Abstract
The aim of the study was to report the outcome of primary localized low-grade fibromyxoid sarcoma (LGFMS), sclerosing epithelioid fibrosarcoma (SEF), and hybrid LGFMS/SEF (H-LGFMS/SEF). Patients with primary localized LGFMS, SEF, or H-LGFMS/SEF, surgically treated with curative intent from January 2000 to September 2022, were enrolled from 14 countries and 27 institutions. Pathologic inclusion criteria were predefined by expert pathologists. The primary endpoint was overall survival (OS). Secondary endpoints were crude cumulative incidence (CCI) of local recurrence (LR), CCI of distant metastases (DM), and post-metastases OS (p-OS). Two hundred ninety-four patients (239 LGFMS, 32 SEF, and 23 H-LGFMS/SEF) were identified. At a median(m-) follow-up (FU) of 57.1 months, 12/294 patients died. The 5- and 10-year OS were 99.0% and 95.9% in LGFMS, 86.2% and 67.0% in SEF, and 84.8% and 84.8% in H-LGFMS/SEF, respectively. Predictors of worse OS included pathology, age at surgery, systemic therapy, and radiotherapy. LR developed in 13/294 (4.4%) patients. The observed m-time to LR was 10.7 months. The 5- and 10-yr CCI-LR were 4.7% in LGFMS and 6.6% in SEF, respectively. There were no LR events in H-LGFMS/SEF. The sole predictor of higher risk of LR was histology. DM developed in 23/294 (7.8%) patients. The observed m-time to DM was 28.2 months. The 5- and 10-yr CCI-DM were 1.3% and 2.7% in LGMFS, 29.9% and 57.7% in SEF, 48.9% and 48.9% in H-LGFMS/SEF, respectively. Predictors of higher risk of DM were histology, systemic therapy, and radiotherapy. Primary localized LGFMS treated with complete surgical resection has an excellent prognosis, while about 50% of H-LGFMS/SEF and SEF develop DM within 5 to 10 years. Very long-term FU is needed to understand absolute cure rates.
Key Words: low-grade fibromyxoid sarcoma, sclerosing epithelioid fibrosarcoma, hybrid forms, localized disease, prognostic factors, survival
Low-grade fibromyxoid sarcoma (LGFMS) and sclerosing epithelioid fibrosarcoma (SEF) are ultra-rare sarcomas.1 They share morphologic, immunohistochemical, and molecular features but are considered distinct though related entities by the current WHO classification, and cases with hybrid morphology are recognized (H-LGFMS/SEF).2 LGFMS and hybrid forms are characterized in the majority of cases by FUS::CREB3L2 fusions,3,4 whereas the most common molecular alteration in pure SEF is an EWSR1::CREB3L1 gene fusion.5 Immunohistochemically, almost all cases of LGFMS and ~70% of SEF show strong diffuse cytoplasmic expression of MUC4.6,7
LGFMS mainly occurs in the deep soft tissue of the extremities of young adults and shows indolent behavior, while SEF primarily affects middle-aged and elderly patients and is more aggressive.8,9 Surgery is considered the standard treatment for localized disease in both subtypes, while radiotherapy is commonly administered when wide excision is not feasible.10 There are a few small series on the molecular profiling of H-LGFMS/SEF,11 but there are no data regarding treatments and outcomes in this specific subtype.
In this global retrospective study within the Ultra-Rare Sarcoma Working Group (URSWG), we aimed to understand the natural history and the prognostic factors of patients with primary localized LGFMS, SEF, or H-LGFMS/SEF.
METHODS
This is an international retrospective multicenter study conducted within the URSWG. All consecutive patients of any age with primary localized LGFMS, SEF, and H-LGFMS/SEF surgically treated with curative intent at the participating institutions between January 2000 and September 2022 were retrospectively identified.
Data retrospectively retrieved included patient gender, age at diagnosis, site of origin, histologic type, neoadjuvant/adjuvant treatments, type of surgery, and margin status.
Eligible patients had a pathologically confirmed diagnosis of LGFMS, SEF, or H-LGFMS/SEF (requiring strong MUC4 immunohistochemical expression and/or 1 of the following: FUS/EWSR1 rearrangement; EWSR1/FUS::CREB3L1/CREB3L2/CREM fusions), as predefined and agreed by a panel of sarcoma expert pathologists within the URSWG. The inclusion criteria are detailed in Supplementary Data-Synopsis.
The indication for radiotherapy (RT) differed among institutions and was generally recommended when a higher risk of relapse was estimated on clinical grounds.
Systemic therapy was rarely administered at the discretion of each expert sarcoma team among institutions.
Statistical Analysis
Patients, disease, and treatment characteristics were summarized using standard descriptive statistics.
The primary endpoint was overall survival (OS). Secondary endpoints were crude cumulative incidence (CCI) of local recurrence (LR), CCI of distant metastases (DM), and post-metastases OS (p-OS). Cox models were fitted to analyze the association between OS and the putative prognostic covariates; Fine and Gray models were used to analyze LR and DM incidence.
OS was defined as the time from diagnosis until death due to any cause. CCI-LR and CCI-DM were estimated in a competing risk setting, that is, considering the first local recurrence/distant metastases as occurring events and including deaths without events among the competing events. p-OS was defined as the time from DM until death due to any cause. Patients alive and without the event of interest were censored at the last known follow-up. The OS and p-OS curves were estimated with the Kaplan-Meier method, and compared with the log-rank test. The CCI curves of LR and DM were compared using the Gray test.
