0.09 | 0.86±0.03 |
| YM-PRF+PDTM | 0.28±0.02 | 1.07±0.01 | 6.39±0.03 | 1.79±0.01 | 2.07±0.03 |
| OM-PRF+PDTM | 0.3±0.02 | 0.93±0.01 | 3.17±0.01 | 1.45±0.01 | 1.42±0.02 |
| YF-PRF+PDTM | 0.28±0.01 | 0.84±0.01 | 2.26±0.03 | 1.19±0.02 | 1.18±0.02 |
| YM-iPRF | 0.39±0.01 | 1.16±0.009 | 11.39±0.02 | 1.99±0.07 | 1.83±0.01 |
| OM-iPRF | 0.12±0.01 | 0.64±0.047 | 1.76±0.15 | 1.42±0.01 | 0.56±0.01 |
| YF-iPRF | 0.2±0.01 | 0.78±0.024 | 1.02±0.02 | 1.18±0.02 | 1.25±0.01 |
| OF-iPRF | 0.38±0.02 | 0.92±0.015 | 7.62±0.02 | 2.11±0.01 | 1.73±0.01 |
| YM-iPRF-PDTM | 0.52±0.03 | 1.41±0.09 | 42.4±0.03 | 2.31±0.008 | 3.05±0.02 |
| OM-iPRF- PDTM | 0.36±0.01 | 1.29±0.01 | 7.92±0.03 | 1.81±0.02 | 2.92±0.01 |
| YF-iPRF- PDTM | 0.41±0.005 | 1.18±0.01 | 4.57±0.02 | 1.44±0.01 | 2.01±0.02 |
| OF-iPRF-PDTM | 0.56±0.007 | 1.3±0.01 | 9.05±0.03 | 2.46±0.01 | 3.07±0.02 |
Figure 1.
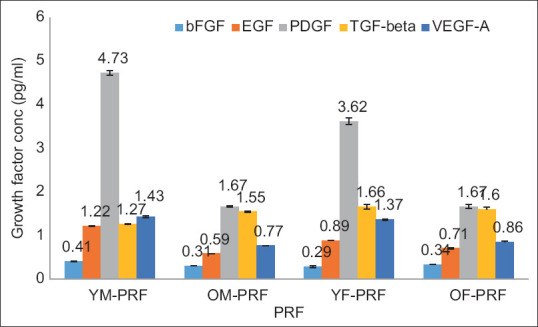
Overlaid bar graph depicting the concentration of growth factors expressed in the PRF samples
Figure 1.1.
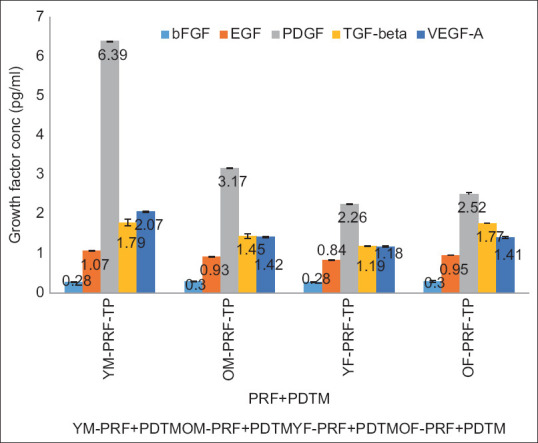
Overlaid bar graph depicting the concentration of growth factors expressed in different PRFs mixed with a partially demineralized tooth matrix
EGF in the PRF group alone was 1.22 pg/ml, 0.59 pg/ml, 0.89 pg/ml, and 0.71 pg/ml, and in combination with PDTM, it was 1.07 pg/ml, 0.93 pg/ml, 0.84 pg/ml, and 0.95 pg/ml in the YM, OM, YF, and OF samples, respectively. EGF expression was high when PDTM was combined with PRF in the OM and OF samples [Table 3; Figures 1 and 1.1].
