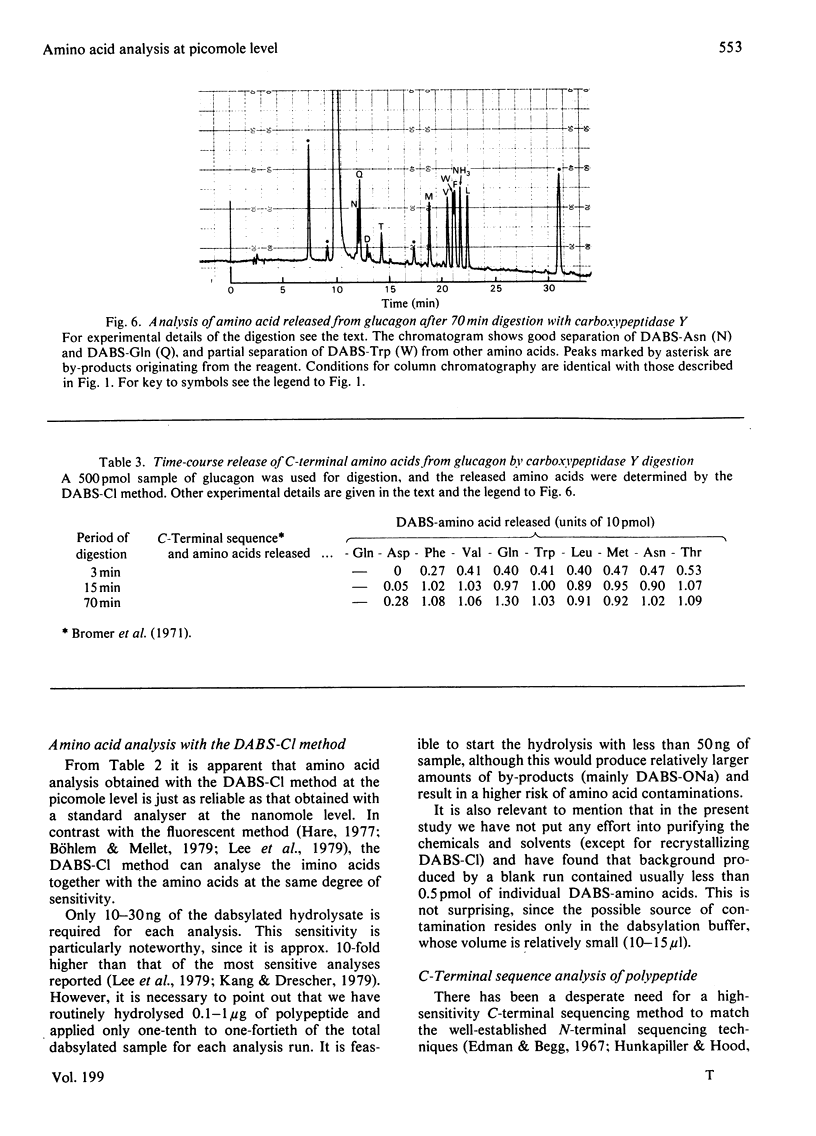
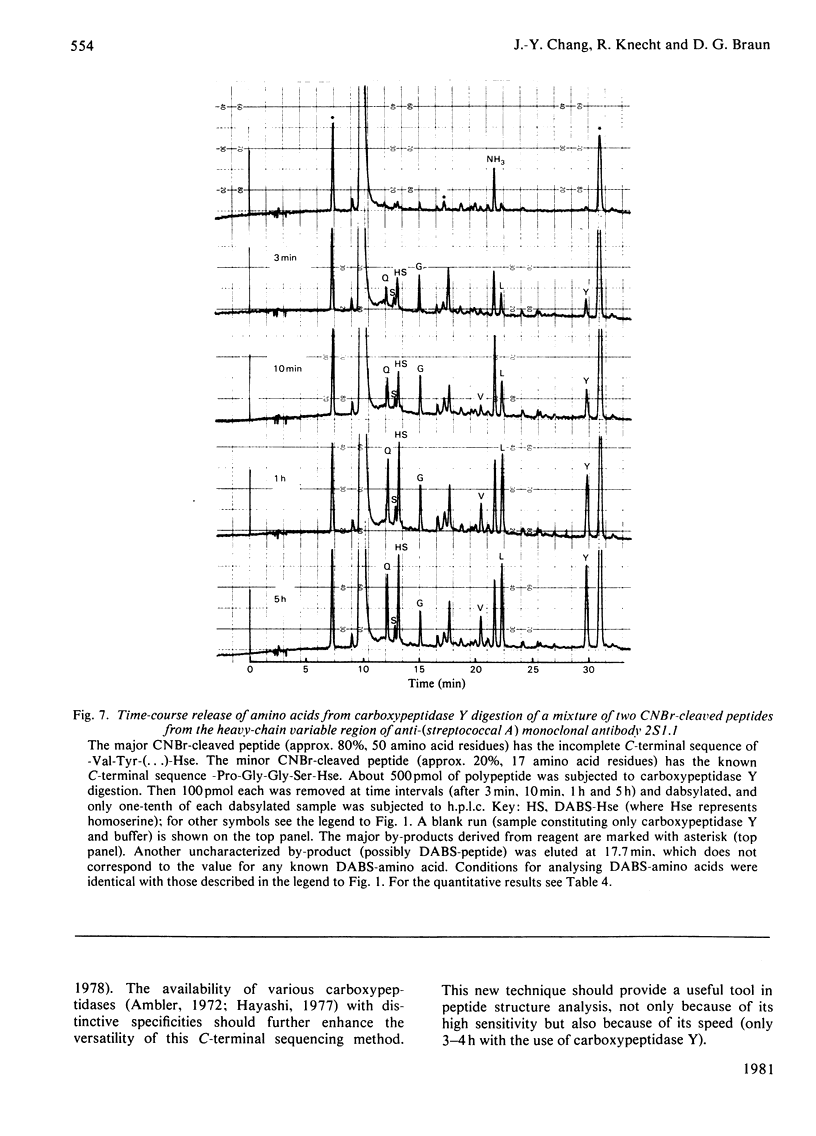
Abstract
Amino acids labelled with dimethylaminoazobenzenesulphonyl chloride can be separated by reversed-phase high-pressure liquid chromatography and detected in the visible region (436 nm). All 19 naturally occurring amino acids can be separated on a Zorbax ODS column by employing two different gradient systems consisting of an acetonitrile/aqueous buffer mixture. As little as 2--5 pmol of an individual dimethylaminoazobenzenesulphonyl-amino acid can be quantitatively analysed with reliability, and only 10--30 ng of the dimethylaminoazobenzenesulphonylated protein hydrolysate is needed for each complete amino acid analysis. This new technique is as sensitive as any of the current amino acid analysis methods involving ion-exchange separation plus fluorescence detection, and is technically much simpler. By the combination of this sensitive amino acid-analysing technique with carboxypeptidase, we have been able to determine the C-terminal sequence of polypeptides at the picomole level.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson J. R., Hare P. E. O-phthalaldehyde: fluorogenic detection of primary amines in the picomole range. Comparison with fluorescamine and ninhydrin. Proc Natl Acad Sci U S A. 1975 Feb;72(2):619–622. doi: 10.1073/pnas.72.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Hare P. E. Subnanomole-range amino acid analysis. Methods Enzymol. 1977;47:3–18. doi: 10.1016/0076-6879(77)47003-4. [DOI] [PubMed] [Google Scholar]