RESULTS
Patient and Tumor Characteristics
Overall, 294 patients with primary and localized LGFMS (239 patients), SEF (32 patients), and H-LGFMS/SEF (23 patients) were surgically treated with curative intent at the participating institutions from January 2000 to September 2022 and were considered for the present study. We excluded 3 patients with primary localized disease (3/297 patients) who did not receive surgery for primary treatment.
Baseline patient, histopathological/molecular, and treatment characteristics are shown in Table 1. Examples of morphologic and immunohistochemical features of the 3 histologic subtypes are shown in Figures 1 and 2.
TABLE 1.
Patient Characteristics
| Histologic subtype | LGFMS | SEF | H-LGFMS/SEF |
|---|---|---|---|
| Number of pts with primary disease (%) | 239/294 (81.3) | 32/294 (10.9) | 23/294 (7.8) |
| Age at surgery (y), median (IQR) | 38.0 (27.5-52.0) | 47.0 (31.8-55.0) | 45.0 (30.0-58.0) |
| Male/female (%) | 119 (49.8)/120 (50.2) | 23 (71.9)/9 (28.1) | 11 (47.8)/12 (52.2) |
| Histopathologic/molecular features | |||
| MUC4 expression (%) | |||
| Positive | 170 (71.1) | 24 (75.0) | 19 (82.6) |
| Negative | 3 (1.3) | 0 (0.0) | 0 (0.0) |
| Unknown | 66 (27.6) | 8 (25.0) | 4 (17.4) |
| Number of pts analyzed for FUS rearrangement (%) | 158/239 (66.1) | 2/32 (6.3) | 16/23 (73.9) |
| Positive | 152/239 (63.6) | 2/32 (6.3) | 8/23 (34.8) |
| FUS::CREB3L2 | 77/152 (50.7) | 1/2 (50.0) | 3/9 (33.3) |
| FUS::CREB3L1 | 6/152 (3.9) | 0/2 (0.0) | 0/9 (0.0) |
| No fusion partner specified | 69/152 (45.4) | 1/2 (50.0) | 5/9 (55.6) |
| Negative | 6/239 (2.5) | 0/32 (0.0) | 8/23 (34.8) |
| Number of pts analyzed for EWSR1 rearrangement (%) | 2/239 (0.8) | 17/32 (53.1) | 9/23 (39.1) |
| Positive | 2/239 (0.8) | 15/32 (46.8) | 5/23 (21.7) |
| EWSR1::CREB3L1 | 2/2 (100.0) | 11/15 (73.3) | 4/5 (80.0) |
| EWSR1-CREB3L2 | 0/2 (0.0) | 2/15 (13.3) | 0/5 (0.0) |
| No fusion partner specified | 0/2 (0.0) | 2/15 (13.3) | 1/5 (20.0) |
| Negative | 0/239 (0.0) | 2/32 (6.3) | 4/23 (17.4) |
| Primary site (%) | |||
| Extremities | 153 (64.0) | 14 (43.8) | 11 (47.8) |
| Abdomen / retroperitoneum | 21 (8.8) | 4 (12.5) | 5 (21.7) |
| Trunk | 33 (13.8) | 4 (12.5) | 3 (13.0) |
| Other | 32 (13.4) | 10 (31.3) | 4 (17.4) |
| Treatments of primary disease | |||
| Surgery (%) | |||
| No | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Yes | 239 (100.0) | 32 (100.0) | 23 (100.0) |
| R0 | 195 (81.6) | 28 (87.5) | 19 (82.6) |
| R1 | 28 (11.7) | 1 (3.1) | 2 (8,7) |
| Missing | 13 (5.4) | 1 (3.1) | 1 (4.3) |
| R2 | 3 (1.3) | 2 (6.3) | 1 (4.3) |
| Radiotherapy (%) | |||
| No | 171 (74.1) | 16 (50.0) | 14 (60.9) |
| Yes | 62 (25.9) | 16 (50.0) | 9 (39.1) |
| Systemic therapies (%) | |||
| No | 235 (98.3) | 24 (75.0) | 22 (95.7) |
| Yes | 4 (1.7) | 8 (25.0) | 1 (4.3) |
| Status at last follow-up (%) | |||
| Alive, No evidence of disease | 211 (88.3) | 19 (59.4) | 17 (73.9) |
| Alive, With evidence of disease | 5 (2.1) | 7 (21.9) | 2 (8.7) |
| Dead | 4 (1.7) | 6 (18.8) | 2 (8.7) |
| Lost to follow-up | 19 (7.9) | 0 (0.0) | 2 (8.7) |
pts indicates patients.
FIGURE 1.
