Abstract
Stem-cell therapy is a revolutionary frontier in modern medicine, offering enormous capacity to transform the treatment landscape of numerous debilitating illnesses and injuries. This review examines the revolutionary frontier of treatments utilizing stem cells, highlighting the distinctive abilities of stem cells to undergo regeneration and specialized cell differentiation into a wide variety of phenotypes. This paper aims to guide researchers, physicians, and stakeholders through the intricate terrain of stem-cell therapy, examining the processes, applications, and challenges inherent in utilizing stem cells across diverse medical disciplines. The historical journey from foundational contributions in the late 19th and early 20th centuries to recent breakthroughs, including ESC isolation and iPSC discovery, has set the stage for monumental leaps in medical science. Stem cells’ regenerative potential spans embryonic, adult, induced pluripotent, and perinatal stages, offering unprecedented therapeutic opportunities in cancer, neurodegenerative disorders, cardiovascular ailments, spinal cord injuries, diabetes, and tissue damage. However, difficulties, such as immunological rejection, tumorigenesis, and precise manipulation of stem-cell behavior, necessitate comprehensive exploration and innovative solutions. This manuscript summarizes recent biotechnological advancements, critical trial evaluations, and emerging technologies, providing a nuanced understanding of the triumphs, difficulties, and future trajectories in stem cell-based regenerative medicine. Future directions, including precision medicine integration, immune modulation strategies, advancements in gene-editing technologies, and bioengineering synergy, offer a roadmap in stem cell treatment. The focus on stem-cell therapy’s potential highlights its significant influence on contemporary medicine and points to a future in which individualized regenerative therapies will alleviate various medical disorders.
Keywords: biotechnology advancements, clinical trials, medical revolution, stem-cell therapy
Overview
Highlights
Stem cell therapy represents a groundbreaking frontier in modern medicine, offering unprecedented potential to address a wide range of debilitating diseases and injuries.
Stem cells possess unique properties, including self-renewal and differentiation into specialized cell types, making them indispensable for regenerative medicine applications.
The historical journey of stem cell research, from foundational contributions in the late 19th and early 20th centuries to recent breakthroughs like the isolation of embryonic stem cells and induced pluripotent stem cells, highlights the monumental progress in medical science.
Stem cell therapy holds promise for treating various conditions, including cancer, neurodegenerative disorders, cardiovascular diseases, spinal cord injuries, diabetes, and tissue damage.
Despite the immense potential, stem cell therapy faces challenges such as immune rejection, tumorigenesis, and the precise manipulation of stem cell behaviors, necessitating innovative solutions for clinical translation.
Recent biotechnological advancements, such as exosome-based therapeutics, single-cell RNA sequencing, and CRISPR technology, have revolutionized stem cell research, offering new opportunities for precise genome editing and therapeutic interventions.
Regulatory considerations are paramount in the clinical translation of stem cell therapies, requiring adherence to strict guidelines and directives to ensure safety and efficacy.
The future of stem cell therapy lies in precision medicine integration, immune modulation strategies, advancements in gene editing technologies, and synergies with bioengineering, paving the way for continued evolution and personalized regenerative therapies.
Stem-cell therapy signifies a pioneering frontier in modern medicine that uses the extraordinary power of stem cells and their revolutionary potential to treat diverse illnesses. Stem cells play a crucial role in regenerative medicine and exhibit the extraordinary ability to differentiate into various cell types and to renew themselves. Their intrinsic capacity to repair and regenerate tissues holds immense promise for revolutionizing therapeutic interventions1,2. The historical journey of stem-cell investigation can be traced to pivotal contributions from visionaries such as Boveri, Häcker, Maximow, and Cohnheim during the late 19th and early 20th centuries3. Their foundational work placed the groundwork for comprehension of the fundamental principles of stem cells and for shedding light on their roles in developmental processes and tissue repair. These early insights have laid the foundation for contemporary stem-cell investigations, fueling a deeper exploration of their biological significance3,4. Important turning points in the history of this field include the identification of ESCs in 1981 by Kaufman and Evans5–7 and Thomson’s discovery of iPSCs in 20078. Although stem-cell therapies have vast and promising potential, several challenges and complexities loom in their clinical translation9. Issues like immunological rejection, tumorigenesis, and precise manipulation of stem-cell behavior for optimal therapeutic outcomes are critical hurdles that necessitate comprehensive exploration and innovative solutions1,10–12. Advances in biotechnology, especially the revolution in exosome-based therapeutics, single-cell RNA sequencing (scRNA-Seq), and CRISPR technology13–15, one of the major developments in genetic engineering, has made precise and effective genome editing possible, which opens new avenues for modified genetic material, leading to advances in a variety of fields such as biotechnology and medicine16,17. Regenerative medicine represents a novel and promising therapeutic approach for individuals with exhausted or nonexistent options for managing their medical condition. Research studies, such as identification, clinical trials, and therapeutic applications on stem-cell have been extensive in recent years because of promising results from preclinical research (Fig. 1). The process of bringing these novel medicinal items from laboratories to the market is governed by strict guidelines and directives issued by qualified regulatory bodies18. Stem cells can be obtained for tissue engineering and cell treatments from four primary sources. The stem cells primary sources are embryonic and fetal tissues, comprising the placenta (including the chorion and amnion), umbilical cord (Wharton jelly), and particular tissues inside the adult, such as blood, skin, skeletal muscle, fat, and bone marrow, and somatic cells that have undergone genetic reprogramming to become distinct from their original state, such as iPSCs19.
Figure 1.
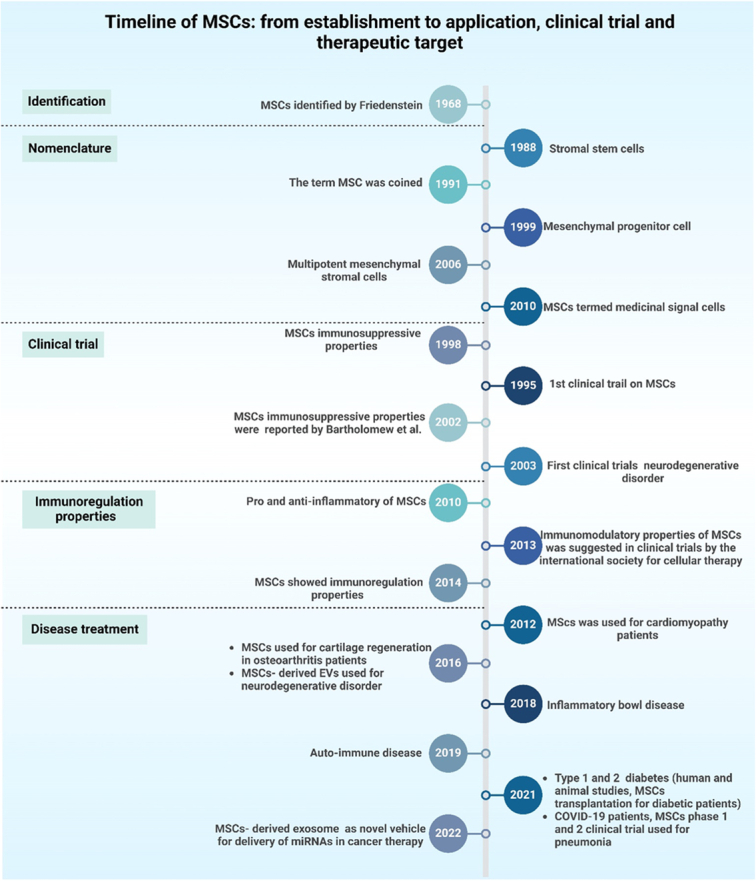
A timeline depicting the introduction of mesenchymal stem cells (MSCs), their early research, and their substantial application in clinical trials, immunoregulation, and disease treatment.
