Abstract
Background:
Autophagy is intimately associated with the development of cardiomyopathy and has received widespread attention in recent years. However, no relevant bibliometric analysis is reported at present. In order to summarize the research status of autophagy in cardiomyopathy and provide direction for future research, we conducted a comprehensive, detailed, and multidimensional bibliometric analysis of the literature published in this field from 2004 to 2023.
Methods:
All literatures related to autophagy in cardiomyopathy from 2004 to 2023 was collected from the Web of Science Core Collection, and annual papers, global publication trends, and proportion charts were analyzed and plotted using GraphPad price v8.0.2. In addition, CtieSpace [6.2.4R (64-bit) Advanced Edition] and VOSviewer (1.6.18 Edition) were used to analyze and visualize these data.
Results:
Two thousand two hundred seventy-nine papers about autophagy in cardiomyopathy were accessed in the Web of Science Core Collection over the last 20 years, comprising literatures from 70 countries and regions, 2208 institutions, and 10 810 authors. China contributes 56.32% of the total publications, substantially surpassing other countries, while the United States is ranked first in frequency of citations. Among the top 10 authors, six are from China, and four are from the United States. Air Force Military Medical University was the institution with the highest number of publications, while the Journal of Molecular and Cellular Cardiology (62 articles, 2.71% of the total) was the journal with the highest number of papers published in the field. Clustering of co-cited references and temporal clustering analysis showed that ferroptosis, hydrogen sulfide mitophagy, lipid peroxidation, oxidative stress, and SIRT1 are hot topics and trends in the field. The principal keywords are oxidative stress, heart, and heart failure.
Conclusion:
The research on autophagy in cardiomyopathy is in the developmental stage. This represents the first bibliometric analysis of autophagy in cardiomyopathy, revealing the current research hotspots and future research directions in this field.
Keywords: autophagy, bibliometric, cardiomyopathy, CiteSpace, VOSviewer
Introduction
Highlights
This study is a comprehensive bibliometric analysis of autophagy in cardiomyopathies which aims to elucidate autophagy and identify hotspots.
China has become a leader in autophagy research in cardiomyopathy, contributing 56.32% of the total publications, substantially surpassing other countries, while the United States is ranked first in frequency of citations.
Air Force Military Medical University was the institution with the highest number of publications, while Journal of Molecular and Cellular Cardiology (62 articles, 2.71% of the total) was the journal with the highest number of papers published in the field.
Ferroptosis, hydrogen sulfide mitophagy, lipid peroxidation, oxidative stress, and SIRT1 are hot topics and trends in the field.
Cardiomyopathy is an organic myocardial lesion caused by different etiologies, including abnormal mechanical activity and/or electrical dysfunction of the heart. Pathologically, it manifests as inappropriate ventricular dilation or hypertrophy, affecting the contraction or relaxation function of the heart, ultimately leading to severe heart failure, atrial or ventricular arrhythmias, or complicated damage to other organs such as the kidneys1,2. The pathogenesis of cardiomyopathy is complex and diverse, and its molecular mechanisms have not been fully elucidated3. Autophagy is now recognized to be instrumental in the etiopathogenesis of cardiomyopathies4. Autophagy is the process of the cell itself breaking down and reusing damaged organelles and proteins, which plays an important role in maintaining intracellular homeostasis and purging abnormal proteins5,6.
Recent studies have shown a strong link between cardiomyopathy and autophagy7. One research study found that autophagy levels typically increased in cardiac tissues of patients with cardiomyopathy8. Autophagy helps remove damaged or abnormal proteins and organelles in order to maintain the normal function of cardiomyocytes6. However, when autophagy is dysregulated, it may also enhance the deterioration of cardiomyopathy9. Accordingly, autophagy may be a therapeutic target for cardiomyopathy. Several researchers have tried to investigate drugs or other ways to modulate the autophagic process in cardiomyocytes to ameliorate cardiomyopathy. Some drugs have been shown to have potential therapeutic effects in animal models of cardiomyopathy10–13.
Although research on the role of autophagy in cardiomyopathy has been accomplished, the current understanding of autophagy in cardiomyopathy is still in its preliminary stages. Moreover, the explosive growth of publications may prevent researchers from fully understanding the key developments and future directions in the field of autophagy in cardiomyopathy. Therefore, it is necessary to conduct a systematic analysis of the hotspots and trends in this special field. Bibliometrics is an emerging approach to knowledge synthesis that identifies quantitative and qualitative attributes of publications and explores salient research trends in the field of study14. With the explosion of scientific research, the metrological analysis of publications has become increasingly important15. Thus, bibliometric analysis has far-reaching implications for the study of disease evolution and cutting-edge trends16–18. As far as we know, there is no bibliometric analysis for the study of autophagy in cardiomyopathy. This study aims to conduct a bibliometric analysis of the number of publications, major contributing countries, institutions, journals, and individuals on autophagy in cardiomyopathy research over the past 20 years. The current research hotspots will be summarized, and existing problems will be identified. The results of this study are expected to suggest directions for investigators on autophagy in cardiomyopathy.
Materials and methods data collection
In this study, we searched the literature related to autophagy in cardiomyopathy from the Web of Science Core Collection (WoSCC) for the last 20 years on January 18, 2024 and performed a comprehensive analysis using bibliometric methods. The search terms included: ((((((((((((((((((((((((((TS=(Cardiomyopathies)) OR TS=(Cardiomyopathy)) OR TS=(Myocardiopathies)) OR TS=(Myocardiopathy)) OR TS=(Myocardial Diseases)) OR TS=(Myocardial Disease)) OR TS=(Disease, Myocardial)) OR TS=(Diseases, Myocardial)) OR TS=(Cardiomyopathies, Secondary)) OR TS=(Cardiomyopathy, Secondary)) OR TS=(Secondary Cardiomyopathies)) OR TS=(Secondary Cardiomyopathy)) OR TS=(Secondary Myocardial Diseases)) OR TS=(Myocardial Diseases, Secondary)) OR TS=(Disease, Secondary Myocardial)) OR TS=(Diseases, Secondary Myocardial)) OR TS=(Myocardial Disease, Secondary)) OR TS=(Cardiomyopathies, Primary)) OR TS=(Cardiomyopathy, Primary)) OR TS=(Primary Cardiomyopathies)) OR TS=(Primary Cardiomyopathy)) OR TS=(Primary Myocardial Diseases)) OR TS=(Myocardial Diseases, Primary)) OR TS=(Primary Myocardial Disease)) OR TS=(Disease, Primary Myocardial)) OR TS=(Diseases, Primary Myocardial)) OR TS=(Myocardial Disease, Primary) AND (((((((((TS=(Autophagy)) OR TS=(Autophagy, Cellular)) OR TS=(Cellular Autophagy)) OR TS=(Autophagocytosis)) OR TS=(Reticulophagy)) OR TS=(ER-Phagy)) OR TS=(ER Phagy)) OR TS=(Nucleophagy)) OR TS=(Ribophagy)) OR TS=(Lipophagy). The literature selection process for this study was based on the following three inclusion criteria: first, the full text of publications related to the role of autophagy in cardiomyopathy was available; second the articles and review manuscript categories were written in English; and third the articles were published in the interval from January 1, 2004, to December 31, 2023. The exclusion criteria were as follows: first the topic was not related to the role of autophagy in cardiomyopathy; and second the article was a conference abstract, news, or briefing paper. Plain text versions of the papers were then exported. Figure 1 shows a flowchart of the search strategy and selection process in this study.
Figure 1.
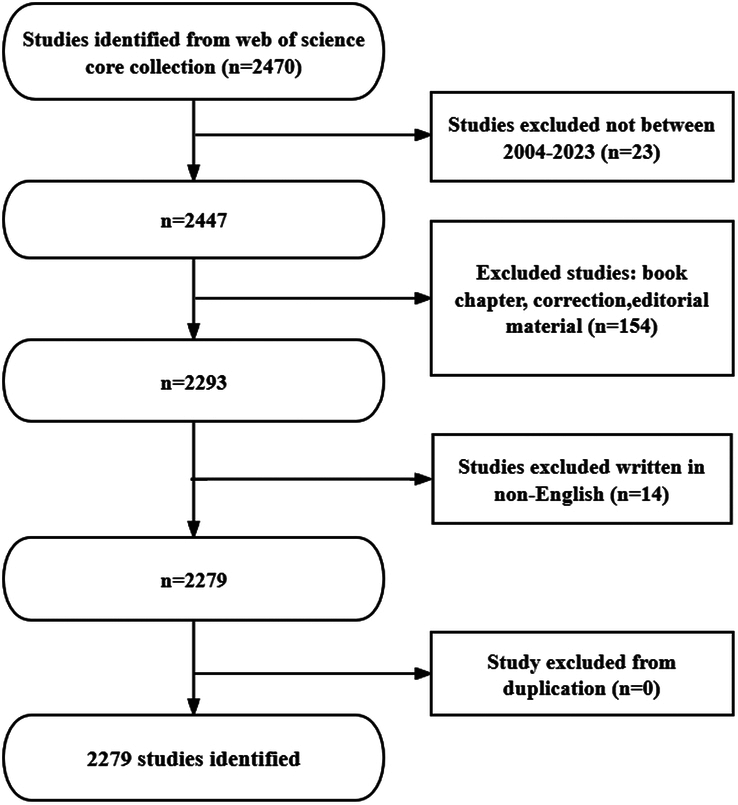
Flowchart of the literature screening process.