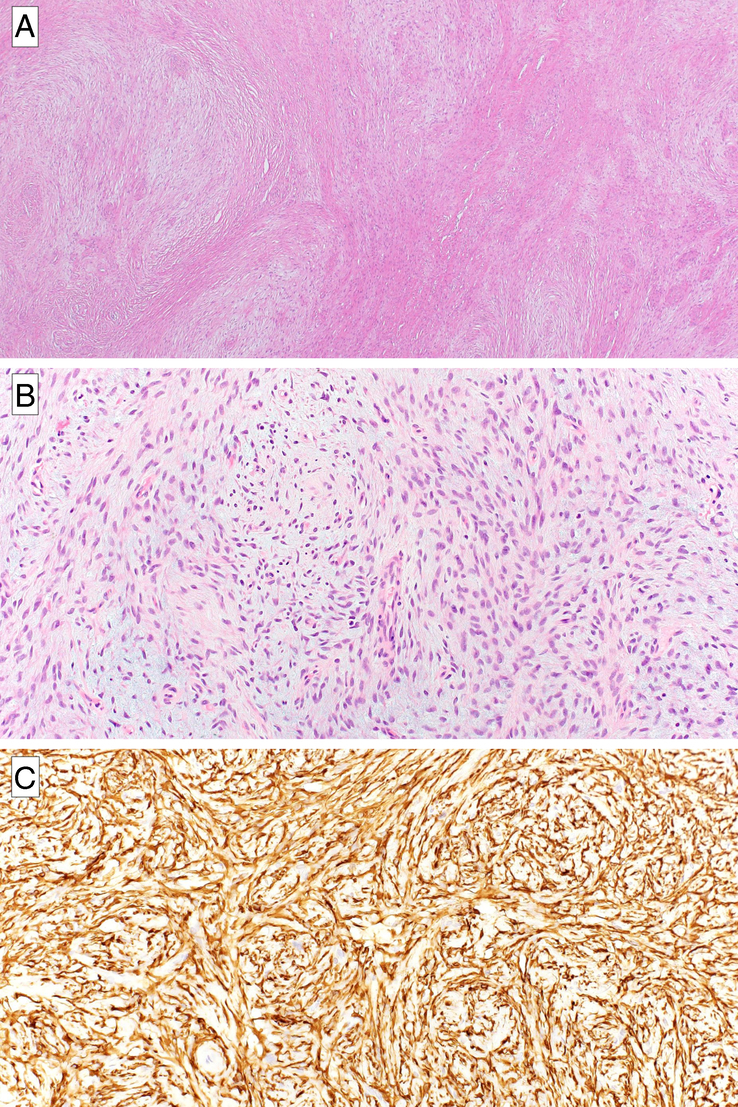
Low-grade fibromyxoid sarcoma: morphology and immunohistochemical features. At low power, the typical alternating multinodular myxoid and collagenous stroma can be appreciated (A). The tumor is characterized by bland spindle cells organized in short fascicles set in a fibromyxoid background (B). Strong and diffuse immunopositivity for MUC4 is observed in almost all cases (C).
FIGURE 2.
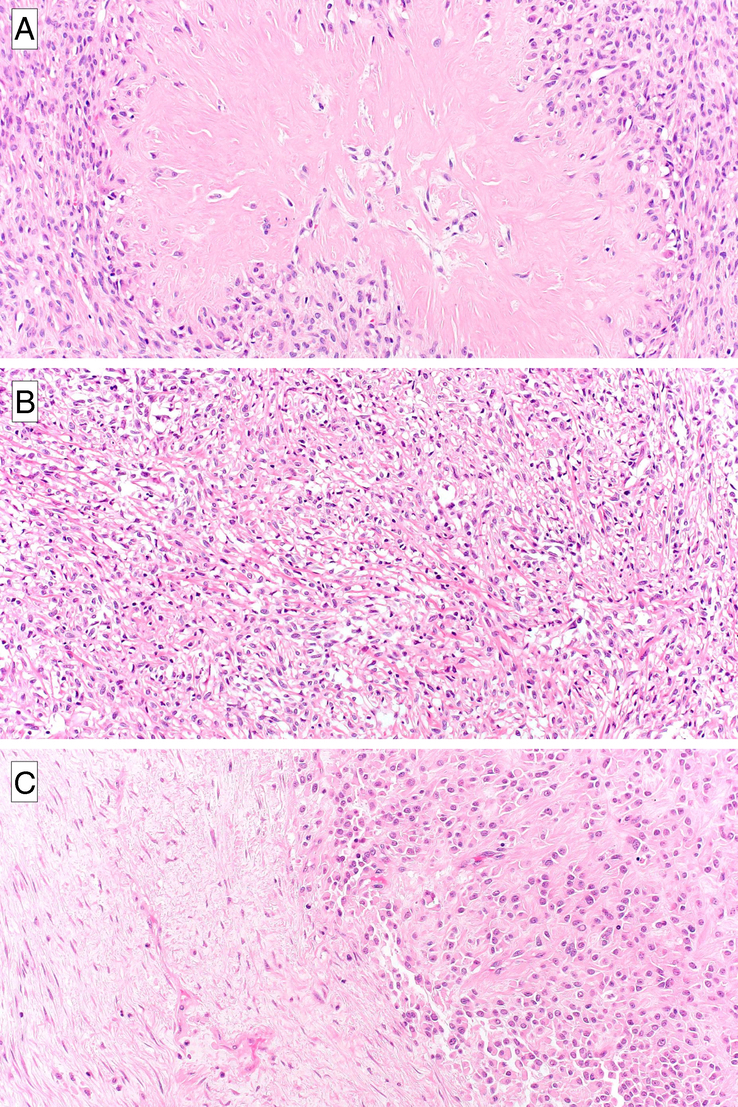
Morphologic features of low-grade fibromyxoid sarcoma, sclerosing epithelioid fibrosarcoma, and hybrid forms. An example of LGFMS showing distinctive large collagenous pseudorosettes composed of a central hyalinized collagenous area surrounded by a collarette of neoplastic cells (A). SEF is composed of epithelioid cells organized in cords and nests set in a collagenous stroma (B). The hybrid form is composed of a combination of SEF and LGFMS areas (C).
Molecular analysis was conducted in 160/239 (67%) LGFMS patients, detecting a positive FUS/EWSR1 rearrangement in 154 cases, including 50% FUS::CREB3L2, 4% FUS::CREB3L1, 1% EWSR1::CREB3L1, and 45% FUS/EWSR1 rearrangements with no fusion partner specified.