Through an extensive synthesis of recent biotechnological advancements, critical evaluations, and emerging technologies, this review offers a nuanced comprehension of the advantages, difficulties, and future trajectories of stem cell-based regenerative therapy. By examining the historical foundations, current landscape, and prospects, this study endeavors to serve as a guide for researchers, clinicians, and stakeholders in navigating the intricate terrain of stem-cell therapy.
Search strategy
An extensive examination of existing literature was performed using the Embase, Web of Science, PubMed, and Scopus databases. The terms ‘stem cell therapy’, ‘medical revolution’, ‘biotechnology advancements’, and ‘clinical trial’ were used in the search. Only articles published in English were included in the search. We assessed the abstracts of each article to determine the relevance of the retrieved papers to the topic. Subsequently, every relevant paper (in vivo, in vitro, and human-based research) was selected as part of the study.
Stem-cell types
Embryonic stem cells (ESCs)
ESCs exhibit characteristics that distinguish them from each other in stem cell biology. Notably, their pluripotency, which is defined by distinct features to differentiate into any human body cell, makes them highly adaptable and has great therapeutic promise20. Additionally, ESCs have a notably high self-renewal capacity, which contributes to their sustained presence and functionality over extended periods21. Potential ESC sources include mice, nonhuman primates, and humans. They are isolated from the blastocysts’ inner cell mass before implantation22,23. Because they are pluripotent cells, they can produce various kinds of cells from fetuses and adults in vivo and in vitro 24–26. Two methods were employed to separate ESCs from blastocysts’ inner cell masses. Microsurgery is the most commonly used surgical approach. Mechanical dissection in the microscopic direction is used to isolate cells of the trophoblastic lineage from the rest of the cell mass. The second approach entails employing an antibody to target trophoblast lineage cells27,28.
Regarding potential applications, the pluripotent nature of ESCs opens avenues for significant contributions to tissue regeneration and repair. Their capacity to undergo differentiation into many cell lineages holds promise for treating degenerative conditions and injuries, making them pivotal players in regenerative medicine. Furthermore, ESCs serve as invaluable tools in disease modeling for research purposes29. By replicating specific cellular environments, researchers can discover more about the workings of various disorders, providing a framework for cellular disease research and aiding in the creation of focused therapies. The unique properties of ESCs are relevant to drug testing and development30. Because of their pluripotency, a variety of cell populations can be created to provide a more complete picture of human cellular responses. This capability is particularly valuable for evaluating drug efficacy and safety and provides a sophisticated model for preclinical testing. Consequently, the multifaceted potential of ESCs dramatically enhances our comprehension of biology, fostering medical research and shaping the landscape of therapeutic innovation31,32.
Adult stem cells (ASCs)
ASCs stand out in the realm of regenerative biology because of their distinctive properties and vital roles in maintaining tissue homeostasis33. Multipotency is the ability of cells to possess various potential fates or abilities to develop into a restricted, diverse array of cellular phenotypes34. ASCs are endogenous stem cells that are crucial for preserving the tissues’ structural integrity, like bone, skin, and blood. They are located in specific niches or tissue sections35. ASCs have been discovered in several tissues, including blood, stomach, muscle, skin, brain, and heart36. They are less potent than ESCs; however, they have demonstrated efficacy in disease treatment. They can be extracted and harvested from individuals and used for tissue regeneration through autologous or allogeneic transplantation37. ASCs have a more specialized differentiation capability than pluripotent cells, such as ESCs, and can help generate particular cell lineages within their original tissue34.
Stem cells’ function in repairing damaged tissues and maintenance is essential throughout an individual’s lifespan38. The unique ability of ASCs to maintain tissue and exhibit multipotency lends itself to a variety of possible uses within the regenerative medicine field39. Tissue-specific regeneration and repair are among the most promising approaches. ASCs can be utilized to regenerate damaged or deteriorated tissues due to their presence in diverse tissues, including the bone marrow, skin, and muscle40. Their capacity to undergo cell type-specific differentiation that is relevant to their native tissues places them at the forefront of tailored regeneration techniques, offering potential treatment options for ailments ranging from degenerative illnesses unique to certain organs to musculoskeletal injuries41.
ASCs are an appealing therapeutic choice for degenerative diseases. Because of their functions in tissue repair and regeneration, they are desirable targets for therapies aimed at slowing the advancement of illnesses marked by cellular degeneration42. Through the utilization of the regenerative capacity of these cells, scientists and medical professionals have investigated ways to create novel treatments that target the root causes of degenerative illnesses with the aim of enhancing patient outcomes and quality of life43. Within the class of ASCs, hematopoietic stem cells are a specific subset essential for bone marrow transplantation44. The immune system and blood regeneration rely on hematopoietic stem cells (HSCs), which are essential due to their versatility in cell differentiation into various blood cell types45. The utilization of these cells in bone marrow transplants represents a cornerstone in hematological therapies, offering a curative approach for conditions like leukemia and other disorders affecting the blood and immune systems46,47. Transplantation of hematopoietic stem cells is a life-saving intervention that reinstates functional blood and immune cell populations in individuals with hematopoietic disorders48.
Perinatal stem cells
Embryonic stem cells are derived from the amniotic fluid, placenta, and umbilical cord and represent a unique category within the spectrum of stem cell types49. Fetal cells possess multipotent capabilities and can differentiate into a restricted type of cells50. These cells are distinctively derived from tissues associated with the prenatal and perinatal stages of development, indicating their specialized origin49. Notably, perinatal stem cells exhibit a hybrid nature, sharing characteristics analogous to those of adults and ESCs. Their dual features make them adaptable and potentially useful for various regenerative medicine applications51. Perinatal stem cells offer a noncontroversial and ethically sound reservoir for therapeutic purposes49. Their properties, which are reminiscent of those of ESCs and ASCs, contribute to their unique regenerative potential. Since these cells undergo cell differentiation into a wide variety of cells, tailored approaches for tissue regeneration and repair are possible52. Perinatal stem cells show promise in furthering regenerative medicine across a range of tissues in terms of prospective uses. They are important components in targeted tissue renewal because of their capacity to specialize in particular cell lineages52.
Moreover, its therapeutic potential can be extended to other conditions, such as cerebral palsy and diabetes. Perinatal stem cells offer a novel and innovative approach to the development of medicines tailored to address the complexities of these disorders by exploiting their regenerative properties and versatile differentiation capabilities53. One notable advantage of perinatal stem cells is their potential for allogeneic transplantation without eliciting immune rejection. The immunomodulatory characteristics of these cells make them well-suited for transplantation across different individuals, eliminating the need for a perfect match between the donor and recipient54. This opens new possibilities for allogeneic stem-cell therapies, providing a feasible and practical approach to transplantation procedures without the intricate challenges associated with immune compatibility.