The PDGF-AB concentrations in the PRF as well as the PRF mixed with PDTM were 4.73 pg/ml, 1.67 pg/ml, 3.62 pg/ml, and 1.67 pg/ml and 6.39 pg/ml, 3.17 pg/ml, 2.26 pg/ml, and 2.52 pg/ml in the YM, OM, YF, and OF samples, respectively, with a slight increase in the concentration of the PRF combined with PDTM [Table 3; Figures 1 and 1.1].
The levels of TGF-beta recorded in the PRF and PRF combined with PDTM were 1.27 pg/ml, 1.55 pg/ml, 1.66 pg/ml, and 1.6 pg/ml and 1.79 pg/ml, 1.45 pg/ml, 1.19 pg/ml, and 1.77 pg/ml, respectively, in the YM, OM, YF, and OF samples. Increased levels of TGF-beta were observed in the combination samples from the YM and OF groups [Table 3; Figures 1 and 1.1].
The VEGF concentrations in the PRF and PDTM groups were 1.45 pg/ml, 0.77 pg/ml, and 1.37 pg/ml; 0.86 pg/ml; 2.07 pg/ml; 1.42 pg/ml; 1.18 pg/ml; and 1.41 pg/ml; and the YM, OM, YF, and OF samples, respectively. Increased levels of VEGF were observed in the combination samples, with the exception of the YF samples [Table 3; Figures 1 and 1.1]. In the i-PRF group and the i-PRF with PDTM group, the growth factor levels were relatively greater in the i-PRF mixed with PDTM group.
The observed concentrations of bFGF in i-PRF were 0.39 pg/ml, 0.12 pg/ml, 0.2 pg/ml, and 0.38 pg/ml, and those in combination with PDTM were 0.52 pg/ml, 0.36 pg/ml, 0.41 pg/ml, and 0.56 pg/ml for the YM, OM, YF, and OF samples, respectively [Table 3; Figures 2 and 2.1].
Figure 2.
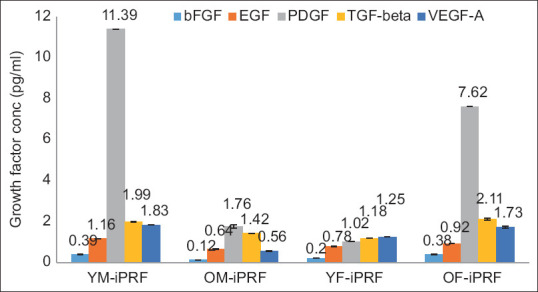
Overlaid bar graph depicting the concentrations of growth factors expressed in different i-PRF at 700 rpm
Figure 2.1.
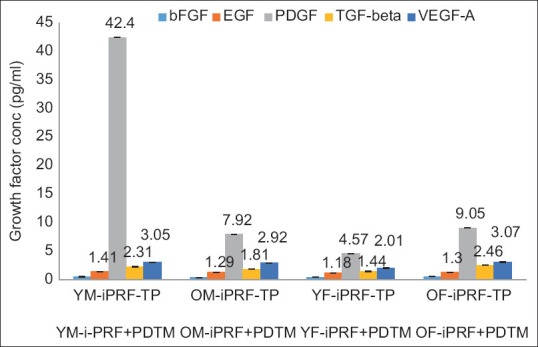
Overlaid bar graph depicting the concentration of growth factors expressed in different i-PRFs mixed with partially demineralized tooth matrix
The EGF levels in i-PRF were 1.16 pg/ml, 0.64 pg/ml, 0.78 pg/ml, and 0.92 pg/ml, and those in PDTM were 1.41 pg/ml, 1.29 pg/ml, 1.18 pg/ml, and 1.3 pg/ml for the YM, OM, YF, and OF samples, respectively. EGF expression was increased in the combination samples [Table 3; Figures 2 and 2.1].
The PDGF-AB concentrations in i-PRF and its combination were 1.39 pg/ml, 1.76 pg/ml, 1.02 pg/ml, and 7.62 pg/ml and 42.4 pg/ml, 7.92 pg/ml, 4.57 pg/ml, and 9.05 pg/ml in the YM, OM, YF, and OF samples, respectively, indicating relatively high PDGF contents when PDTM was combined with i-PRF [Table 3; Figures 2 and 2.1].