For SEF, molecular analysis was performed in 19/32 (59%) patients, with EWSR1 rearrangement detected in 15 cases. This included 65% EWSR1::CREB3L1, 12% EWSR1::CREB3L2, and 23% EWSR1 rearrangements with no fusion partner specified.
Similarly, in H-LGFMS/SEF, analysis was conducted in 17/23 patients (74%), revealing a positive FUS/EWSR1 rearrangement in 13 cases. Among these, 31% were EWSR1::CREB3L1, 23% were FUS::CREB3L2, and 46% were FUS/EWSR1 rearrangements with no fusion partner specified.
Thirteen out of 294 (4%) patients (4/239 LGFMS, 8/32 SEF, and 1/23 H-LGFMS/SEF) underwent systemic therapy, with the majority (10/13) receiving doxorubicin-based treatment. Among patients who received neoadjuvant systemic treatments and were evaluable for response (3/8 SEF, 1/4 LGFMS, and 0/1 H-LGFMS/SEF), 3 SEF patients were treated with doxorubicin-ifosfamide and had stable disease and 1 LGFMS patient was treated with oral cyclophosphamide and had stable disease. No radiologic responses were observed.
Eighty-seven out of 294 (30%) patients (62/239 LGFMS, 16/32 SEF, and 9/23 H-LGFMS/SEF) received radiotherapy, administered in postoperative setting in 29/87 cases (19/29 after R0 surgery, 5/29 after R1 surgery, 2/29 after R2 surgery, and 3/29 unknown margins).
Median(m-) follow-up (FU) was 57.1 months (interquartile range [IQR]: 20.7, 87.0) overall, 55.7 months (IQR: 19.5, 87.0) in LGFMS, 57.7 months (IQR: 37.1, 90.5) in SEF, and 58.5 months (IQR: 15.9, 81.6) in H-LGFMS/SEF.
Overall Survival
Overall, m-OS was not reached (IQR: 191.4-not reached, Fig. 3). The corresponding 5- and 10-year OS estimates were 96.5% (95% CI: 93.9-99.2) and 92.0% (CI: 87.0-97.2), respectively. m-OS, and 5- and 10-year estimates of OS according to pathology are shown in Table 2. LGFMS was associated with better OS (96.1% 10-year OS) compared with SEF (67.0%) and H-LGFMS/SEF (84.8%). In the overall cohort, older age at surgery (P value 0.0050), histologic subtype (P value 0.0022), administration of radiotherapy (P value 0.0292), and systemic therapy (P value 0.0091) were associated with OS in univariable analysis (Supplementary Table 1A, Supplemental Digital Content 1, http://links.lww.com/PAS/B960), while only age at surgery (P value 0.0027) was significantly associated with OS in multivariable analysis (Supplementary Table S1B, Supplemental Digital Content 1, http://links.lww.com/PAS/B960).
FIGURE 3.
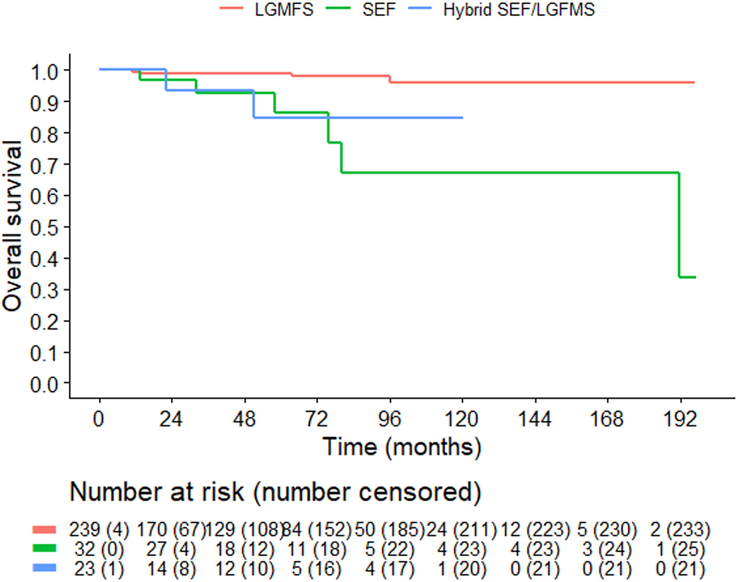
Overall survival according to histologic subtype. LGFMS indicates low-grade fibromyxoid sarcoma; SEF, sclerosing epithelioid fibrosarcoma.
TABLE 2.