In summary, perinatal stem cells signify a distinct and highly promising category of stem cells with hybrid properties. Their application in regenerative medicine, therapeutic interventions for specific conditions, and allogeneic transplantation underscore their potential to reshape the landscape of stem cell-based therapies.
Induced pluripotent stem cells (iPSCs)
The iPSCs represent a revolutionary category in stem-cell studies and are characterized by properties that mirror those of ESCs55. Several human and mouse investigations have utilized fibroblasts and skin cells as the primary sources of adult cells. It has been discovered that adult brain stem cells have been identified as the primary cell type in investigations of reprograming cells56. Another study reported that murine bone marrow mononuclear cells can be reprogrammed more effectively than mouse embryonic fibroblasts57. Notably, iPSCs and their embryonic counterparts possess the capacity to undergo pluripotency to differentiate into distinct kinds of specialized cells58. One important way to iPSCs is to distinguish them from ESCs by their source, in which in order to create iPSCs, adult cells are reprogrammed. This methodology provides a novel means of addressing ethical concerns regarding the use of ESCs in scientific investigation59. Personalized medicine could undergo significant transformations if adult cells are reprogrammed to become iPSCs. The advancement of individualized cellular therapeutics involves the process of cellular reprogramming for individual patients is one of the main uses of iPSCs60. The iPSCs have the remarkable ability to transform into a wide variety of disease-specific cell types during cell reprogramming. This personalized approach improves the integrity and efficiency of cell-based treatments and offers a potential path in order to treat numerous illnesses and traumas.
Furthermore, iPSCs play a pivotal role in disease modeling in personalized medicine61. The capacity to generate iPSCs from individuals with particular genetic conditions has enabled researchers to create in vitro disease models. These models are extremely invaluable tools for understanding disease mechanisms at the cellular level and enable the exploration of targeted therapeutic interventions62. iPSC-based disease modeling advances the field of personalized medicine by enabling a more accurate and customized approach to medical research, thus opening the door for customized treatments. Beyond illness modeling and customized treatments, iPSCs have a major impact on toxicity assessments and drug development63. The pluripotent characteristics of iPSCs allow the generation of diverse cellular phenotypes, providing a flexible platform for evaluating the safety and effectiveness of pharmaceuticals. iPSC-based assays offer a more thorough understanding of how pharmaceuticals interact with various cell types, which helps identify possible side effects and directs advancements in the creation of remedies that are both safer and more effective64.
In conclusion, iPSCs offer a revolutionary approach to stem-cell investigation, owing to their pluripotent characteristics and the origin of adult cell reprogramming. Their applications in patient-specific cell therapies, disease modeling for personalized medicine, and drug discovery underscore their potential to revolutionize medical treatment and contribute to advancements in personalized healthcare.
Stem cells mechanisms of action
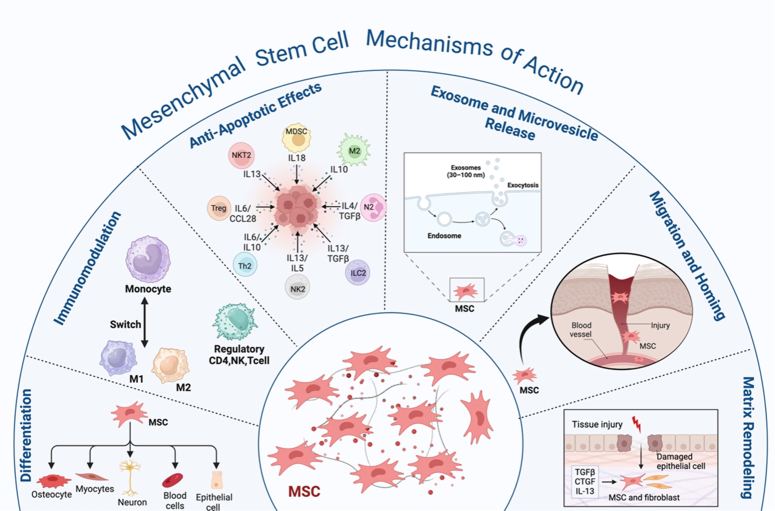
Stem cells secrete numerous factors and exosomes that are responsible for immunomodulatory, antiapoptotic, antibacterial, and microbial properties. In addition to the ability for repair, communication, and regeneration (Fig. 2).
Figure 2.
The schematic diagram represents the mesenchymal stem cells’ mechanism of action and their interaction with immune cells, including differentiation, immunomodulation, antiapoptotic effects, exosome and microvesicle release, migration and homing, and matrix remodeling.
Stem cells’ immunomodulatory actions have undergone extensive research when contrasted with other stem cell types65,66. Stem cells have a role in suppressing acute-phase responses by suppressing excessive activation of macrophages and T cells and initiating the secretion of inflammatory cytokines. This could decrease the likelihood of a cytokine storm67. Toll-like receptors (TLRs) present in MSCs detect injury signals and initiate immunomodulatory responses68. MSCs exhibit immunomodulatory properties via paracrine activity and direct intercellular communication facilitated by several bioactive compounds like cytokines, chemokines, and growth factors. These molecules affect both adaptive and innate immunity. MSCs can prevent the activation of T-cells via several immunomodulatory substances, such as TGF-β1, PGE2, and HLA-G5. They also utilize molecules that are linked to a membrane, such as VCAM-1, PD-L1, and Gal-169,70. MSCs regulate NK cell cytotoxicity by reducing the expression of IFN-γ71. Cytokines are crucial for preserving the ability of ESCs to reproduce. This is achieved through the action of a specific cytokine called leukemia inhibitory factor (LIF), which belongs to the class of cytokines known as interleukin-672. The iPSCs can modulate the immune system, as demonstrated through their capacity to suppress the rapid increase of responder T cells in modified combined leukocyte reactions in vitro 73.
In addition, apoptosis serves as a protective process within the immunological response of the host to combat pathogens and has a crucial function in interactions between the host and pathogens71. MSCs can inhibit apoptosis, which may occur due to pathogens, low oxygen levels, mechanical stress, or radiation. For instance, the ability of MSCs to avoid cell death (antiapoptotic effects) has been investigated in cardiac ischemia, neurological conditions, and respiratory ailments74. In addition, during apoptosis caused by hypoxia, MSCs stimulate the expression of certain proteins, including HGF, VEGF, and TGF-β1, with the potential to prevent endothelial cell death75. Additional variables contribute to the antiapoptotic effect of MSCs, such as IL-6 and IGF-1, which results in enhanced secretion of SFRP2 protein76.
Stem cells exert their antimicrobial activity by secreting molecules and direct cell-to-cell interactions, namely by releasing antimicrobial peptides (AMPs). The antimicrobial activities are carried out by specific AMPs like the family of lipocalins (Lcn2), hepcidin, and b-defensins (hBD-1, hBD-2, and hBD-3)77,78. Stem cells boost their antimicrobial activity by upregulating LL-37, a peptide that is stimulated by bacteria and inhibits bacterial growth79.