The levels of TGF-beta recorded in i-PRF and its combination with PDTM were 1.99 pg/ml, 1.42 pg/ml, 1.18 pg/ml, and 2.11 pg/ml and 2.31 pg/ml, 1.81 pg/ml, 1.44 pg/ml, and 2.46 pg/ml, respectively, in the YM, OM, YF, and OF groups, with increases in the combination group [Table 3; Figures 2 and 2.1].
The VEGF concentrations in i-PRF and i-PRF mixed with PDTM were 1.83 pg/ml, 0.56 pg/ml, 1.25 pg/ml, and 1.73 pg/ml and 3.05 pg/ml, 2.92 pg/ml, 2.01 pg/ml, and 3.07 pg/ml in the YM, OM, YF, and OF samples, respectively [Table 3; Figures 2 and 2.1].
DISCUSSION
Growth factors play a significant role in tissue healing and regeneration. The most common autogenous graft material used for bone regeneration is the iliac crest, which contains TGF-β, FGF, VEGF, PDGF, IGF, and BMPs.[17] Bone morphogenic proteins (BMPs) and growth factors present in demineralized bone matrix in large quantities, especially BMP-4 and TGF-β1; however, their release varies on the basis of donor conditions.[18] The current study also revealed that the maximum number of growth factors released was PDGF-AB, followed by TGF-β and VEGF.
The demineralized dentin matrix (DDM) contains BMPs and growth factors expressed in picograms (pg); therefore, issues of toxicity or ectopic mineralization are rare. Studies on DDMs via ELISA have demonstrated the presence of TGF-β, VEGF, FGF-2, PDGF, BMPs, and inulin-like growth factor-1 (IGF-1). The results of the current study revealed that TGF-β was followed by FGF-2, VEGF, PDGF, and IGF-1.[19] In the present study, the release of growth factors when the PDTM was mixed with i-PRF and PRF was as follows: PDGF-AB, TGF-β, h VEGF, EGF, and b FGF. IGF-1 was not assessed in the current study because a study by Richard D. Finkelman reported that IGF-I was the least expressed.[20]
When the platelet-rich fibrin matrix (PRFM) and PRF were compared in vitro, the release of PDGF, VEGF, EGF, FGF, TGF-β, and IGF was found to be significantly greater in the PRFM initially, but the release of growth factors from the PRF was sustained up to 23 days.[21] The results of the present study, however, revealed the release of growth factors in the order of PDGF-AB, TGF-β, VEGF, EGF, and bFGF in PRF.
Another in vitro study on the release of growth factors in PRF at different time intervals revealed the sustainable release of growth factors, highest being IGF-1, followed by TGF-β1, PDGF-AB, VEGF, and EGF.[22] In the present study, IGF-1 release was not assessed, but there was a change in the order of release of growth factors which were PDGF-AB, TGF-β, VEGF, and EGF.
The release of growth factors from i-PRF resulted in increased concentrations of PDGF-AB and TGF-β9, which was in accordance with our study, in which PDGF-AB and TGF-β were highly expressed in i-PRF. Many studies on i-PRF have concluded that, at different centrifugation forces, there is greater release of VEGF and TGF-β1 growth factor[6,12]; these results are similar to those of the current study, which revealed similar values of VEGF and TGF-β1.
The correlation of growth factor release with donor age and sex in PRP revealed that the release of PDGF-AB and TGF-β was more similar to that reported in the present study, but there was no significant difference in age or sex.[5] In the present study, however, there was a statistically significant difference in terms of sex and age, with young males and old females in the TGF-β group (P = 0.001), old females in the PDGF-AB group (P = 0.001) in the i-PRF and young females in the TGF-β (P = 0.003) and PDGF-AB (P = 0.001) groups in the PRF.
A study performed by Gummaluri SS compared the clinical efficacy of titanium-prepared platelet-rich fibrin (T-PRF) and leucocyte platelet-rich fibrin (L-PRF) for infrabony defects and concluded that T-PRF filled the defect better than L-PRF clinically and radiographically; however, this difference was not significant.[23] In the present study, the addition of PDTM to platelet concentrates resulted in a statistically significant release of growth factors, especially with i-PRF, which can promote the treatment of bony defects.