Data analysis
GraphPad Prism v8.0.2 was used to analyze and plot yearly publication trends and trends in publications from different countries. In addition, CtieSpace [6.2.4R (64-bit) Advanced Edition] and VOSviewer (version 1.6.18) were used to analyze these data and visualize the scientific knowledge graph. VOSviewer created by van Eck and Waltman19, is a free JAVA-based software for analyzing large amounts of literature data and displaying it in a map format. In order to visualize the results of research in a particular field by mapping the co-citation network of literature, Prof. Chaomei Chen developed the CiteSpace (6.1.6R) software20, which envisions the use of an experimental framework to study new concepts and evaluate existing technologies. This enables users to understand areas of knowledge better, research frontiers and trends, and predict their future research progress.
Results
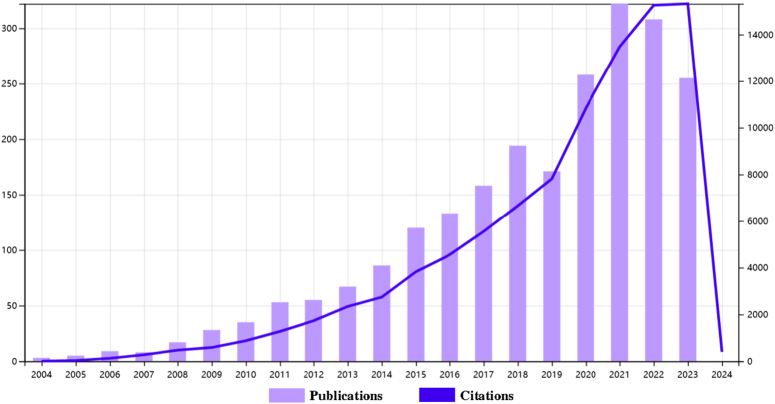
The results showed that from January 1, 2004 to December 31, 2023, the WoSCC database contained 2279 publications on the role of autophagy in cardiomyopathy (Fig. 1).
The literature involved 70 countries and regions, 2208 institutions, and 10 810 authors. As shown, the number of papers published each year has slowly increased since 2004. We categorize the 20 years of publications into three phases (Fig. 2): the number of papers increased slowly during 2004–2008, with less than 20 publications per year, indicating that this field was not noticed by researchers, and the number of publications gradually increased from 2009 to 2014, indicating that this field gradually entered the field of researchers, and the number of publications in this field increased rapidly after 2015 and reached a peak in 2021, which indicates that the field has received widespread attention after 2015.
Figure 2.
Trends in annual publications and annual total citations.
Countries and institutions
The data show that 70 countries and regions have researched the role of autophagy in cardiomyopathy. Figure 3A–B shows the annual publication volume of the top 10 countries in the past 20 years, as shown the top five countries in this field are China, USA, Italy, Japan, and Germany. China accounts for 56.32% of the total number of publications, which is far more than other countries. Among the top 10 countries/regions in terms of the number of publications, the number of citations of the papers published in the United States is 44 994 (Table 1), which is much higher than that of all the other countries/regions, and its citation/publication ratio (68.90) ranks fourth among all the countries/regions, which indicates that the quality of the papers published in the United States is generally high. China is the first country in terms of the number of publications (1287) and ranks second in terms of the number of citations (30 959), and its citation/publication ratio (24.06) ranks at the back of the list, indicating that the quality of its published papers is generally low. The network of cooperation between countries is shown in Figure 3C: the United States cooperates closely with France, Italy, Germany, and the United Kingdom, while China cooperates more closely with Australia, India, and Japan. With a large number of publications, high citation frequency, and centrality of 0.13, China is the leading country in this field. In recent years, the amount of articles published by countries such as the United States and Japan has increased rapidly, which may be related to their cooperation with China.
Figure 3.
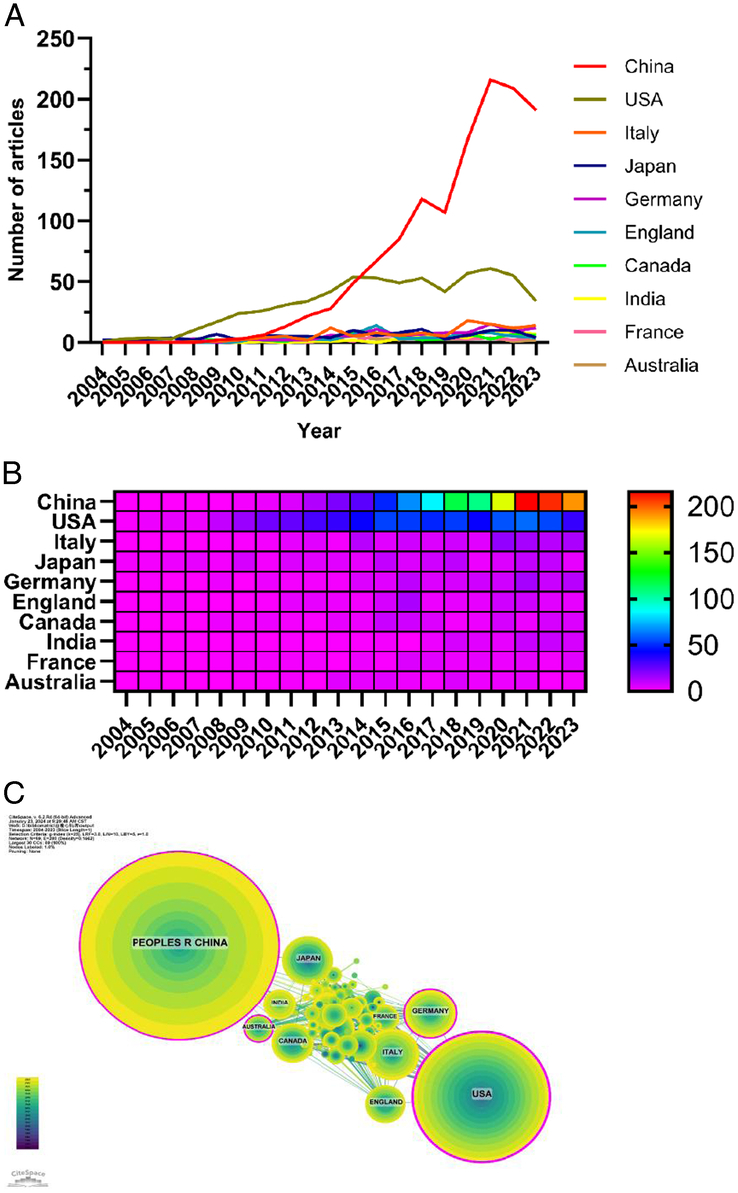
Contributions of countries to the research of autophagy in cardiomyopathy. (A) Line graph of national communications. (B) Hotmap of national issuances. (C) Network map of cooperation among countries.
Table 1.
The top 10 countries contributing to publication in autophagy of cardiomyopathy.
| Ranks | Country/region | Article counts | Centrality | Percentage | Citation | Citation per publication |
|---|---|---|---|---|---|---|
| 1 | China | 1287 | 0.13 | 56.32 | 30959 | 24.06 |
| 2 | USA | 653 | 0.00 | 18.58 | 44994 | 68.90 |
| 3 | Italy | 118 | 0.11 | 5.16 | 6305 | 53.43 |
| 4 | Japan | 110 | 0.01 | 4.81 | 12490 | 113.55 |
| 5 | Germany | 100 | 0.05 | 4.38 | 6701 | 67.01 |
| 6 | England | 73 | 0.04 | 3.19 | 5416 | 74.19 |
| 7 | Canada | 69 | 0.02 | 3.02 | 3467 | 50.25 |
| 8 | India | 50 | 0.05 | 2.19 | 1290 | 25.80 |
| 9 | France | 45 | 0.05 | 1.97 | 3104 | 68.98 |
| 10 | Australia | 43 | 0.03 | 1.88 | 2523 | 58.67 |
Two thousand two hundred eight institutions systematically published articles on the role of autophagy in cardiomyopathy. Among the top 10 institutions in terms of publications, six were from China, and four were from the United States (Table 2, Fig. 4). Air Force Military Medical University published the most literature (84 papers, 3325 citations, 39.58 citations/paper). Fudan University (81 papers, 2510 citations, 30.99 citations/paper) ranked second, the University of Texas System (61 papers, 4187 citations, 68.64 citations/paper) ranked third, and the University of California System (59 papers, 2795 citations, 47.37 citations/paper) ranked fourth, Shandong University (56 papers, 1472 citations, 26.29 times/paper) ranked fifth.