Regeneration and restoration of damaged tissues rely heavily on stem cells because of their distinctive ability to suppress aberrant immune responses, their capacity to transform into specific tissues, and produce certain substances that stimulate the host’s reparative and regenerative systems80. Furthermore, the micro-vesicles and exosomes generated from stem cells are important for stem-cell communication and regeneration. Lipids, proteins, nucleic acids, including RNA and micro RNA, and signaling molecules are among the many bioactive compounds that are transported within the extracellular vesicles (EVs) emitted by stem cells of the body81. Compounds secreted by stem cells facilitate tissue regeneration by promoting the growth and specialization of stem/progenitor cells in the immediate vicinity. In addition, they control the placement of molecules in the extracellular matrix, activate pathways that prevent scarring, and promote the development of new blood vessels82,83. MSCs release soluble paracrine factors, including ANGPT1, HGF, EGF, VEGF, KGF, PGE2, and interleukin-10 (IL10). These factors can improve the restoration of epithelial and endothelial cells84,85.
Recent advancements in stem-cell research
Recent years have seen remarkable progress in stem-cell research that has greatly expanded our comprehension of stem-cell biology86. One notable milestone was the elucidation of novel mechanisms governing stem cell fate decisions. Researchers have uncovered key signaling pathways and transcription factors that play pivotal roles in directing stem-cell differentiation87,88. A cellular communication system known as the Notch signaling pathway is vital for various physiological and developmental functions89. Researchers have demonstrated the significance of the Notch pathway in determining the outcome of cells by either promoting the renewal of cells or their differentiation into various types of stem cells, including ESCs90, PSCs91, HSCs92, NSCs93, and ISCs94. Other instances of the signaling pathways are the PI3k/AKT signaling95 and TGF-β signaling96. A transcription factor known as NF-κB controls the diverse functions of NF-κB in stem cells and developmental processes97. These findings enhance stem cell manipulation capabilities for specific therapeutic purposes, offering unprecedented opportunities for targeted cell-based interventions98. Recent studies have explored the nuances of lineage commitment and cellular specialization within the framework of stem-cell development. Scientists have identified regulatory networks that govern stem cell differentiation into distinct cell types, shedding light on the molecular events that dictate cell fate99,100.
Researchers have also unveiled insights into the epigenetic modifications associated with reprogramming, enhancing our comprehension of the molecular mechanisms by which somatic cells transform into pluripotent states101. For example, studies proved that gene expression and cellular identity are influenced by changes in DNA methylation patterns during the formation of iPSCs102. Modification of histones through acetylation and methylation, which affect chromatin structure and gene regulation, also play significant roles in reprogramming. This new understanding of epigenetic pathways helps clarify the complex processes involved in pluripotency induction and cellular reprogramming.
These advancements have contributed to improvements in iPSC-based methods for pharmaceutical innovation, disease modeling, and customized regenerative medicine62. Another significant stride in stem-cell research pertains to the tissue regeneration field103.
Transplantation of stem cells has great potential as a medicine applied to numerous illnesses. In neurology clinical trials, scientists are presently investigating stem cell therapy’s feasibility for the purpose of alleviating neurological disorders, such as Alzheimer’s and Parkinson’s104. Additionally, investigations are being conducted on stem-cell therapy for cardiovascular illnesses, orthopedic conditions, hematological conditions, and diabetes. The adaptability of stem cells, coupled with advancements in delivery techniques, positions them as potential game-changers in regenerative medicine105. Emerging applications include the use of stem cells in immunotherapy, where they are engineered to target and treat certain cancers106. Furthermore, continuous investigations have investigated the possibility of using stem cells to regulate the immune system in disorders like autoimmune illnesses107. As these clinical applications progress from research to practice, the landscape of healthcare is poised to undergo significant transformation.
Neural stem-cell transplants have been administered to patients with PD in a clinical trial. In addition to improving motor system function, the data demonstrated a slowing of the disease’s progression and suggested the prospects of stem cells for neurological regeneration108,109. Individuals with heart failure participated in a cardiac stem-cell clinical trial. The outcomes showed less scar tissue, increased angiogenesis, and improved heart function, indicating the effectiveness of stem-cell treatment in promoting the regrowth of cardiac tissue110,111. Additionally, bone marrow-derived MSCs (BM-MSCs) have been utilized in a clinical study of osteoarthritis. Patients experience decreased pain, improved joint function, and evidence of cartilage regeneration, demonstrating the therapeutic prospects of stem cells in orthopedic applications112–114.
Treating leukemia with HSC transplantation (HSCT) has proven beneficial. Patients undergoing this procedure achieve complete remission and hematopoietic system reconstitution, leading to prolonged survival and improved quality of life46,115. Furthermore, clinical trials utilizing iPSCs to generate pancreatic progenitor cells have demonstrated promise for the treatment of diabetes. Patients exhibit restored insulin production and improved glycemic control, suggesting a regenerative approach to diabetes management116,117 (Table 1) (Fig. 3).
Table 1.
The advancement in stem-cell therapies in various diseases.
| Conditions | Stem-cell type | Models Human/Animal | Application and study design | Main outcomes | Ref. |
|---|---|---|---|---|---|
| Cell differentiation, reprogramming, and regeneration | Wharton’s jelly-MSCs (WJMSCs) | NA | Investigate the effect of nanostructures on WJMSCs that are undergoing motor neuron lineage differentiation when combined with sonic hedgehog and retinoic acid | WJMSCs are a desirable source of stem cells for producing and restoration of motor neurons | 118 |
| iPSCs | Mice | Create modified human EVs that can initiate reprogramming-based vasculogenic therapies without relying on viral vectors or progenitor cells | Reprogramming was utilized to create induced endothelial cells (iECs) from iPSCs | 119 | |