Outcome According to Histologic Subtype
| LGFMS (239 pts) | SEF (32 pts) | H-LGFMS/SEF (23 pts) | |
|---|---|---|---|
| Median follow-up, mo (IQR) | 55.7 (19.5-87.0) | 57.7 (37.1-90.5 | 58.5 (15.9-81.6) |
| OS | |||
| m-OS (months) | NR | 191.4 (CI: 80.1-NR) | NR |
| 5-yr OS | 99.0% (CI: 97.6-100.0) | 86.2% (CI: 72.1-100.0) | 84.8% (CI: 67.4-100.0) |
| 10-yr OS | 96.1% (CI: 91.9-100.0) | 67.0% (CI: 45.3-99.2) | 84.8% (CI: 67.4-100.0) |
| LR | |||
| Number of events | 10 | 3 | 0 |
| Median time to LR (mo) | 5.4 (IQR: 1.3-33.5) | 24 (IQR: 17-24) | — |
| 5-yr CCI | 4.7% (CI: 2.4-9.0) | 6.6% (CI: 3.6-31.8) | — |
| 10-yr CCI | 4.7% (CI: 2.4-9.0) | 6.6% (CI: 3.6-31.8) | — |
| DM | |||
| Number of events | 3 | 12 | 8 |
| Median time to DM (mo) | 47.5 (IQR: 27.6-63.1) | 32.0 (IQR: 25-97) | 20 (IQR: 14.7-28.6) |
| 5-yr CCI | 1.3% (CI: 0.3-5.2 | 29.9% (CI: 16.4-54.5) | 48.9% (CI: 28.5-83.9) |
| 10-yr CCI | 2.7% (CI: 0.8-9.3) | 57.7% (CI:30.5-109.1) | 48.9% (CI: 28.5-83.9) |
| p-OS | |||
| m-p-OS (mo) | NR (CI: NR-NR) | 83.2 (CI: 42.1-NR) | 34.0 (CI: 34.0-NR) |
| 5-yr OS | 100% (CI: 100.0-100.0) | 64.6% (CI: 38.1-100.0) | 43.8% (CI: 10.7-100.0) |
H indicates hybrid; LR, local recurrence; m, median; NR, not reached; pts, patients.
Local Recurrence
Overall, 13/294 (4.4%) patients experienced LR (10/13 LGFMS, 3/10 SEF, and 0/10 H-LGFMS/SEF). The observed median time to LR was 10.7 months (IQR: 1.8-26.1). The corresponding 5- and 10-year CCI-LR were 5.1% (CI: 2.9-9.0).
The number of events, median time to LR, and 5- and 10-year estimates of CCI-LR according to histologic subtype are shown in Table 2. CCI-LR curves of LR are shown in Fig. 4.
FIGURE 4.
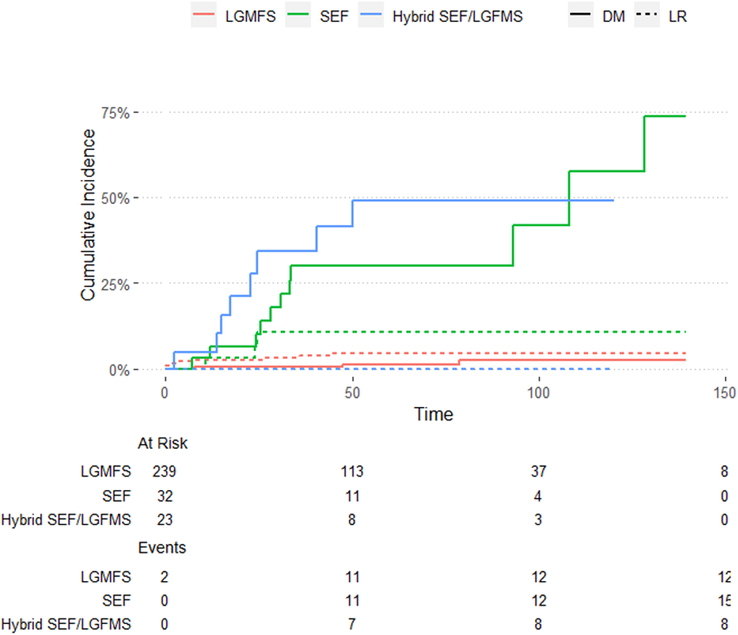
Local recurrence and distant metastases according to histologic subtype. SEF indicates sclerosing epithelioid fibrosarcoma; LR, local recurrence; DM, distant metastases.
In the overall cohort, SEF histologic subtype (P value <0.0001) was significantly associated with CCI-LR in both univariate and multivariate analysis (Supplementary Table 2A, B, Supplemental Digital Content 2, http://links.lww.com/PAS/B961), while the administration of systemic therapy was the only variable significantly associated with CCI-LR on multivariate analysis (P value <0.0001) (Supplementary Table 2B, Supplemental Digital Content 2, http://links.lww.com/PAS/B961).
Distant Metastases
Overall, 23/294 (7.8%) patients experienced DM (3/239 LGFMS, 12/32 SEF, and 8/23 H-LGFMS/SEF). The observed median time to DM was 28.2 months (IQR: 16.2-48.8). The corresponding 5- and 10-year CCI-DM were 8.6% (CI: 5.5-13.4) and 14.0% (CI: 8.2-22.6), respectively.
The number of events, median time to DM, and 5- and 10-year estimates of CCI-DM according to histologic subtype are shown in Table 2. CCI-DM curves of DM are shown in Figure 4.
In the H-LGFMS/SEF group, 3/8 FUS-positive and 3/4 EWSR1-positive patients developed DM; the corresponding 3-year CCI-DM was 37.5% (CI: 10.5-100) and 66.7% (CI: 22.3-100), respectively.
In the overall cohort, SEF and H-LGFMS/SEF histology (P value <0.0001) was significantly related to CCI-DM in both univariate and multivariate analysis, while the administration of radiotherapy and systemic therapy (P value <0.0001) were significantly related to CCI-DM only on univariate analysis (Supplementary Table 3A, Supplemental Digital Content 3, http://links.lww.com/PAS/B962), but not in multivariable analysis (Supplementary Table 3B, Supplemental Digital Content 3, http://links.lww.com/PAS/B962).
Three of 239 patients with LGFMS developed DM: 1 had DM 7.8 months postsurgery for the primary disease, subsequently receiving systemic therapies (pazopanib, liposomal doxorubicin, and pembrolizumab), and was alive with disease (AWD) at the latest follow-up. Another patient developed DM 47.5 months after primary surgery, underwent twice a complete metastasectomy, and was AWD at the latest follow-up. The third patient developed DM 78.7 months post-primary surgery, underwent complete metastasectomy, and was alive without disease at the latest follow-up.