Figure 4.
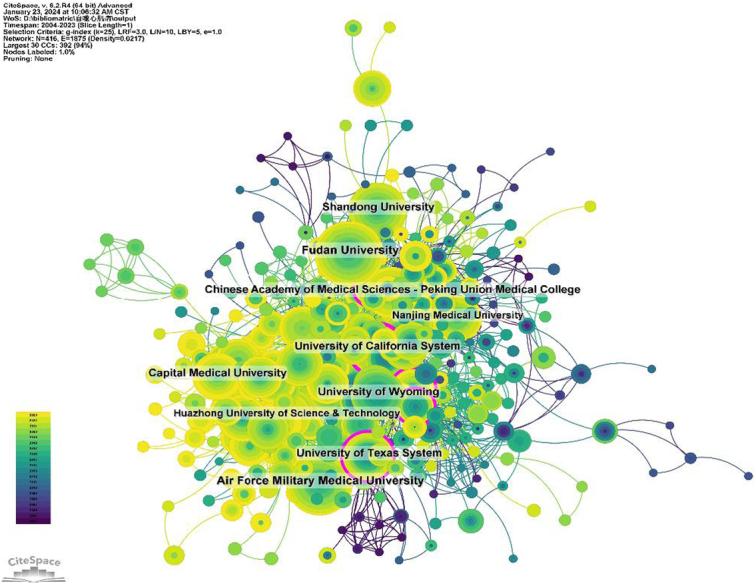
Institutional collaboration network map.
Table 2.
The top 10 institutions contributed to publications in the autophagy of cardiomyopathy research.
| Ranks | Institution | Country | Number of studies | Total citations | Average citation |
|---|---|---|---|---|---|
| 1 | Air Force Military Medical University | USA | 84 | 3325 | 39.58 |
| 2 | Fudan University | China | 81 | 2510 | 30.99 |
| 3 | University of Texas System | USA | 61 | 4187 | 68.64 |
| 4 | University of California System | USA | 59 | 2795 | 47.37 |
| 5 | Shandong University | China | 56 | 1472 | 26.29 |
| 6 | Chinese Academy of Medical Sciences – Peking Union Medical College | China | 55 | 1946 | 35.38 |
| 7 | University of Wyoming | USA | 53 | 2438 | 46.00 |
| 8 | Capital Medical University | China | 50 | 1210 | 24.20 |
| 9 | Nanjing Medical University | China | 43 | 959 | 22.30 |
| 10 | Huazhong University of Science & Technology | China | 43 | 974 | 22.65 |
Journals
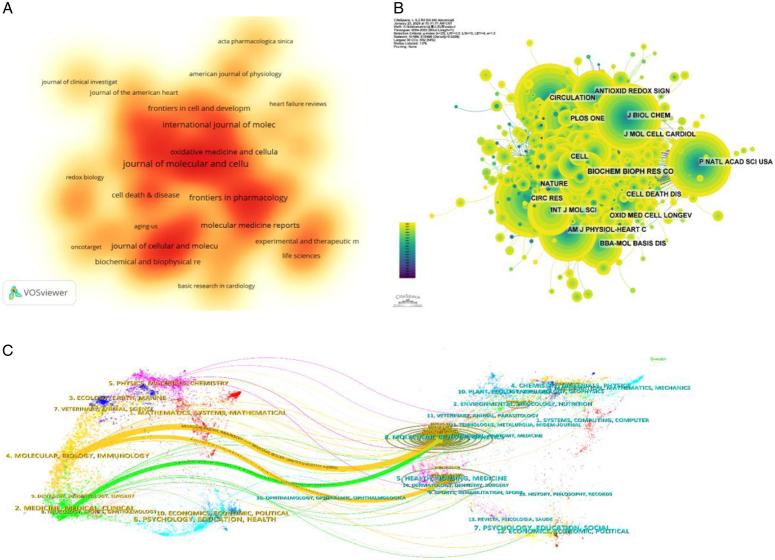
The top 10 most productive and most cited journals are listed in Tables 3 and 4, respectively. Journal of Molecular and Cellular Cardiology (62 articles, 2.71%) is the most published journal in this field, followed by Frontiers in Cardiovascular Medicine (51 articles, 2.23%), Frontiers in Pharmacology (48 articles, 2.10%), Biomedicine & Pharmacotherapy (44 articles, 1.93%), and International Journal of Molecular Sciences (44 articles, 1.93%). Figure 5A is a map of the density of magazine issues. Among the top 10 most prolific journals, Circulation Research had the highest IF of 20.1. All of these journals were categorized in either Q1 or Q2 regions. Journal impact is determined by how often it is co-cited, which indicates whether the journal has had a significant impact on the scientific community. According to Figure 5B and Table 4, the journal with the highest number of co-citations is Circ Res (1608), followed by Circulation (1501) and J BIOL CHEM (1342). Among the top 10 most co-cited journals, Nature was cited 1240 times with the highest IF among the top 10 journals (64.8). Among the co-cited journals, all journals were in the Q1/Q2 region.
Table 3.
The top 10 journals that contributed to publications in the field of autophagy of cardiomyopathy.
| Ranks | Journal | Article counts | Percentage(2285) | IF | Quartile in category |
|---|---|---|---|---|---|
| 1 | Journal of Molecular and Cellular Cardiology | 62 | 2.71 | 5.0 | Q2 |
| 2 | Frontiers in Cardiovascular Medicine | 51 | 2.23 | 3.6 | Q2 |
| 3 | Frontiers in Pharmacology | 48 | 2.10 | 5.6 | Q1 |
| 4 | Biomedicine & Pharmacotherapy | 44 | 1.93 | 7.5 | Q1 |
| 5 | International Journal of Molecular Sciences | 44 | 1.93 | 5.6 | Q1 |
| 6 | Autophagy | 43 | 1.88 | 13.3 | Q1 |
| 7 | Biochimica et Biophysica Acta-Molecular Basis of Disease | 42 | 1.84 | 6.2 | Q1 |
| 8 | Journal of Cellular and Molecular Medicine | 41 | 1.79 | 5.3 | Q2 |
| 9 | Circulation Research | 39 | 1.71 | 20.1 | Q1 |
| 10 | Oxidative Medicine and Cellular Longevity | 35 | 1.53 | 7.31 | Q2 |
Table 4.
The top 10 co-cited journals associated with autophagy of cardiomyopathy.
| Ranks | Cited journal | Co-citation | IF (2022) | Quartile in category |
|---|---|---|---|---|
| 1 | Circ Res | 1608 | 20.1 | Q1 |
| 2 | Circulation | 1501 | 37.8 | Q1 |
| 3 | J Biol Chem | 1342 | 4.8 | Q2 |
| 4 | Autophagy | 1317 | 13.3 | Q1 |
| 5 | J Mol Cell Cardiol | 1290 | 5.0 | Q2 |
| 6 | Nature | 1240 | 64.8 | Q1 |
| 7 | P Natl Acad Sci USA | 1237 | 11.1 | Q1 |
| 8 | PLOS ONE | 1192 | 3.7 | Q2 |
| 9 | Cell | 1140 | 64.5 | Q1 |
| 10 | Cardiovasc Res | 1117 | 10.9 | Q1 |
Figure 5.
Analysis of journals. (A) Density map of magazine issues. (B) Journal co-citation network map. (C) Journal dual stacked chart.
The thematic distribution of scholarly publications is shown by a double map overlay. (Fig. 5C). The colored tracks indicate citation links, with citing journals on the left and cited journals on the right. Based on the displayed results, we identified three main colored citation paths: studies published in molecular/biology/immunology were mainly cited by studies published in journals in the fields of molecular/biology/genetics and health/nursing/medicine, whereas medicine/medical/clinical published studies are mainly cited by studies published in journals in the field of molecular/biology/genetics.
Authors
Of all the authors who have published literature related to the role of autophagy in cardiomyopathy, Table 5 lists the 10 authors with the most publications. The top 10 authors published a total of 253 papers, accounting for 11.07% of all papers in this field. Ren, Jun published the most research papers with 60, followed by Zhang, Yingmei (32) and Sadoshima, Junichi (30). Further analysis shows that the top 10 authors are six from China and four from the United States. CiteSpace visualizes the network between authors (Fig. 6A). Figure 6B and Table 5 show the top 10 authors with the highest number of co-citations and citations, respectively. One hundred twenty authors have been cited more than 50 times in total, indicating that their research is highly reputable and influential. The largest nodes are associated with the most co-cited authors, including Mizushima N (429 citations), Levine B (360 citations), and Nakai A (331 citations).