| hUCMSCs | Rats | Developed biocompatible nanoparticles made of layered double hydroxide and optimized the elemental compositions of ions to improve the process of chondrogenic differentiation in hUCMSCs | New perspectives on treating intervertebral degeneration IDD | 120 | |
| Alzheimer disease | Corpus cerebrospinal fluid produced from iPSC (CNSC-SE) | Transgenic (5×FAD) mice | Administered 5 μg/g CNSC-SE produced from iPSC throughout 12 weeks | The human iPSC-derived CNSC-SE is utilized for neurogenesis and dendritic morphogenesis | 121 |
| Neural stem/progenitor cell (NSPC) | Rats and mice | Mice and rats treated with NSPCs | NSPC therapy may enhance cognitive performance and delay the onset of AD | 122 | |
| Parkinson disease | ADMSCs | Rats | The subjects were categorized into four distinct categories: control, sham, treatment cell, and lesion. The treatment cell group injected intravenous injection of adipose-derived MSCs (ADMSCs) | ADMSs can treat Parkinson’s could enhance the density of neurons that express TH protein | 123 |
| BM-MSCs | Mice | In vivo by utilizing a neurotoxin-induced model 6-hydroxydopamine (6-OHDA) exposure and assessed the impact of BM-MSC secretome in living organisms by comparing the effects of secretome administration through two different routes | The capacity of BM-MSCs’ secretome to inhibit dopaminergic neuronal death | 124 | |
| Cardiovascular diseases, | iPSC-derived cardiomyocyte (iPSC-CM) | Animal model | The injected cell dosages ranged from 2×105 to 4×108. The duration of the follow-up period varied between 1 and 12 weeks | The iPSC-CM therapy is a secure and helpful technique to improve cardiac function in individuals with infarction | 125 |
| hADSC | Rats | hADSCs were injected in various doses (between 2×105 to 4×108), and patients were followed up for 1-10 weeks | The hADSC is able to improve cardiac function, less ventricular remodeling, reduce fibrosis, and increase angiogenesis | 126 | |
| Orthopedic conditions (knee osteoarthritis) | Autologous-ADMSC | Human | Thirty cases were categorized into three distinct categories: two categories were treated with either a single injection of ADMSCs (100×106) or two injections of ADMSCs ((100×106) at the start and 6 months later), and the 3rd category was the control | ADMSC therapy is a safe and successful treatment that may also stop disease development | 127 |
| Hematological disorders | Allogeneic stem-cell transplantation (allo-SCT) | Human | One hundred four cases were given allo-SCT | Allo-SCT showed significant implications for patients | 128 |
| HLA haploidentical- HSCT (haploid-HSCT) | Human | One hundred three patients have severe aplastic anemia; patients received HLA-HSCs together with allogenic MSCs after a preparatory treatment regimen consisting of busulfan, fludarabine, cyclophosphamide, and antithymocyte globulin | Safe and effective haplo-HSCT could help children and adolescents with severe aplastic anemia | 129 | |
| Diabetes | UC-MSCs | Human | Seventy-three patients were allocated at random to either receive intravenous injections of UC-MSCs. The infusion was administered three times, with 4 weeks for each administration. Patients were monitored for 48 weeks | UC-MSCs are a useful strategy for improving the time to onset of type Ⅱ diabetes | 130 |
| Cancer | MSC-exosomes | NA | Four distinct cell lines were utilized, namely ACHN, LNCaP, 5637, and PC3, which are well-established models for prostate tumors that are sensitive to hormones that affect the kidneys and bladder. The cell lines were subjected to several doses of exosomes produced from MSCs | MSC-exosomes have anticancer effects | 131 |
| MSCs | Mice | The M/LPV/O2 is used in both laboratory and animal studies, which is a liposomal formulation of an oxygen-loading perfluorocarbon and a sonosensitizer verteporfin coated with a membrane of MSCs | MSCs are able to be a therapeutic approach to treat oral squamous cell carcinoma | 132 | |
| Autoimmune diseases | MSCs | Mice | The positive group was treated with lupus, and the negative group was normal mice, Naïve MSCs (N-MSCs), Lactobacillus strains, delbrueckii (D-MSCs) or rhamnosus (R-MSCs) were coincubated for 48 h, then intravenously injected in separate groups | Combining MSCs with Lactobacillus strains may help alleviate symptoms resembling lupus | 133 |
| MSCs | Rat | Extracted (MSCs) from rats, then they produced gas vesicles and incubated them with MSCs to achieve intracellular labeling of the MSCs, then tested in vivo and in vitro | MSCs are an innovative threptic method for treating Rheumatoid arthritis | 134 | |
| COVID-19 | hUCMSCs | Human | Patients and control groups received hUCMSC therapy, a three-month follow-up study | hUCMSCs were suggested to be an initial success and relative safety for individuals suffering from COVID-19 | 135 |
| hUCMSCs | Human | The study consists of 40 patients; 20 patients were administered an intravenous infusion of UC-MSCs at a dosage of 1×106/kg body weight, diluted in a 100 ml saline solution (SS) (0.9%). Another set of 20 patients got a 100 ml SS (0.9%) as a control | Intravenous hUCMSCs are used as adjuvant therapy for individuals suffering from COVID-19 | 136 |
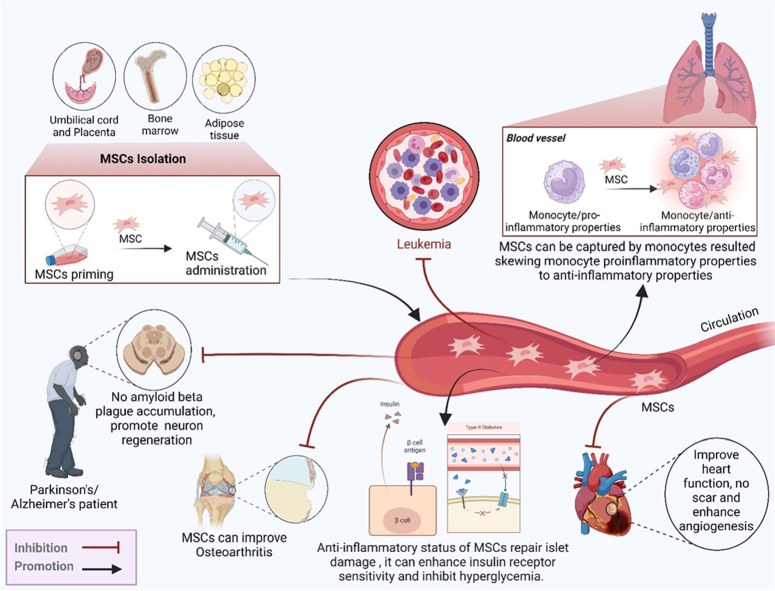
Figure 3.
MSC sources, such as bone marrow, adipose tissue, and placenta, and their role in the therapy of different diseases. MSCs improve and combat diseases including pneumonia, leukemia, neuron diseases, osteoarthritis hear diseases, and the two types of diabetes. MSCs have immunoregulator and anti-inflammatory properties.
In combating the COVID-19 pandemic, universal vaccination remains the primary strategy; however, uncertainties persist regarding the duration of vaccine protection and the inability of any vaccine to provide absolute immunity137. Stem-cell therapy has arisen as a potential substitute, building on successes observed in severe H7N9 avian influenza138,139. Stem cells, particularly those derived from human umbilical cord stem cells (hUCMSCs), are effective and safe for treating severe COVID-19, demonstrating their potential in over 100 international clinical trials140. Allogeneic MSCs, notably hUCMSCs, contribute to anti-inflammatory responses, tissue repair, and the modulation of immune functions, showcasing their therapeutic promise141. Challenges include difficulties in recruitment due to the evolving clinical landscape, lack of preclinical data, and variations in stem-cell properties. Despite these hurdles, stem-cell therapy, especially considering advancements in organoid technology for better modeling of viral effects, has significant clinical potential142. Despite current limitations and technological challenges, the continuous advancement of stem-cell treatment offers optimism in the fight to preserve lives and improve treatment results for individuals with severe COVID-19 infection (Fig. 4).