- Hayashi R. Carboxypeptidase Y in sequence determination of peptides. Methods Enzymol. 1977;47:84–93. doi: 10.1016/0076-6879(77)47010-1. [DOI] [PubMed] [Google Scholar]
- Hill D. W., Walters F. H., Wilson T. D., Stuart J. D. High performance liquid chromatographic determination of amino acids in the picomole range. Anal Chem. 1979 Jul;51(8):1338–1341. doi: 10.1021/ac50044a055. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Direct microsequence analysis of polypeptides using an improved sequenator, a nonprotein carrier (polybrene), and high pressure liquid chromatography. Biochemistry. 1978 May 30;17(11):2124–2133. doi: 10.1021/bi00604a016. [DOI] [PubMed] [Google Scholar]
- Lee H. M., Forde M. D., Lee M. C., Bucher D. J. Fluorometric microbore amino acid analyzer: the construction of an inexpensive, highly sensitive instrument using o-phthalaldehyde as a detection agent. Anal Biochem. 1979 Jul 15;96(2):298–307. doi: 10.1016/0003-2697(79)90585-2. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Drescher D. G. Derivatization of cysteine and cystine for fluorescence amino acid analysis with the o-phthaldialdehyde/2-mercaptoethanol reagent. J Biol Chem. 1979 Jul 25;254(14):6248–6251. [PubMed] [Google Scholar]
- Lin J. K., Chang J. Y. Chromophoric labeling of amino acids with 4-dimethylaminoazobenzene-4'-sulfonyl chloride. Anal Chem. 1975 Aug;47(9):1634–1638. doi: 10.1021/ac60359a007. [DOI] [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. Procedures for the chromatographic determination of amino acids on four per cent cross-linked sulfonated polystyrene resins. J Biol Chem. 1954 Dec;211(2):893–906. [PubMed] [Google Scholar]