Table 5.
The top 10 authors and co-cited authors in the autophagy of cardiomyopathy research.
| Rank | Author | Count | Location | Rank | Co-cited author | Citation |
|---|---|---|---|---|---|---|
| 1 | Ren, Jun | 60 | China | 1 | Mizushima N | 429 |
| 2 | Zhang, Yingmei | 32 | China | 2 | Levine B | 360 |
| 3 | Sadoshima, Junichi | 30 | USA | 3 | Nakai A | 331 |
| 4 | Hill, Joseph a | 24 | USA | 4 | Matsui Y | 323 |
| 5 | Wang, Xuejun | 22 | China | 5 | Klionsky DJ | 310 |
| 6 | Robbins, Jeffrey | 19 | USA | 6 | Sciarretta | 309 |
| 7 | Sciarretta | 18 | USA | 7 | Zhang Y | 227 |
| 8 | Sun, Dongdong | 18 | China | 8 | Kanamori H | 213 |
| 9 | Wang, Haichang | 15 | China | 9 | Kim J | 212 |
| 10 | zhang | 15 | China | 10 | Zhu Hx | 207 |
Figure 6.
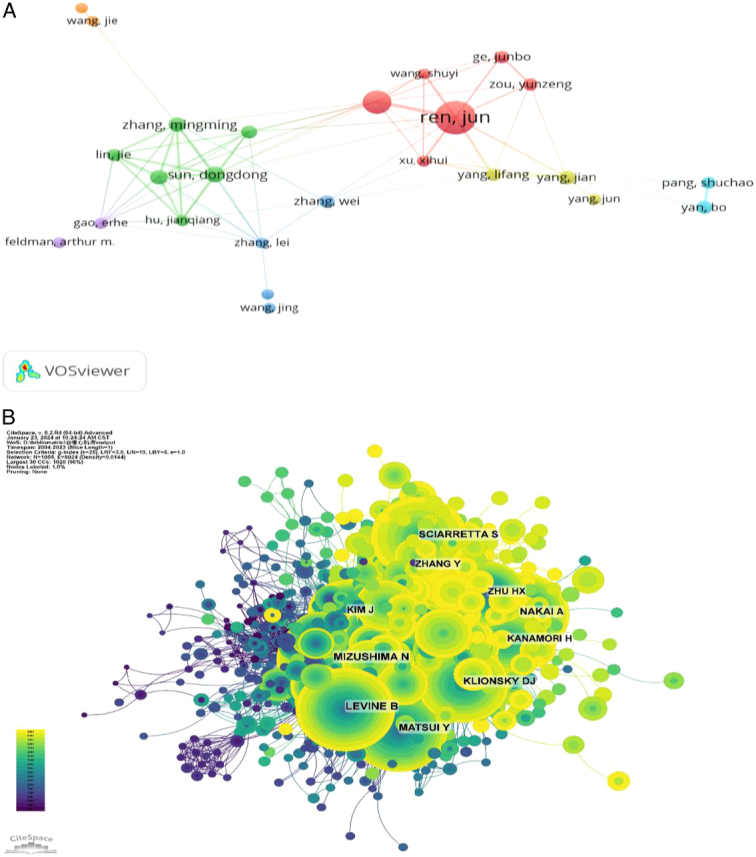
Contributions of the authors of investigations on autophagy in cardiomyopathy. (A) Top 10 author collaboration network chart for publications on autophagy in cardiomyopathy. (B) Top 10 most co-cited and cited authors.
Literatures
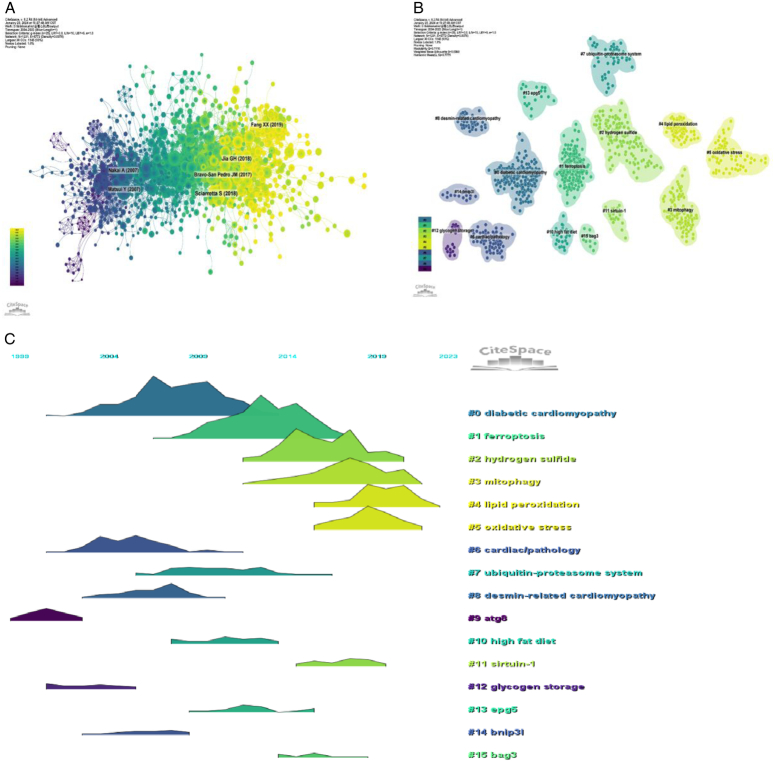
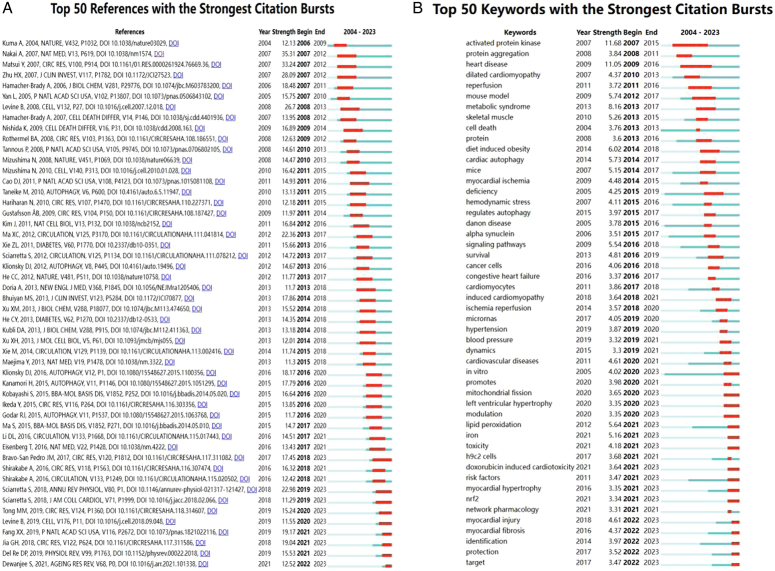
The co-cited reference network contained 1231 nodes and 5772 links, using a 1-year time slice with a time frame from 2004 to 2023 (Fig. 7A). According to the top 10 most co-cited articles (Table 6), the article entitled ‘The Role of Autophagy in the Heart’ in Annual Review Of Physiology (IF=18.2) was the most co-cited reference and Sciarretta, Sebastiano was the first author of the article.
Figure 7.
Co-citation reference analysis. (A) Co-cited literature network map. (B–C) Analysis of co-cited reference clustering and time-clustering.
Table 6.