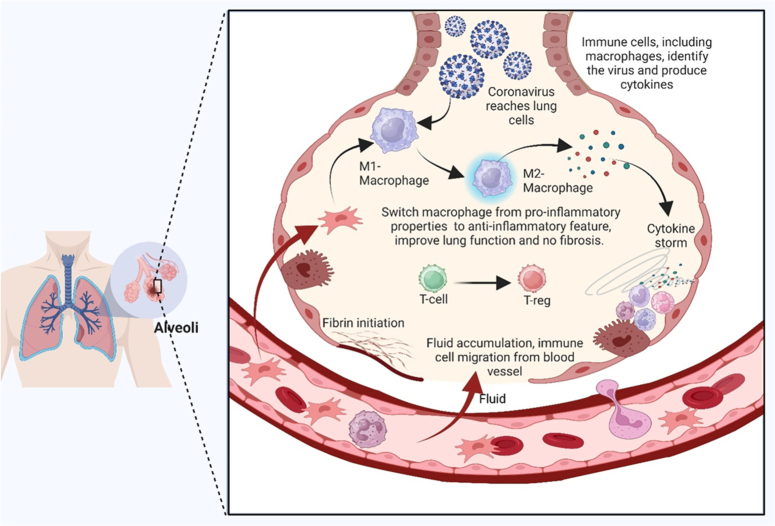
Figure 4.
Potential and mechanism of action of mesenchymal stem cell treatment for COVID-19 pneumonia using MSCs, which have immunoregulatory characteristics, can help control the cytokine storm and COVID-19 lung injury. Mesenchymal stromal cells (MSCs) play an important role in a number of processes, including preventing neutrophil infiltration and transforming hyperactivated T cells into regulatory T cells (Tregs). They also promote the production of anti-inflammatory cytokines, such as prostaglandin E2 (PGE2), transforming growth factor beta (TGF), indoleamine 2,3-dioxygenase (IDO), and interleukin 10 (IL-10). Nevertheless, MSCs play a crucial function by stimulating the synthesis of growth factors by endothelial and epithelial cells, which in turn inhibits fibrosis and boosts the infusion of alveolar fluid.
Stem-cell therapy in specific medical fields
Regenerative medicine with stem cells has investigated significant capacity across diverse medical specialties, offering innovative solutions for previously challenging conditions143. Patients’ stem cells are harvested for autologous stem-cell treatment. Autologous stem cells that have been cultured are cultivated in the lab before transplantation. These cells have the potential to be categorized into modified and unmodified expanded autologous stem cells. Allogeneic stem cells are classified similarly to autologous stem cells, but they come from healthy donors18. Autologous stem cells can be readily acquired and do not cause immunological rejection after infusion. Allogeneic stem cells provide multiple benefits, including the ability to select a donor, availability from different sources, minimal likelihood of causing an immune response, and the convenience of being readily available. Allogeneic MSCs are also immunogenic, indicating that they can trigger an immunological response. These cells can generate a memory response in the immune system under specific circumstances144–146.
Regenerative medicine can restore, repair, or regenerate impaired tissues or organs by harnessing the unique characteristics of stem cells147. This topic includes a range of approaches that seek to leverage the extraordinary capacity of stem cells for medical applications. Although stem cells possess the capacity to undergo self-renewal and differentiate into various distinct cell types, they hold great promise as therapeutic agents against various illnesses and wounds148. Regenerative medicine aims to create novel methods to repair damaged tissues caused by disease, injury, or aging using stem cells to restore normal function and structure to damaged organs or tissues149. These therapies have great potential to revolutionize medical treatments, particularly in areas where conventional medicine falls short of providing effective remedies or cures150.
This emerging field presents a promising avenue for personalized cancer treatments, as researchers have delved into harnessing the unique attributes of stem cells to create innovative strategies for cancer management and potential cures. These investigations signify a significant paradigm shift in oncology, offering a progressive outlook for tailored therapies and potential breakthroughs in cancer treatment151,152. Stem cell-based cancer treatments are becoming increasingly promising. Because stem cells can locate and target primary and metastatic tumors, and serve as innovative delivery approaches. In preclinical animal models, stem cells modified to express different cytotoxic chemicals consistently reduced tumor size and increased survival153,154. They have also been used to reduce side effects and improve primary medicinal efficacy by acting as carriers of viruses and nanoparticles. Additionally, stem cells have the potential for utilization in immunotherapy, anticancer drug screening, regenerative medicine, and cancer stem cell-targeted therapy for diverse forms of malignancies, including lung cancer, breast cancer, and osteosarcoma155.
Regenerative strategies in orthopedics include advanced osteonecrosis of the hip joint, intervertebral hernias, osteoporosis, targeted joint injuries, cartilage restoration, and bone healing through stem-cell and tissue-engineering methodologies156,157. Recent investigations have shown innovative approaches, like MSC therapy, platelet-rich plasma (PRP) injections, and biocompatible scaffolds infused with growth factors158. These methods aim to optimize cartilage repair and bone regeneration, offering promising outcomes under musculoskeletal conditions159,160. Research has focused on refining MSC isolation techniques, deciphering the crucial signaling pathways involved in tissue regeneration, and developing bioactive materials that enhance healing161.
In the cardiology field, innovative approaches, including stem-cell therapy and bioengineered cardiac patches, are being explored to mend and regenerate impaired heart tissues after cardiac events such as myocardial infarctions162. Current research has been focused on different stem-cell types, including iPSCs and cardiac progenitor cells, to regenerate impaired heart muscles and restore cardiac function. Furthermore, research has focused on creating bioengineered cardiac patches using cell-based structures and biomaterials that resemble genuine heart tissue163.
In the field of neurology, ongoing investigations have delved into the domain of medicines based on stem cells developed to fight diseases affecting the nervous system, including Parkinson’s and Alzheimer’s164,165. Studies have focused on using stem cell-derived neurons to replace and regenerate impaired nerve cells166. Recent studies have shown that there are numerous varieties of stem cells, including neural stem cells and iPSCs, with the aim of producing functional neurons capable of integrating into damaged neural networks167,168.
Regenerative medicine in dermatology represents a dynamic frontier of research, particularly concerning stem-cell applications in the skin169. Stem cells residing in the skin tissues offer promising avenues for innovative therapeutic strategies that target various dermatological conditions and injuries170. Their remarkable regenerative potential holds immense promise for advancing wound healing, addressing burns, and managing skin disorders such as psoriasis and vitiligo171,172. Additionally, stem cell use in cosmetic dermatology for antiaging treatments and improving skin quality underscores their diverse clinical utility173. Researchers have actively explored methods to harness the inherent regenerative abilities of stem cells with the aim of developing tailored and effective therapies for combating skin-related diseases and facilitating cosmetic enhancements. This transformative approach involves tissue engineering techniques utilizing stem cells, biomaterials, and growth factors to create skin substitutes that promote tissue regeneration and repair174–177.
Due to their potential function, an enormous amount of curiosity about stem cells has persisted in rejuvenating the retina and addressing corneal damage, particularly in diseases such as macular degeneration178. Noteworthy studies featured in journals such as ‘Investigative Ophthalmology and Visual Science’ and ‘British Journal of Ophthalmology’ delve into the strides made in utilizing stem cells for ocular regeneration179,180. Studies have employed stem-cell therapies to restore retinal cells and heal corneal injuries, presenting encouraging pathways for managing vision-related ailments181. These studies signify a burgeoning field of ophthalmology research, offering promising prospects for innovative treatments aimed at addressing ocular disorders and enhancing vision182.