SUMMARY AND CONCLUSION
Within the limitations of the study, the following conclusions were drawn:
The i-PRF samples from young males significantly released growth factors from h VEGF (P = 0.033) and TGF-β (P = 0.001), whereas in old males, bFGF (P = 0.002) and h VEGF (P = 0.001) were more abundant in the PRF samples.
In the PRF samples from older females, PDGF (P = 0.001), VEGF (P = 0.001), and TGF-β (P = 0.001) were significant, whereas in young females, were significant for TGF-β (P = 0.003) and PDGF-AB (P = 0.001).
When the partially demineralized tooth matrix of all the groups was mixed with i-PRF and PRF, the release of growth factors was significantly greater in the i-PRF groups than in the PRF groups.
Further studies need to be performed to evaluate BMP release from PDTM, PRF, and i-PRF, which should be evaluated clinically.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
M.S. Ramaiah University of Applied Sciences, for providing me with the opportunity to conduct the study. Faculty of Dental Sciences, Department of Oral and Maxillofacial Surgery for providing the samples for the study, and Department of Oral and Maxillofacial Pathology for their valuable inputs for conducting ELISA.
M.S. Ramaiah University of Applied Sciences, Research Department and Pro-Vice Chancellor (Research) Dr. Govind R Kadambi for providing seed money for procuring “The Smart Dentin Grinder” used in the study.
Sterlix Lab, Peenya, and Bangalore Karnataka for providing their laboratory facilities for conducting the study under trained technicians and sterile environments.
REFERENCES
- 1.Clark RDJ, Smith AJ. Angiogenic growth factors in human dentine matrix. Arch Oral Biol. 2000;45:1013–6. doi: 10.1016/s0003-9969(00)00075-3. [DOI] [PubMed] [Google Scholar]
- 2.Borsani E, Bonazza V, Buffoli B, Cocchi MA, Castrezzati S, Scarì G, et al. Biological characterization and in vitro effects of human concentrated growth factor preparation: An innovative approach to tissue regeneration. Biol Med. 2015;7:1–11. [Google Scholar]
- 3.Burnouf T, Goubran HA, Chen TM, Ou KL, El-Ekiaby M, Radosevic M. Blood-derived biomaterials and platelet growth factors in regenerative medicine. Blood Rev. 2013;27:77–89. doi: 10.1016/j.blre.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Qiao J, An N, Ouyang X. Quantification of growth factors in different platelet concentrates. Platelets. 2017;28:774–8. doi: 10.1080/09537104.2016.1267338. [DOI] [PubMed] [Google Scholar]
- 5.Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Cranio-Maxillofac Surg. 2002;30:97–102. doi: 10.1054/jcms.2002.0285. [DOI] [PubMed] [Google Scholar]
- 6.El Bagdadi K, Kubesch A, Yu X, Al-Maawi S, Orlowska A, Dias A, et al. Reduction of relative centrifugal forces increases growth factor release within solid platelet-rich-fibrin (PRF)-based matrices: A proof of concept of LSCC (low speed centrifugation concept) Eur J Trauma Emerg Surg. 2019;45:467–79. doi: 10.1007/s00068-017-0785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu CL, Lee SS, Tsai CH, Lu KH, Zhao JH, Chang YC. Platelet-rich fibrin increases cell attachment, proliferation and collagen-related protein expression of human osteoblasts. Aust Dent J. 2012;57:207–12. doi: 10.1111/j.1834-7819.2012.01686.x. [DOI] [PubMed] [Google Scholar]
- 8.Kim J, Ha Y, Kang NH. Effects of growth factors from platelet-rich fibrin on the bone regeneration. J Craniofac Surg. 2017;28:860–5. doi: 10.1097/SCS.0000000000003396. [DOI] [PubMed] [Google Scholar]
- 9.Miron RJ, Fujioka-Kobayashi M, Hernandez M, Kandalam U, Zhang Y, Ghanaati S, et al. Injectable platelet rich fibrin (i-PRF): Opportunities in regenerative dentistry? Clin Oral Investig. 2017;21:2619–27. doi: 10.1007/s00784-017-2063-9. [DOI] [PubMed] [Google Scholar]