The top 10 cited references in the field of autophagy of cardiomyopathy.
| Ranks | Cited references | Total citations |
|---|---|---|
| 1 | Sciarretta S, Maejima Y, Zablocki D, et al. The Role of Autophagy in the Heart. Annu Rev Physiol, 2018,80:1–26. | 95 |
| 2 | Jia G, Hill MA, Sowers JR. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ Res, 2018,122(4):624–638. | 87 |
| 3 | Nakai A, Yamaguchi O, Takeda T, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med, 2007,13(5):619–24. | 69 |
| 4 | Matsui Y, Takagi H, Qu X, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res, 2007,100(6):914–22. | 65 |
| 5 | Bravo-San Pedro JM, Kroemer G, Galluzzi L. Autophagy and Mitophagy in Cardiovascular Disease. Circ Res, 2017,120(11):1812–24. | 64 |
| 6 | Fang X, Wang H, Han D, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A, 2019,116(7):2672–2680. | 63 |
| 7 | Klionsky DJ, Abdelmohsen K, Abe A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy, 2016,12(1):1–222. | 59 |
| 8 | Zhu H, Tannous P, Johnstone JL, et al. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest, 2007,117(7):1782–93. | 55 |
| 9 | Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell, 2008,132(1):27–42. | 55 |
| 10 | Li DL, Wang ZV, Ding G, et al. Doxorubicin Blocks Cardiomyocyte Autophagic Flux by Inhibiting Lysosome Acidification. Circulation, 2016,133(17):1668–87. | 54 |
We performed co-citation reference clustering and temporal clustering analyses (Fig. 7B–C). We found that diabetic cardiomyopathy (cluster0), cardiac/patholohy (cluster6), atg8 (cluster9), and glycogen storage (cluster11) were the hotspots in early-stage research. ubiquitin-proteasome system (cluster7), desmin-related cardiomyopathy (cluster8), high-fat diet (cluster10), epg5 (cluster13), and bag3 (cluster15) are hotspots for mid-term research. Ferroptosis (cluster1), hydrogen sulfide (cluster2), mitophagy (cluster3), lipid peroxidation (cluster4), oxidative stress (cluster5), sirtuin-1 (cluster11) are hot topics and trends in the field.
Keywords
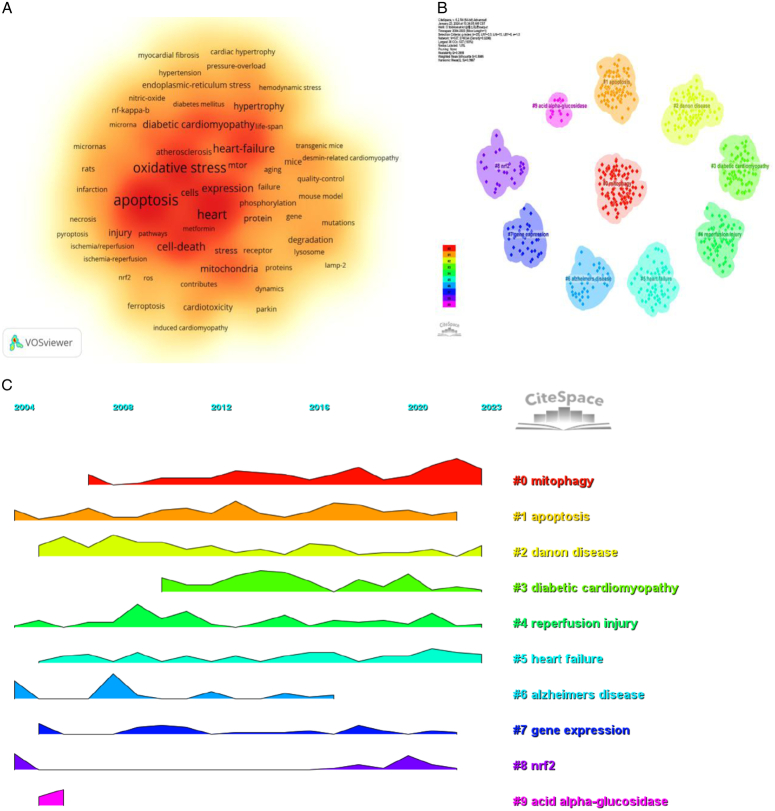
By analyzing keywords, we can get a quick overview of a field and its direction. According to the co-occurrence of keywords in VOSwiever, the most popular keyword was apoptosis (629), followed by oxidative stress (515), heart (405), and heart failure (288) (Table 7, Fig. 8A). We removed useless keywords and constructed a network containing 177 keywords with at least 24 occurrences, yielding a total of six different clusters. Cluster 1 (red) contains 47 keywords, including protein, mice, deficiency, mitophagy, mitochondria, lamp-2, mutations, ubiquitin, gene, muscle, aging, receptor, fusion, mouse model, association, failure, degradation, mitochondrial autophagy, selective autophagy. Group 2 (green) comprised 40 keywords, including oxidative stress, cell death, mechanism, myocardial ischemia, atherosclerosis, induced apoptosis, necrosis, NF-kappa-b, smooth muscle cells, reperfusion injury, pyroptosis, ros, ferroptosis, iron. Group 3 contains 36 keywords (in blue), including heart failure, in vivo, hypertension, diabetic cardiomyopathy, diabetes, mTOR, obesity, life-span, rapamycin, insulin-resistance, cardiac remodeling, metabolism. Group 4 contains 35 keywords (yellow), including apoptosis, expression, dysfunction, stress, pathway, injury, activation, protections, hypoxia, inhibition, inflammation, rats, microRNAs, survival, cancer, angiogenesis. Group 5 contains 15 keywords (purple), including Akt, heart, ischemia, mechanisms, protection, risk, roles, and target. Group 6 contains four keywords (in purple), including er stress, endoplasmic reticulum stress, and unfolded response. A volcano map was created with CiteSpace to visualize the research hotspots over time (Fig. 8B–C).
Figure 8.
Keywords analysis. (A) Keyword density map. (B–C) Keyword clustering volcano maps created with CiteSpace.
Co-cited references and keywords
Using CiteSpace, we derived the 50 most reliable citation bursts in the field of the role of autophagy in cardiomyopathy. One of the most cited references (35.31) is ‘The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress’ published in Nature Medicine. The first author of the article is Atsuko Nakai, and all 50 references were published between 2004 and 2023, suggesting that these papers have been cited frequently over the last 20 years. Importantly, nine of these papers are currently at peak citation (Fig. 9A), implying that the study of the role of autophagy in cardiomyopathy will continue to be of interest in the future. Among the 543 strongest mutated keywords in the field, we focused on those 50 keywords with the strongest mutations (Fig. 9B), which represent the current research hotspots in the field and represent possible future research directions.
Figure 9.
Citation burst and keyword Burst Analysis. (A) Top 50 references with the strongest citation bursts. (B) Top 50 keywords with the strongest citation bursts.
Discussion
Cellular autophagy has been increasingly found to be a target in the pathogenesis and development of cardiomyopathies, and the modulation of autophagy may be a breakthrough for the treatment of cardiomyopathies in the future. In this paper, we analyzed the literature related to autophagy in cardiomyopathy in the past 20 years by bibliometric methods and made a detailed analysis of the publication trend, geographical distribution, international and institutional cooperation network, and research hotspots, so as to point out the direction of future researchers.
Trend of publications
The number of publications in this field has increased rapidly since 2015 and will reach a peak in 2021, which indicates that this field has received extensive attention after 2015. Literature survey identifies important roles for autophagy and mitochondrial autophagy in regulating cardiac dynamic homeostasis and adaptation to stress8,21. So the role of cellular autophagy in cardiomyopathy has been widely studied, which is a hot spot and focus of research in recent years.
Countries/institutions and their cooperation
The role of autophagy in cardiomyopathy has been studied in 70 countries and regions, and the top five countries are China, the United States, Italy, Japan, and Germany. The number of publications in China accounted for 56.32% of the total number of publications, which was much higher than that in other countries, indicating that China has made a greater contribution to the study of autophagy in cardiomyopathy. The number of citations in the United States was 44 994 (Table 1), which was much higher than that of all the other countries/regions, and the citation/publication ratio of the papers in the United States (68.90) ranked fourth among all the countries/regions, which indicated that the quality of the papers published in the United States was generally high. Despite China’s rapid development and dominance in this field, the quality of its papers is not high, so there is a need to improve regional cooperation to increase academic impact. Among the top 10 institutions in terms of publications, six are from China, and four are from the United States. After further analysis, we found that domestic and foreign institutions prefer to collaborate with units within their own countries, so we call for strengthening cooperation between domestic and foreign institutions and breaking down academic barriers.
Citation information
Journal impact is identified by the number of co-citations it receives, which indicates whether or not the journal has had a significant impact on the scientific community. According to Figure 8 and Table 4, the journal with the highest number of co-citations is Circ Res (1608), followed by Circulation (1501) and J Biol Chem (1342). Among the top 10 most co-cited journals, Nature was cited 1240 times with the highest IF among the top 10 journals (64.8). Of the journals that were co-cited, all journals were in the Q1/Q2 region. Six of the top 10 authors are from China, and four are from the United States. The largest nodes are associated with the most co-cited authors, including Mizushima N (429 citations), Levine B (360 citations), and Nakai A (331 citations). The above suggests that Chinese scholars have invested a lot in their research work, but the quality of their depth of research needs to be improved, and that it is necessary to provide the quality of their research by collaborating with American scholars.