Stem-cell utilization in oncology, regenerative medicine, and disease therapeutics is an expanding field of research and innovation151. Research has focused on leveraging stem cells for targeted cancer therapies and exploring their potential for cellular reprogramming and immune cell modulation to combat tumors183. The immunomodulatory potential of stem cells presents a compelling avenue in biomedical research, particularly in addressing autoimmune disorders and graft-versus-host disease (GVHD) and improving transplantation outcomes184. Stem cells show a remarkable ability to influence immune cell behavior and function, offering promising prospects for novel therapeutic interventions185. This intersection of immunology and stem-cell biology promises not only innovative treatments but also deeper insights into the complex mechanisms governing immune system regulation and dysregulation. This rapidly expanding field has an enormous potential to improve our knowledge of immune-related disorders and provide efficient treatment plans186.
Stem-cell utilization in hematology is a dynamic area of scientific inquiry and clinical application in the regenerative medicine field and therapeutic interventions for diseases187. Leveraging the potential of stem cells to regenerate is the main goal of research, particularly in HSCs, for transplanting bone marrow and exploring its role in immune cell therapies to combat various blood-related ailments188. This growing field represents a promising avenue for innovative treatments, emphasizing the pivotal role of stem cells in revolutionizing hematology by offering potential cures and personalized therapeutic solutions for blood disorders, thereby marking a transformative shift in disease management189,190.
Stem-cell research offers the potential for addressing illnesses such as inflammatory bowel disease (IBD)191,192 and managing various gastrointestinal disorders193. Researchers are investigating stem cell-based approaches to repair gastrointestinal tract injuries, manage ulcers, and alleviate the symptoms of chronic conditions like ulcerative colitis and Crohn’s disease194,195. Despite ongoing investigations, the clinical application of stem-cell therapies in gastroenterology remains the subject of clinical trials and extensive research, emphasizing the need for further exploration and understanding of their efficacy and safety in treating many immunopathological diseases (Fig. 5)196.
Figure 5.
MSCs inhibit many immunopathological disease conditions, including skin infection, inflammatory bowel disease, and endocrine hormone disorders; they also suppress tumor cells, the aging process, and reproductive infertility.
Ongoing investigations explore the potential of stem cells in restoring lung tissue damaged by diseases like serious respiratory disease or chronic obstructive pulmonary disease (COPD)197. Researchers have investigated the capacity of stem cells to restore impaired lung tissue, alleviate COPD symptoms, and target conditions such as idiopathic pulmonary fibrosis198. Despite extensive research, the use of stem-cell therapies in pulmonology requires further examination to establish their safety, effectiveness, and long-term effects on respiratory illnesses199. Although this emerging field shows promise for future treatment, it requires thorough comprehension and robust clinical validation200.
Stem-cell research in reproductive medicine opens new avenues for treating infertility and addressing various reproductive system disorders201. Stem cells, whether derived from embryonic, adult, or induced pluripotent sources, hold promise for regenerating and repairing damaged reproductive tissues202. This area of study covers various aspects of reproductive health, including the restoration of ovarian function, addressing endometrial issues, and potentially aiding fertility preservation. Research endeavors detailed in publications such as the ‘Journal of Assisted Reproduction and Genetics’ and ‘Fertility and Sterility’, explore the potential of interventions utilizing stem cells to revolutionize infertility treatments and offer new hope to individuals facing reproductive health challenges. These advancements represent a burgeoning field that may reshape the landscape of reproductive medicine and provide innovative solutions for the treatment of infertility and related disorders201,203.
Stem-cell research in endocrinology presents a promising avenue for managing endocrine disorders such as diabetes by focusing on the generation of insulin-producing cells and regenerating pancreatic tissues204. Through various studies documented in journals like ‘Diabetes’ and ‘Endocrine Reviews’, researchers work to create functional beta cells or islet-like structures that can secrete insulin by utilizing the regeneration ability of stem cells205. This pioneering field aims to address deficiencies observed in traditional diabetes management by offering cell-based therapies that can potentially restore insulin production and regulate glucose levels206. The exploration of stem-cell therapies in endocrinology has heralded a new era of diabetes treatment, offering hope for more effective and sustainable management strategies for this chronic condition207.
In dentistry, cutting-edge research has focused on the innovative utilization of stem cells to regenerate crucial dental tissues, including tooth enamel, dentin, and dental pulp208. This revolutionary exploration seeks to redefine conventional approaches to dental care by offering transformative treatments for prevalent conditions such as cavities, gum diseases, and dental trauma209. Utilizing their unique regenerative stem-cell capacities, scientists aim to generate interventions that induce the natural regeneration and repair of diseased or impaired dental tissues, potentially revolutionizing the oral healthcare landscape210,211. This promising field of study in dentistry holds the potential to pave the way for novel therapeutic strategies that offer patients improved outcomes and enhanced oral health212.
In the domains of trauma and wound healing, intensive research efforts have focused on uncovering the regenerative processes of stem cells to address the complexities of chronic wounds, burns, and traumatic injuries213. Stem cells exhibit promising capabilities in fostering tissue regeneration and mitigating scarring by influencing cell differentiation and supporting repair mechanisms in damaged tissues214,215. This exploration of stem cell-based interventions aims to revolutionize conventional wound care approaches by fostering natural tissue regeneration, accelerating healing processes, and minimizing scarring, thereby offering renewed hope to patients with challenging wounds and traumatic injuries216. In the quest for more potent treatment approaches to enhance patient outcomes and accelerate recovery, the potential of stem cells in trauma and wound healing serves as a ray of hope217,218. Stem-cell regenerative medicine is a dynamic and expansive field, continuously expanding its applications across various medical disciplines to address a wide spectrum of health conditions and diseases219 (Table 2).
Table 2.
Stem-cell therapy is utilized in specific medical fields.