Post-metastases Overall Survival
Median-p-OS and 5-year estimates of p-OS according to histologic subtype are shown in Table 2. Kaplan-Meyer curves are shown in Figure 5.
FIGURE 5.
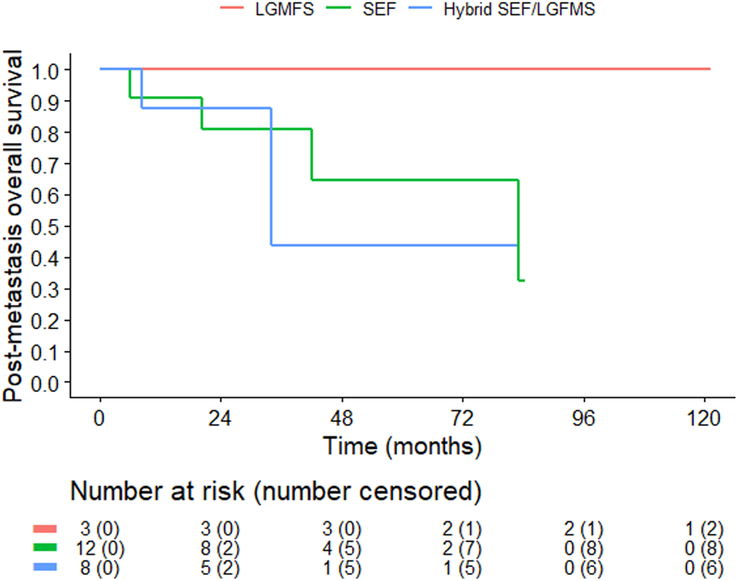
Post-metastases overall survival according to histologic subtype. The graph represents the overall survival in patients who had a distant metastasis. Patients in the LGFMS subgroup who had an event (Fig. 3) are not represented in this curve because they did not have DM. LGFMS indicates low-grade fibromyxoid sarcoma; SEF; sclerosing epithelioid fibrosarcoma.
DISCUSSION
In this international, multicentric retrospective series, we report data on the largest cohort to date of patients affected by primary, localized, resectable LGFMS, SEF, and H-LGFMS/SEF observed and treated at several sarcoma referral centers over a >20-year time span. We found that localized LGFMS is associated with an excellent prognosis, while about 50% of H-LGFMS/SEF and SEF develop DM within 5 years and 10 years. This suggests that the biology and clinical behavior of H-LGFMS/SEF more closely mimics SEF than LGFMS. No significant prognostic factors other than age and histologic subtype were identified for any of the endpoints analyzed.
This was a retrospective study with inherent limitations. The relatively short m-FU (57 mo) did not allow for a comprehensive analysis of the natural history of the disease, particularly of LGFMS, which may also metastasize many years after diagnosis (observed median time to DM=47.5 mo), with events occurring many months after the m-FU of the study (1 of the 3 patients who developed DM did so 78.7 mo after surgery). Because of the restricted number of events, our ability to effectively study the correlation between putative prognostic factors and survival in all histologies is limited. Moreover, among the 27 institutions that took part in the study, the approach to disease was heterogeneous. Consequently, we were not able to thoroughly analyze either the optimal systemic therapy or radiation schedule.
However, to the best of our knowledge, this is one of the largest series of this ultra-rare sarcoma type. We tried to homogenize the population by selecting cases with pathologically confirmed diagnoses based on predefined criteria and only treated by an expert sarcoma team (Supplementary Data-Synopsis).
The chance of cure for localized LGFMS is high with surgery alone with OS close to 100% at 10 years. Patients are usually young (median age 48 y). Hence, as also suggested by the French study,8 they should be followed for at least 10 years after surgery to monitor for disease recurrence in this young population, as metastases were also seen well above the m-FU. In a 33 LGFMS series from the U.T.M.D. Anderson Cancer Center, 21/33 patients recurred after intervals of up to 15 years.12
Conversely, the chance of cure for localized H-LGFMS/SEF and SEF does not exceed 50%. These tumors are more aggressive, tend to recur earlier, and do not seem to significantly benefit from currently available therapies. Indeed, the activity of conventional systemic treatments is very limited,8,9,13 as we reported in patients with metastatic H-LGFMS/SEF (paper under submission), and also in this study, we did not see any responses to chemotherapy in the neoadjuvant setting (anthracycline-based regimens and oral cyclophosphamide). Therefore, current systemic therapy does not have a clearly defined role for localized LGFMS, SEF, and H-LGFMS.
The LR risk is limited in all histologies. Surgery may be sufficient to control the primary disease. In terms of anatomic locations, there is a nonsignificant trend in favor of better local control in extremities compared with chest and retroperitoneum/abdomen and other sites (Supplementary Table 2, Supplemental Digital Content 2, http://links.lww.com/PAS/B961). This trend is consistent with what is observed in all other sarcoma types and is related to the different anatomic constraints of extremities versus trunk and the ability to perform a wide resection.
RT was administered in around 30% to 40% of cases in the 3 histologies and was not significantly associated with survival benefit or reduction of relapse risk in this cohort. Therefore, the administration of RT may not be needed in all cases, regardless of size, location, or histologic type. As mentioned, all our patients were treated in sarcoma referral centers by a team of expert surgeons who performed a macroscopic complete resection in 90% to 95% of cases. This may well be one of the reasons why the administration of adjuvant radiotherapy was limited and did not influence the LR risk.