The 50 most reliable citation bursts in the field of the role of autophagy in cardiomyopathy were derived through CiteSpace. One of the most cited references (35.31) is ‘The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress’ published in Nature Medicine. The first author of the article is Atsuko Nakai, which argued that autophagy, an evolutionarily conserved process of mass degradation of cytoplasmic components, is a cellular survival mechanism in starved cells22,23. Although alterations in autophagy have been observed in a variety of cardiac disorders, including myocardial hypertrophy24–26 and heart failure27–29, whether autophagy plays a beneficial or a detrimental role in the heart is still unclear. Loss of cardiac-specific autophagy leads to cardiomyopathy in mice30,31. In adult mice, temporal control cardiac-specific deletion of Atg5 (autophagy-associated 5), a protein required for autophagy, leads to cardiac hypertrophy, left ventricular dilatation, and systolic dysfunction, accompanied by elevated levels of ubiquitination32,33. In addition, Atg5-deficient hearts show disorganized sarcomere architecture, mitochondrial mislocalization, and aggregation34. On the other hand, hearts lacking Atg5 early in cardiogenesis specifically did not exhibit this cardiac phenotype under baseline conditions but developed cardiac dysfunction and left ventricular dilatation after 1 week of pressure overload treatment35. These results suggest that autophagy in the heart is a homeostatic mechanism that maintains cardiomyocyte size and overall cardiac structure and function under baseline conditions, whereas upregulation of autophagy in the failing heart is an adaptive response that protects cells from hemodynamic stress.
According to the top 10 most co-cited articles (Table 6), the article entitled ‘The role of autophagy in the heart’ in Annual Review Of Physiology (IF=18.2) is the most co-cited reference with Sciarretta, Sebastiano as the first author of the article, autophagy is an evolutionarily conserved mechanism of cytoplasmic degradation within the cell6,36,37. Autophagy is a major regulator of cardiac homeostasis and function38,39. Autophagy protects cardiac structure and function under baseline conditions and is activated during stress, thereby limiting damage in most cases40. Autophagy reduces damage and protects cardiac function during ischemia41,42. It also reduces chronic ischemic remodeling and mediates cardiac adaptation to pressure overload by limiting the accumulation of misfolded proteins, mitochondrial dysfunction, and oxidative stress. Impaired autophagy is associated with diabetes and aging-induced cardiac abnormalities. Defective autophagy leads to cardiac proteinopathy and doxorubicin-induced cardiomyopathy43. However, under certain stress conditions, such as reperfusion injury, massive activation of autophagy may be detrimental to the heart44. Our study supports recent evidence that autophagy and mitophagy play important roles in regulating cardiac homeostasis and adaptation to stress.
Research hotspots and frontiers
Identifying research hotspots and frontiers is crucial for comprehending the evolution. We performed co-citation reference clustering and temporal clustering analysis (Fig. 7B–C). We found that diabetic cardiomyopathy (cluster0), cardiac/patholohy (cluster6), atg8 (cluster9), and glycogen storage (cluster11) were the early research hotspots. Ubiquitin-proteasome system (cluster7), desmin-related cardiomyopathy (cluster8), high-fat diet (cluster10), epg5 (cluster13), and bag3 (cluster15) are hotspots for mid-term research. Ferroptosis (cluster1), hydrogen sulfide (cluster2), mitophagy (cluster3), lipid peroxidation (cluster4), oxidative stress (cluster5), sirtuin-1 (cluster11) are the hot topics and trends in the field. Similarly, keyword-based analysis of the keywords shows that the most popular keyword is apoptosis (629) followed by oxidative stress.
Hotspot1: cellular autophagy proactively interacted with ferroptosis in cardiomyopathy
Ferroptosis is a newly discovered type of programmed cell death, and current studies have shown that autophagy plays a crucial role in ferroptosis, which has been studied and shown to be involved in the regulation of iron-dependent lipid peroxidation and ROS accumulation during ferroptosis45–47. The interrelationship between autophagy and ferroptosis has received increasing attention, providing a new concept in cell death regulation48. There is growing evidence that autophagy leads to ferroptosis, at least under certain conditions49 and that molecular chaperone-mediated autophagy is associated with ferroptosis50; there is also increasing attention to autophagy-associated regulation of ferroptosis in cardiomyopathies. We found that in the mouse model of septic cardiomyopathy: miR-130b-3p inhibits the activation of autophagy and attenuates ferroptosis in cardiomyocytes by down-regulating the expression of AMPK/mTOR signaling pathway in a mouse model septic cardiomyopathy51 and the transcriptional activation of ELAVL1 by FOXC1 may promote ferroptosis through the regulation of autophagy, leading to myocardial injury52; AP39 can inhibit mitophagy through the PINK1/Parkin pathway, fight cardiomyocyte death, iron and ameliorate myocardial ferroptosis and myocardial infarction in rats53. circRNA1615 inhibits ferroptosis by regulating autophagy in cardiomyocytes through the miRNA152-3p/LRP6 molecular axis. Diabetes leads to autophagy deficiency54 and Nrf2-mediated defenses while turning on Nrf2-operated pathological programs to promote ferroptosis in cardiomyocytes, thereby worsening the progression of diabetic cardiomyopathy55. The above-mentioned studies show that regulation of autophagy and ferroptosis in cardiomyopathy varies over time, and thus noncoding RNA, mitochondrial autophagy, therapeutic strategies for crosstalk between molecular chaperone-mediated autophagy and ferroptosis, and changes in both at different stages of the disease could provide new research directions for the prevention and treatment of cardiomyopathies56.
Hotspot2: oxidative stress and autophagy in cardiomyopathy
The regulation of autophagy by oxidative stress is complex and variable57–60. Products of lipid peroxidation can additively bind to specific mitochondrial and autophagy-associated proteins, driving cellular dysfunction in the form of autophagic cell death61. During myocardial ischemia and reperfusion, autophagy signaling (e.g., AMP-activated protein kinase and Akt-mTOR signaling) is affected by lipid peroxidation products, which interfere with upstream regulators62. Lipid peroxidation products may induce lysosomal dysfunction and lipofuscinogenesis, leading to reduced autophagic activity63. A growing number of studies have confirmed that reduced GPX4 activity or iron overload leads to ferroptosis64–66, and inhibition of GPX4 leads to an increase in ROS67, whereas overexpression of GPX4 reduces ROS and thus prevents cell death68. Above, we summarized the complex relationship between oxidative stress and cellular autophagy as well as ferroptosis; therefore, investigating the role of autophagy in cardiomyopathy is closely related to different cell deaths, and their interactions are worth further exploration in cardiomyopathy.
Hotspot3: Sirtuin-1
SIRT1 is considered a promising new target for the treatment of cardiovascular diseases69–71. It has been shown that SIRT1 regulates autophagy by interacting with and deacetylating autophagy-associated proteins Atg5, Atg7, and Atg872. It promotes mitochondrial autophagy and inhibits cardiomyocyte ferroptosis by increasing NAD levels and activating the SIRT-PINK1 and SIRT1-GPX4 signaling pathways, ultimately attenuating cardiomyocyte injury73. In response to ER stress, SIRT1 activation promotes cardiomyocyte survival by enhancing autophagy through activation of the EEF2K/EEF2 pathway. These results suggest that SIRT1 via the IRE1α pathway promotes autophagy through AMPK activation and reduces hypoxia-induced apoptosis, protecting cardiomyocytes from hypoxic stress74. SIRT1 attenuates cardiac dysfunction by inhibiting transcriptional factors and increases SERCA2a, ERK1/2/Homer1, eNOS, PGC-1α, and AMPK71. The above studies indicated that SIRT1 is closely related to cardiovascular diseases, especially the prevention and treatment of cardiomyopathy, and the effect of SIRT1 on cardiomyopathy through the regulation of Fe death is a hotspot of current research, of which the in-depth mechanism study is still unclear and needs to be further explored.
Strengths and limitations of the study
In this study, we first conducted a multidimensional and detailed bibliometric analysis of 2279 articles in the WoSCC database on autophagy in cardiomyopathy in the past 20 years, and summarized and analyzed the current status and characteristics of autophagy in cardiomyopathy, identified current problems and future research directions of autophagy in cardiomyopathy for the researchers, which can help to promote the development of the research. And also analyzed the cooperation among the research institutes, problems, and cooperation among countries, as well as provided feasible suggestions to solve the problems. However, the fact that literature data can be affected by a variety of factors, such as self-citation, language bias, etc., may affect the validity of the bibliometric analysis. In addition, bibliometrics mainly analyzes quantitative data, but not qualitative factors, such as research design and research methodology. Therefore, it is not possible to do a comprehensive evaluation of the quality of the research.