- 10.Eren G, Gürkan A, Atmaca H, Dönmez A, Atilla G. Effect of centrifugation time on growth factor and MMP release of an experimental platelet-rich fibrin-type product. Platelets. 2016;27:427–32. doi: 10.3109/09537104.2015.1131253. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Zhang Y, Choukroun J, Ghanaati S, Miron RJ. Effects of an injectable platelet-rich fibrin on osteoblast behavior and bone tissue formation in comparison to platelet-rich plasma. Platelets. 2018;29:48–55. doi: 10.1080/09537104.2017.1293807. [DOI] [PubMed] [Google Scholar]
- 12.Choukroun J, Ghanaati S. Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients'own inflammatory cells, platelets and growth factors: The first introduction to the low speed centrifugation concept. Eur J Trauma Emerg Surg. 2018;44:87–95. doi: 10.1007/s00068-017-0767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujioka-Kobayashi M, Miron RJ, Hernandez M, Kandalam U, Zhang Y, Choukroun J. Optimized platelet-rich fibrin with the low-speed concept: Growth factor release, biocompatibility, and cellular response. J Periodontol. 2017;88:112–21. doi: 10.1902/jop.2016.160443. [DOI] [PubMed] [Google Scholar]
- 14.Dohan Ehrenfest DM, Pinto NR, Pereda A, Jiménez P, Corso MD, Kang BS, et al. The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, and fibrin architecture of a leukocyte-and platelet-rich fibrin (L-PRF) clot and membrane. Platelets. 2018;29:171–84. doi: 10.1080/09537104.2017.1293812. [DOI] [PubMed] [Google Scholar]
- 15.Marcazzan S, Weinstein RL, Del Fabbro M. Efficacy of platelets in bone healing: A systematic review on animal studies. Platelets. 2018;29:326–37. doi: 10.1080/09537104.2017.1327652. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt-Schultz TH, Schultz M. Intact growth factors are conserved in the extracellular matrix of ancient human bone and teeth: A storehouse for the study of human evolution in health and disease. Biol Chem. 2005;386:767–76. doi: 10.1515/BC.2005.090. [DOI] [PubMed] [Google Scholar]
- 17.Schmidmaier G, Herrmann S, Green J, Weber T, Scharfenberger A, Haas NP, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39:1156–63. doi: 10.1016/j.bone.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 18.Blum B, Moseley J, Miller L, Richelsoph K, Haggard W. Measurement of bone morphogenetic proteins and other growth factors in demineralized bone matrix. Orthopedics. 2004;27:S161–5. doi: 10.3928/0147-7447-20040102-17. [DOI] [PubMed] [Google Scholar]
- 19.Gao X, Qin W, Wang P, Wang L, Weir MD, Reynolds MA, et al. Nanostructured demineralized human dentin matrix to enhance bone and dental repair and regeneration. Appl Sci. 2019;9:1013. [Google Scholar]
- 20.Finkelman RD, Mohan S, Jennings JC, Taylor AK, Jepsen S, Baylink DJ. Quantitation of growth factors IGF-I, SGF/IGF-II, and TGF-β in human dentin. J Bone Miner Res. 1990;5:717–23. doi: 10.1002/jbmr.5650050708. [DOI] [PubMed] [Google Scholar]
- 21.Chatterjee A, Debnath K. Comparative evaluation of growth factors from platelet concentrates: An in vitro study. J Indian Soc Periodontol. 2019;23:322–8. doi: 10.4103/jisp.jisp_678_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su CY, Kuo YP, Tseng YH, Su CH, Burnouf T. In vitro release of growth factors from platelet-rich fibrin (PRF): A proposal to optimize the clinical applications of PRF. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2009;108:56–61. doi: 10.1016/j.tripleo.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Gummaluri SS, Bhattacharya HS, Astekar M, Cheruvu S. Evaluation of titanium-prepared platelet-rich fibrin and leucocyte platelet-rich fibrin in the treatment of intrabony defects: A randomized clinical trial. J Dent Res Dent Clin Dent Prospects. 2020;14:83–91. doi: 10.34172/joddd.2020.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