The main driver of prognosis is the DM risk, which is low in LGFMS and high in H-LGFMS/SEF and SEF. The 5-year OS was 100% in LGFMS, 64.6% in SEF, and 43.8% in H-LGFMS/SEF, and survival does not seem to be strongly correlated with the need for RT or systemic therapy.
We observed that the molecular profile of LGFMS and SEF was consistent with previous reports. Indeed, the most common alteration in LGFMS was FUS::CREB3L2, and in SEF was EWSR1::CREB3L1, as reported.3–5,14,15 Molecular analysis of H-LGFMS/SEF revealed different molecular alteration involving FUS and EWSR1 genes, in particular 62% (8 patients) had positive FUS rearrangements and 31% (4 patients) EWSR1::CREB3L1 gene fusion. Comparing these 2 small subsets of H-LGFMS/SEF, 3/8 (37.5%) FUS-positive and 3/4 (75%) EWSR1-positive patients developed DM, with 3-year estimate DM of 37.5% (CI: 10.5-100) and 66.7% (CI: 22.3-100), respectively. In another series, including 8 cases from Memorial Sloan-Kettering Cancer Center, all H-LGFMS/SEF exhibited the FUS::CREB3L2 fusion.11 While H-LGFMS/SEF and LGFMS both exhibit the FUS::CREB3L2 fusion, the clinical behavior of H-LGFMS/SEF more closely resembles that of SEF. However, we may speculate that FUS-positive H-LGFMS/SEF are less similar to SEF than EWSR1-positive H-LGFMS/SEF and test patients at diagnosis to inform management.
An interesting point of discussion is whether H-LGFMS/SEF is one separate entity or, conversely, part of a spectrum with SEF and/or LGFMS. In this study, SEF and H-LGFMS/SEF showed similar clinical behavior with a similar metastatic risk. On the other hand, the analysis of the metastatic series revealed that H-LGFMS/SEF more closely mimicked the behavior of LGFMS. Starting from these preliminary observations, a prospective observational study (also with a translational substudy) is planned to better characterize these 3 entities and the correlation of their outcome with the molecular profile.
In conclusion, this study, along with the French series,8 helps clarify the natural history of these ultra-rare sarcomas. This study highlights the importance and impact of investigators from multiple institutions and countries coming together to advance the knowledge and treatment of ultra-rare sarcomas. Our data show that localized LGFMS have a high chance of cure with surgery alone, while localized SEF and H-LFGMS/SEF have a more aggressive behavior and higher metastatic risk. Long-term follow-up is essential to assess absolute cure rates and possibly identify prognostic factors.
Supplementary Material
ACKNOWLEDGMENTS
The authors are deeply grateful to Barbara Rapp, from the Connective Tissue Oncology Society (CTOS) for her support in organizing the Ultra-Rare Sarcoma Working Group meeting.
Footnotes
The cost was partially supported by funding from the Italian Ministry of Health, Ricerca Corrente and 5×1000 funds for health care research.
Conflicts of Interest and Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.ajsp.com.
Contributor Information
Claudia Giani, Email: claudia.giani@istitutotumori.mi.it.
Abdulazeez Salawu, Email: abdulazeez.salawu@uhn.ca.
Silva Ljevar, Email: Silva.ljevar@istitutotumori.mi.it.
Ryan A. Denu, Email: radenu@mdanderson.org.
Andrea Napolitano, Email: Andrea.Napolitano@rmh.nhs.uk.
Emanuela Palmerini, Email: emanuela.palmerini@ior.it.
Elizabeth A. Connolly, Email: Elizabeth.Connolly@lh.org.au.
Koichi Ogura, Email: koogura@ncc.go.jp.
Daniel D. Wong, Email: Daniel.WongChungLung@health.wa.gov.au.
Roberto Scanferla, Email: roberto.scanferla91@gmail.com.
Evan Rosenbaum, Email: rosenbae@mskcc.org.
Jyoti Bajpai, Email: dr_jyotibajpai@yahoo.co.in.
Zola Chia-Chen Li, Email: zola.ccli@gmail.com.
Susie Bae, Email: Susie.Bae@petermac.org.
Lorenzo D’Ambrosio, Email: lorenzo.dambrosio@unito.it.
Steve Bialick, Email: steven.bialick@med.miami.edu.
Andrew J. Wagner, Email: Andrew_Wagner@dfci.harvard.edu.
Hanna Koseła-Paterczyk, Email: Hanna.Kosela-Paterczyk@pib-nio.pl.
Giacomo G. Baldi, Email: giacomogiulio.baldi@uslcentro.toscana.it.
Antonella Brunello, Email: antonella.brunello@ioveneto.it.
Yeh Chen Lee, Email: YehChen.Lee@health.nsw.gov.au.
Herbert H. Loong, Email: h_loong@clo.cuhk.edu.hk.
Sosipatros Boikos, Email: Sosipatros.A.Boikos@medstar.net.
Fernando Campos, Email: fernandoaugustobc@gmail.com.
Carlo M. Cicala, Email: carlocicala@vhio.net.
Robert G. Maki, Email: bobmakimd@gmail.com.
Nadia Hindi, Email: nhindi@atbsarc.org.
Costanza Figura, Email: costanza.figura@gmail.com.
Shahd S. Almohsen, Email: ShahdSalehM.AlMohsen@sinaihealth.ca.
Sheyaskumar Patel, Email: spatel@mdanderson.org.
Robin L. Jones, Email: robin.jones4@nhs.net.
Toni Ibrahim, Email: toni.ibrahim@ior.it.