Conclusions
This study is a comprehensive bibliometric analysis of autophagy in cardiomyopathies from 2004 to 2023. This manuscript aims to elucidate autophagy in cardiomyopathies and identify hotspots through systematic bibliometric analysis and visualization. China has become a leader in autophagy research in cardiomyopathy. Initial progress has been made in the study of the mechanisms of autophagy in the development and progression of cardiomyopathies and the exploration of autophagy-associated proteins and genes, ‘oxidative stress,’ ‘apoptosis,’ ‘ferroptosis,’ and ‘SIRT1’ are the hot topics in this field. The interaction of autophagy with ferroptosis and apoptosis has been a prominent theme in the study of autophagy in cardiomyopathy in recent years. In summary, this study provides valuable information to summarize the research progress of autophagy in cardiomyopathy and explore the future research direction.
Ethical approval
All data used in this work are publicly available from studies with relevant participant consent and ethical approval.
Consent
None.
Source of funding
None.
Author contribution
L.J.P. was pivotal in conceptualization, supervision, and the review and editing process. Z.X.H. and S.B. were instrumental in the formal analysis and drafting of the original manuscript. Z.Q.F., W.X.G., and L.K.N. played crucial roles in data acquisition. Z.Q.F., Z.X.H., and W.J.C. carried out the statistical analysis. All authors have actively contributed to the article and have approved the final version submitted for publication.
Conflicts of interest disclosure
The authors declare no conflicts of interest.
Research registration unique identifying number (UIN)
None.
Guarantor
Jianping Luo.
Data availability statement
The raw data underpinning the conclusions of this article will be made accessible by the authors without undue reservation. For further inquiries, please contact the corresponding author.
Provenance and peer review
None.
Presentation
None.
Table 7.
The top 10 keywords.
| Ranks | Keyword | Counts | Rank | Keyword | Counts |
|---|---|---|---|---|---|
| 1 | Apoptosis | 629 | 11 | Inflammation | 197 |
| 2 | Oxidative stress | 515 | 12 | Dysfunction | 192 |
| 3 | Heart | 405 | 13 | Heart failure | 165 |
| 4 | Heart failure | 288 | 14 | Protects | 164 |
| 5 | Activation | 268 | 15 | Mitochondria | 161 |
| 6 | Cell death | 268 | 16 | Injury | 150 |
| 7 | Mechanisms | 253 | 17 | Mitophagy | 146 |
| 8 | Expression | 238 | 18 | Myocardial infarction | 139 |
| 9 | Inhibition | 219 | 19 | Cells | 136 |
| 10 | Diabetic cardiomyopathy | 212 | 20 | Hypertrophy | 136 |
Acknowledgements
The authors thank CiteSpace and VOSviewer for free access.
Footnotes
X.Z. and B.S. contributed equally.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 11 July 2024
Contributor Information
Xianghui Zeng, Email: zengxianghui0501@163.com.
Bin Shu, Email: subin3392@163.com.
Qingfeng Zeng, Email: zengqf2239@163.com.
Xianggui Wang, Email: wangxianghuirmyy@163.com.
Kening Li, Email: lkn990816@163.com.
Jincheng Wu, Email: 1285694277@qq.com.
Jianping Luo, Email: luojianping@mail.gzsrmyy.com.
References
- 1.Hollenberg SM, Singer M. Pathophysiology of sepsis-induced cardiomyopathy. Nat Rev Cardiol 2021;18:424–434. [DOI] [PubMed] [Google Scholar]
- 2.Zeng X, Zeng Q, Zhou L, et al. Prevalence of chronic kidney disease among US adults with hypertension, 1999 to 2018. Hypertension 2023;80:2149–2158. [DOI] [PubMed] [Google Scholar]
- 3.Ciarambino T, Menna G, Sansone G, et al. Cardiomyopathies: an overview. Int J Mol Sci 2021;22:7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bravo-San Pedro JM, Kroemer G, Galluzzi L. Autophagy and mitophagy in cardiovascular disease. Circ Res 2017;120:1812–1824. [DOI] [PubMed] [Google Scholar]
- 5.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science 2000;290:1717–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell 2011;147:728–741. [DOI] [PubMed] [Google Scholar]
- 7.Tong M, Saito T, Zhai P, et al. Alternative mitophagy protects the heart against obesity-associated cardiomyopathy. Circ Res 2021;129:1105–1121. [DOI] [PubMed] [Google Scholar]
- 8.Sciarretta S, Maejima Y, Zablocki D, et al. The role of autophagy in the heart. Annu Rev Physiol 2018;80:1–26. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda S, Zablocki D, Sadoshima J. The role of autophagy in death of cardiomyocytes. J Mol Cell Cardiol 2022;165:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu B, Song H, Fan M, et al. Luteolin attenuates sepsis‑induced myocardial injury by enhancing autophagy in mice. Int J Mol Med 2020;45:1477–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanamori H, Naruse G, Yoshida A, et al. Metformin enhances autophagy and provides cardioprotection in δ-sarcoglycan deficiency-induced dilated cardiomyopathy. Circ Heart Fail 2019;12:e005418. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Yan S, Liu X, et al. PRMT4 promotes ferroptosis to aggravate doxorubicin-induced cardiomyopathy via inhibition of the Nrf2/GPX4 pathway. Cell Death Differ 2022;29:1982–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madonna R, Moscato S, Cufaro MC, et al. Empagliflozin inhibits excessive autophagy through the AMPK/GSK3β signalling pathway in diabetic cardiomyopathy. Cardiovasc Res 2023;119:1175–1189. [DOI] [PubMed] [Google Scholar]
- 14.Guler AT, Waaijer CJ, Palmblad M. Scientific workflows for bibliometrics. Scientometrics 2016;107:385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He T, Zou J, Sun K, et al. Global research status and frontiers on autophagy in hepatocellular carcinoma:a comprehensive bibliometric and visualized analysis. Int J Surg 2024;110:2788–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng C, Kuang L, Zhao J, et al. Bibliometric and visualized analysis of ocular drug delivery from 2001 to 2020. J Control Release 2022;345:625–645. [DOI] [PubMed] [Google Scholar]
- 17.Wei N, Xu Y, Li Y, et al. A bibliometric analysis of T cell and atherosclerosis. Front Immunol 2022;13:948314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Zhao S, Tan L, et al. Frontier and hot topics in electrochemiluminescence sensing technology based on CiteSpace bibliometric analysis. Biosens Bioelectron 2022;201:113932. [DOI] [PubMed] [Google Scholar]
- 19.van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010;84:523–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci USA 2004;101 Suppl 1(Suppl 1):5303–5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Wang L, Guan YQ. Role and mechanisms of cardiomyocyte autophagy in cardiovascular diseases. Zhonghua Xin Xue Guan Bing Za Zhi 2016;44:275–277. [DOI] [PubMed] [Google Scholar]
- 22.González-Rodríguez P, Füllgrabe J, Joseph B. The hunger strikes back: an epigenetic memory for autophagy. Cell Death Differ 2023;30:1404–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pedro J, Sica V, Madeo F, et al. Acyl-CoA-binding protein (ACBP): the elusive 'hunger factor' linking autophagy to food intake. Cell Stress 2019;3:312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song C, Qi H, Liu Y, et al. Inhibition of lncRNA Gm15834 attenuates autophagy-mediated myocardial hypertrophy via the miR-30b-3p/ULK1 axis in mice. Mol Ther 2021;29:1120–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Wang J, Yang X. Functions of autophagy in pathological cardiac hypertrophy. Int J Biol Sci 2015;11:672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu M, Wan CX, Huang SH, et al. Oridonin protects against cardiac hypertrophy by promoting P21-related autophagy. Cell Death Dis 2019;10:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shirakabe A, Zhai P, Ikeda Y, et al. Drp1-dependent mitochondrial autophagy plays a protective role against pressure overload-induced mitochondrial dysfunction and heart failure. Circulation 2016;133:1249–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang Y, Xu W, Liu Y, et al. Autophagy protects mitochondrial health in heart failure. Heart Fail Rev 2024;29:113–123. [DOI] [PubMed] [Google Scholar]
- 29.Raffa S, Forte M, Gallo G, et al. Atrial natriuretic peptide stimulates autophagy/mitophagy and improves mitochondrial function in chronic heart failure. Cell Mol Life Sci 2023;80:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alcalai R, Arad M, Wakimoto H, et al. LAMP2 cardiomyopathy: consequences of impaired autophagy in the heart. J Am Heart Assoc 2021;10:e018829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun S, Lu J, Lai C, et al. Transcriptome analysis uncovers the autophagy-mediated regulatory patterns of the immune microenvironment in dilated cardiomyopathy. J Cell Mol Med 2022;26:4101–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Changotra H, Kaur S, Yadav SS, et al. ATG5: a central autophagy regulator implicated in various human diseases. Cell Biochem Funct 2022;40:650–667. [DOI] [PubMed] [Google Scholar]
- 33.Xu CN, Kong LH, Ding P, et al. Melatonin ameliorates pressure overload-induced cardiac hypertrophy by attenuating Atg5-dependent autophagy and activating the Akt/mTOR pathway. Biochim Biophys Acta Mol Basis Dis 2020;1866:165848. [DOI] [PubMed] [Google Scholar]
- 34.Xie X, Bi HL, Lai S, et al. The immunoproteasome catalytic β5i subunit regulates cardiac hypertrophy by targeting the autophagy protein ATG5 for degradation. Sci Adv 2019;5:eaau0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ljubojević-Holzer S, Kraler S, Djalinac N, et al. Loss of autophagy protein ATG5 impairs cardiac capacity in mice and humans through diminishing mitochondrial abundance and disrupting Ca2+ cycling. Cardiovasc Res 2022;118:1492–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klionsky DJ, Petroni G, Amaravadi RK, et al. Autophagy in major human diseases. EMBO J 2021;40:e108863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu S, Yao S, Yang H, et al. Autophagy: regulator of cell death. Cell Death Dis 2023;14:648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rabinovich-Nikitin I, Love M, Kirshenbaum LA. Intersection of autophagy regulation and circadian rhythms in the heart. Biochim Biophys Acta Mol Basis Dis 2022;1868:166354. [DOI] [PubMed] [Google Scholar]
- 39.De Meyer GR, De Keulenaer GW, Martinet W. Role of autophagy in heart failure associated with aging. Heart Fail Rev 2010;15:423–430. [DOI] [PubMed] [Google Scholar]
- 40.Shirakabe A, Ikeda Y, Sciarretta S, et al. Aging and autophagy in the heart. Circ Res 2016;118:1563–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popov SV, Mukhomedzyanov AV, Voronkov NS, et al. Regulation of autophagy of the heart in ischemia and reperfusion. Apoptosis 2023;28:55–80. [DOI] [PubMed] [Google Scholar]
- 42.Shen Y, Liu X, Shi J, et al. Involvement of Nrf2 in myocardial ischemia and reperfusion injury. Int J Biol Macromol 2019;125:496–502. [DOI] [PubMed] [Google Scholar]
- 43.Asnani A. Activating autophagy to prevent doxorubicin cardiomyopathy: the timing matters. Circ Res 2021;129:801–803. [DOI] [PubMed] [Google Scholar]
- 44.Zhang M, Sui W, Xing Y, et al. Angiotensin IV attenuates diabetic cardiomyopathy via suppressing FoxO1-induced excessive autophagy, apoptosis and fibrosis. Theranostics 2021;11:8624–8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu PC, Choo YL, Wei SY, et al. Contribution of autophagy to cellular iron homeostasis and stress adaptation in alternaria alternata. Int J Mol Sci 2024;25:1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang B, Wang Y, Zhang J, et al. ROS-induced lipid peroxidation modulates cell death outcome: mechanisms behind apoptosis, autophagy, and ferroptosis. Arch Toxicol 2023;97:1439–1451. [DOI] [PubMed] [Google Scholar]
- 47.Zhou B, Liu J, Kang R, et al. Ferroptosis is a type of autophagy-dependent cell death. Semin Cancer Biol 2020;66:89–100. [DOI] [PubMed] [Google Scholar]
- 48.Tang D, Chen X, Kang R, et al. Ferroptosis: molecular mechanisms and health implications. Cell Res 2021;31:107–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu J, Kuang F, Kroemer G, et al. Autophagy-dependent ferroptosis: machinery and regulation. Cell Chem Biol 2020;27:420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu Z, Geng Y, Lu X, et al. Chaperone-mediated autophagy is involved in the execution of ferroptosis. Proc Natl Acad Sci USA 2019;116:2996–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qi Z, Liu R, Ju H, et al. microRNA-130b-3p attenuates septic cardiomyopathy by regulating the AMPK/mTOR signaling pathways and directly targeting ACSL4 against ferroptosis. Int J Biol Sci 2023;19:4223–4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen HY, Xiao ZZ, Ling X, et al. ELAVL1 is transcriptionally activated by FOXC1 and promotes ferroptosis in myocardial ischemia/reperfusion injury by regulating autophagy. Mol Med 2021;27:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang T, Yang Q, Lai Q, et al. AP39 inhibits ferroptosis by inhibiting mitochondrial autophagy through the PINK1/parkin pathway to improve myocardial fibrosis with myocardial infarction. Biomed Pharmacother 2023;165:115195. [DOI] [PubMed] [Google Scholar]
- 54.Li RL, Fan CH, Gong SY, et al. Effect and mechanism of LRP6 on cardiac myocyte ferroptosis in myocardial infarction. Oxid Med Cell Longev 2021;2021:8963987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zang H, Wu W, Qi L, et al. Autophagy inhibition enables Nrf2 to exaggerate the progression of diabetic cardiomyopathy in mice. Diabetes 2020;69:2720–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen X, Tsvetkov AS, Shen HM, et al. International consensus guidelines for the definition, detection, and interpretation of autophagy-dependent ferroptosis. Autophagy 2024;20:1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dodson M, Wani WY, Redmann M, et al. Regulation of autophagy, mitochondrial dynamics, and cellular bioenergetics by 4-hydroxynonenal in primary neurons. Autophagy 2017;13:1828–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ornatowski W, Lu Q, Yegambaram M, et al. Complex interplay between autophagy and oxidative stress in the development of pulmonary disease. Redox Biol 2020;36:101679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei Y, Ni L, Pan J, et al. The roles of oxidative stress in regulating autophagy in methylmercury-induced neurotoxicity. Neuroscience 2021;469:175–190. [DOI] [PubMed] [Google Scholar]
- 60.Yun HR, Jo YH, Kim J, et al. Roles of autophagy in oxidative stress. Int J Mol Sci 2020;21:3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gęgotek A, Skrzydlewska E. Lipid peroxidation products ' role in autophagy regulation. Free Radic Biol Med 2024;212:375–383. [DOI] [PubMed] [Google Scholar]
- 62.Ma H, Guo R, Yu L, et al. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: role of autophagy paradox and toxic aldehyde. Eur Heart J 2011;32:1025–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krohne TU, Kaemmerer E, Holz FG, et al. Lipid peroxidation products reduce lysosomal protease activities in human retinal pigment epithelial cells via two different mechanisms of action. Exp Eye Res 2010;90:261–266. [DOI] [PubMed] [Google Scholar]
- 64.Seibt TM, Proneth B, Conrad M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic Biol Med 2019;133:144–152. [DOI] [PubMed] [Google Scholar]
- 65.Forcina GC, Dixon SJ. GPX4 at the crossroads of lipid homeostasis and ferroptosis. Proteomics 2019;19:e1800311. [DOI] [PubMed] [Google Scholar]
- 66.Dixon SJ, Pratt DA. Ferroptosis: a flexible constellation of related biochemical mechanisms. Mol Cell 2023;83:1030–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jia M, Qin D, Zhao C, et al. Redox homeostasis maintained by GPX4 facilitates STING activation. Nat Immunol 2020;21:727–735. [DOI] [PubMed] [Google Scholar]
- 68.Ursini F, Maiorino M. Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radic Biol Med 2020;152:175–185. [DOI] [PubMed] [Google Scholar]
- 69.Liu ZH, Zhang Y, Wang X, et al. SIRT1 activation attenuates cardiac fibrosis by endothelial-to-mesenchymal transition. Biomed Pharmacother 2019;118:109227. [DOI] [PubMed] [Google Scholar]
- 70.Prola A, Pires Da Silva J, Guilbert A, et al. SIRT1 protects the heart from ER stress-induced cell death through eIF2α deacetylation. Cell Death Differ 2017;24:343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karbasforooshan H, Karimi G. The role of SIRT1 in diabetic cardiomyopathy. Biomed Pharmacother 2017;90:386–392. [DOI] [PubMed] [Google Scholar]
- 72.Zhao N, Li Y, Wang C, et al. DJ-1 activates the Atg5-Atg12-Atg16L1 complex via Sirt1 to influence microglial polarization and alleviate cerebral ischemia/reperfusion-induced inflammatory injury. Neurochem Int 2022;157:105341. [DOI] [PubMed] [Google Scholar]
- 73.Yu H, Gan D, Luo Z, et al. α-Ketoglutarate improves cardiac insufficiency through NAD+-SIRT1 signaling-mediated mitophagy and ferroptosis in pressure overload-induced mice. Mol Med 2024;30:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pires Da Silva J, Monceaux K, Guilbert A, et al. SIRT1 protects the heart from er stress-induced injury by promoting eEF2K/eEF2-dependent autophagy. Cells 2020;9:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data underpinning the conclusions of this article will be made accessible by the authors without undue reservation. For further inquiries, please contact the corresponding author.