Rooshdiya Karim, Email: rooshdiya.karim@health.nsw.gov.au.
Akira Kawai, Email: akawai@ncc.go.jp.
Richard Carey-Smith, Email: richard.careysmith@health.wa.gov.au.
Richard Boyle, Email: rich_boyle@hotmail.com.
Silvia M. Taverna, Email: silvia.taverna@istitutotumori.mi.it.
Alexander J. Lazar, Email: alazar@mdanderson.org.
Elizabeth G. Demicco, Email: elizabeth.demicco@sinaihealth.ca.
Judith V.M.G. Bovee, Email: j.v.m.g.bovee@lumc.nl.
Angelo P. Dei Tos, Email: angelo.deitos@unipd.it.
Christopher Fletcher, Email: alessandro.gronchi@istitutotumori.mi.it.
Daniel Baumhoer, Email: daniel.baumhoer@usb.ch.
Marta Sbaraglia, Email: marta.sbaraglia@aopd.veneto.it.
Inga-Marie Schaefer, Email: imschaefer@me.com.
Rosalba Miceli, Email: rosalba.miceli@istitutotumori.mi.it.
Alessandro Gronchi, Email: alessandro.gronchi@istitutotumori.mi.it.
Silvia Stacchiotti, Email: silvia.stacchiotti@istitutotumori.mi.it.
REFERENCES
- 1.Stacchiotti S, Frezza AM, Blay JY, et al. Ultra-rare sarcomas: a consensus paper from the Connective Tissue Oncology Society community of experts on the incidence threshold and the list of entities. Cancer. 2021;127:2934–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Classification of Tumours Editorial Board . Soft Tissue and Bone Tumours. International Agency for Research on Cancer; 2020. (WHO Classification of Tumours series, 5th ed. Vol. 3). [Google Scholar]
- 3.Bejarano PA, Padhya TA, Smith R, et al. Hyalinizing spindle cell tumor with giant rosettes—a soft tissue tumor with mesenchymal and neuroendocrine features. An immunohistochemical, ultrastructural, and cytogenetic analysis. Arch Pathol Lab Med. 2000;124:1179–1184. [DOI] [PubMed] [Google Scholar]
- 4.Panagopoulos I, Storlazzi CT, Fletcher CDM, et al. The chimeric FUS/CREB3l2 gene is specific for low-grade fibromyxoid sarcoma. Genes Chromosomes Cancer. 2004;40:218–228. [DOI] [PubMed] [Google Scholar]
- 5.Arbajian E, Puls F, Magnusson L, et al. Recurrent EWSR1-CREB3L1 gene fusions in sclerosing epithelioid fibrosarcoma. Am J Surg Pathol. 2014;38:801–808. [DOI] [PubMed] [Google Scholar]
- 6.Doyle LA, Möller E, Dal Cin P, et al. MUC4 is a highly sensitive and specific marker for low-grade fibromyxoid sarcoma. Am J Surg Pathol. 2011;35:733–741. [DOI] [PubMed] [Google Scholar]
- 7.Doyle LA, Wang WL, Dal Cin P, et al. MUC4 is a sensitive and extremely useful marker for sclerosing epithelioid fibrosarcoma: association with FUS gene rearrangement. Am J Surg Pathol. 2012;36:1444–1451. [DOI] [PubMed] [Google Scholar]
- 8.Blay JY, Tlemsani C, Toulmonde M, et al. Sclerosing Epithelioid Fibrosarcoma (SEF) versus Low Grade Fibromyxoid Sarcoma (LGFMS): presentation and outcome in the nationwide NETSARC+ series of 330 patients over 13 years. Eur J Cancer Oxf Engl 1990. 2024;196:113454. [DOI] [PubMed] [Google Scholar]
- 9.Chew W, Benson C, Thway K, et al. Clinical characteristics and efficacy of chemotherapy in sclerosing epithelioid fibrosarcoma. Med Oncol Northwood Lond Engl. 2018;35:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martínez-Trufero J, Cruz Jurado J, Gómez-Mateo MC, et al. Uncommon and peculiar soft tissue sarcomas: multidisciplinary review and practical recommendations for diagnosis and treatment. Spanish group for Sarcoma research (GEIS-GROUP). Part I. Cancer Treat Rev. 2021;99:102259. [DOI] [PubMed] [Google Scholar]
- 11.Prieto-Granada C, Zhang L, Chen HW, et al. A genetic dichotomy between pure sclerosing epithelioid fibrosarcoma (SEF) and hybrid SEF/low-grade fibromyxoid sarcoma: a pathologic and molecular study of 18 cases. Genes Chromosomes Cancer. 2015;54:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans HL. Low-grade fibromyxoid sarcoma: a clinicopathologic study of 33 cases with long-term follow-up. Am J Surg Pathol. 2011;35:1450–1462. [DOI] [PubMed] [Google Scholar]
- 13.Chamberlain F, Engelmann B, Al-Muderis O, et al. Low-grade fibromyxoid sarcoma: treatment outcomes and efficacy of chemotherapy. Vivo Athens Greece. 2020;34:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang WL, Evans HL, Meis JM, et al. FUS rearrangements are rare in ‘pure’ sclerosing epithelioid fibrosarcoma. Mod Pathol. 2012;25:846–853. [DOI] [PubMed] [Google Scholar]
- 15.Memon RA, Granada CNP, Patel C, et al. Gastric sclerosing epithelioid fibrosarcoma harboring a rare FUS-CREM fusion. Int J Surg Pathol. 2021;29:565–570. [DOI] [PubMed] [Google Scholar]