| Disease | Therapeutic agents | Models Human/Animal | Application | Outcomes | Ref. |
|---|---|---|---|---|---|
| Injury | Bone marrow- MSCs (BMSCs) | NA | In vivo, autophagy development and HaCaT cell migration in hypoxic BMSC-derived conditioned media were assessed by assessing autophagy-related protein expression | The BMSC-based therapy can be a highly efficient treatment method for diabetic wounds | 220 |
| Aging | ADSC-containing medium (ADSC-CM) | Human | In vivo, in 25 cases with wrinkles and aging face, Moisturizers containing ADSC-CM, for three weeks, on certain facial areas, with or without 2% niacinamide | The ADSC-CM, after laser treatment, when combined with niacinamide, has antiaging effects on the skin | 221 |
| Breast cancer | hUCMSCs | Mouse | In vitro/ In vivo, the suppressive impact of hUCMSCs on CSCs was evaluated by the application of the colony formation test on soft agar and the cell proliferation assay on the Cell Counting Kit-8 | The proliferation of breast cancer stem cells (CSCs) was considerably repressed by hUCMSC, which also induced tumor cell death and reduced the activity of the protein kinases PI3K and AKT | 222 |
| Lung cancer | MSCs-derived EVs | Human/mice | In vivo, 65 NSCLC tissues were taken, an NSCLC cell line was employed, and hBMSCs were implanted in 6-well plates at a density of 1×105 cells per well. The effect of miRNA-598 on tumor growth and metastasis was tested in animals | Discovered that miR-598-containing EVs produced from MSCs suppress the migration and proliferation of NSCLC cells both in vivo and in vitro via targeting Thrombospondin-2 (THBS2) | 223 |
| Osteosarcoma | MSCs | In vivo/ ex vivo, Adenoviruses expressing the osteoprotegerin OPG gene were employed to modify MSCs. These modified MSCs, referred known as MSCs-OPG, were then administered to the tail vein of mice with osteosarcoma | The administration of MSC-osteoprotegerin treatment resulted in a decrease in tumor development and a suppression of bone degradation. The MSCs can transport osteoprotegerin to tumor locations | 224 | |
| Orthopedics | MSCs /PRP | Human | In vivo, 3 groups of 47 patients were randomly assigned to receive intra-articular injections: corticosteroid (n=17), autologous BM-derived MSCs (n=16), and autologous BM-derived MSCs plus PRP (n=14). The outcomes were evaluated by comparing the subjects’ baseline range of motion with the KOOS | The MSC is effective in decreasing and function improved of Knee osteoarthritis symptoms | 225 |
| Osteonecrosis | HUCMSCs | Rats | Next, HUCMSCs were given locally to the femoral heads of rats. Using histological staining, micro-CT, Luminex, and immunofluorescence staining, bone healing of the necrotic area in the femoral head was examined 4-weeks and 8 weeks following surgery to assess the therapeutic impact of HUCMSCs | HUCMSCs are able to survive and positively affect osteonecrosis of the femoral head (ONFH) patients, which could provide an option to treat those patients | 157 |
| Intervertebral hernias IVD | MSCs | Human | In this work, MSCs were extracted from disk samples that were clinically classified as H-IVD and D-IVD. With an immunophenotypic profile resembling that of MSCs, H-IVD-MSCs, and D-IVD-MSCs demonstrated multipotent mesenchymal differentiation potential while exhibiting positive for chondrogenic, osteogenic, and adipogenicity markers | The expression of osteopontin takes place in IVD-MSCs and is essential to the pathophysiological mechanism underlying human disk degeneration | 226 |
| Osteoporosis | ASCs | Mice | The radiographic and histological investigation was conducted four weeks after the in vitro implantation of the ASC-collagen I hydrogel composite into mice. *ASCs isolated from individuals with osteoporosis | Provide sufficient osteogenic activity and present novel opportunities for bone tissue engineering associated with osteoporosis cases | 227 |
| Cardiac patches | MSCs | Rats | Implantation of the MSC into the infarct area | The creation of artificial tissue using MSC and decellularized umbilical artery | 228 |
| Neurodegenerative disease | Neural stem cells (NSCs) | Mouse | NSCs were injected into mice APP/PS1 transgenic to analyze the cognitive function and to measure GFAP, Iba-1, and TLR4 activation | NSCs reduced cognitive deficits in mice and reduced inflammatory injury | 229 |
| Psoriasis | hE-MSCs | Mouse | In vivo, evaluate hEMSCs therapeutic potential in skin disease immune-mediated inflammation | The potential of hEMSCs to modulate the immune system in psoriasis | 230 |
| Vitiligo | MSCs | Human | Cocultured melanocytes with MSCs and tested the capacity of MSCs to inhibit the AKT/ PI3K/ PTEN pathway in melanocytes | MSCs could be a promising treatment for vitiligo | 231 |
| Ocular disorders | Healthy donors’ ASCs-derived MSCs | Human | Seven individuals’ lacrimal glands were injected with ASCs in one eye to test safety and objectively improve dry eye symptoms | ASCs are employed as a therapeutic alternative with anti-inflammatory properties to treat dry eye conditions | 232 |
| GVHD | MSCs | Human | Sixty-two patients divided into 53 adults and 9 children, MSC therapy was intravenously injected in 4 separate doses of 1×106 cells/kg | MSCs are a safe and effective therapeutic alternative for treating refractory GVHD | 233 |
| Blood disorders | HSCs | Mice | Investigated the potential of HSCs derived from β654-ER mice to treat β-thalassemia through repeated HSC transplantation | HSCs managed to enhance the mice’s survival rate | 234 |
| Crohn’s disease | MSCs | Human | In ex vivo, 19 patients were divided into 15 cases and 4 control, using a 22G needle, 75 million mesenchymal stem cells were injected following curettage and primary fistula tract closure on days 0 and 3 | BMSCs provide a safe alternative therapy for Crohn’s disease | 235 |
| COPD | HUC-MSCs | Rat | The mice were divided into 3 groups: COPD + UC-MSCs, COPD + vehicle, and Control group. The assessment of lung function alterations following UC-MSCs therapy was conducted on a weekly basis for 6 weeks | UCMSCs could improve lung function and decrease inflammatory response | 236 |
| Infertility | hUCMSCs | Mice | Twenty rats were categorized into 10 treatment groups and 10 control groups; mice were treated with hUCMSCs; following a 4-week treatment period, the 5 mice were selected randomly to study the organ morphology and pathology, proliferation, inflammatory cytokines, and apoptosis, specifically in the fallopian tube | The anti-inflammatory and antiapoptotic properties of hUCMSCs were leveraged to enhance fertility | 237 |
| MSCs | Human | Ten cases suffered from premature ovarian failure and MSCs were injected into the ovaries using laparoscopic techniques. The endometrial fractional biopsy was subjected to histological and immunohistochemical staining and evaluation | MSCs are effective in treating premature ovarian failure | 238 | |
| Diabetes Mellitus | hUCMSCs | Mouse | The mice were categorized into four groups: the normal control, the type II diabetes mellitus group, the hUCMSC treatment alone (UCMSC) group, and the hUCMSCs pretreated with melatonin (UCMSC/Mel) group | hUCMSCs helped to reduce insulin resistance and poor glycemic control | 239 |
| Dentistry | Dental pulp stem cell-derived exosomes (DPSC-EXO) | Rat | In vivo, PDLSCs were subjected to DPSC-EXO treatment in a laboratory setting. The researchers assessed the cells’ capacity for cell proliferation, metastasis, and osteogenic potential | DPSC-EXO is a potentially effective treatment for periodontitis | 240 |
| Traumatic Injuries | Human chorionic membrane -MSCs (hCM-MSCs) | Rat | Adult rats treated with hCM-MSCs to investigate the effect of hCM-MSCs on traumatic brain injury | hCM-MSCs greatly reduced neurological impairments and increased neurogenesis and angiogenesis | 241 |
In addition, various types of stimulation have been utilized during stem-cell therapy to enhance differentiation proliferation and improve healing, such as shock wave stimulation242. MSCs are increasingly being acknowledged as valuable resources for various orthopedic applications, and radial shock waves have been shown to substantially enhance the development and regrowth of MSCs in a laboratory setting. Furthermore, this type of stimulation safely accelerates cartilage repair in living organisms, suggesting positive results for clinical applications243. IR is a type of high-energy radiation that has enough energy to dislodge firmly bound electrons from atoms, leading to the creation of ions. In addition to being a carcinogen, IR is also used as a therapeutic option for patients with cancer. However, there is increasing data showing that extranuclear components, such as mitochondria, play a significant role in the cellular response to IR, and the mitochondrial function of MSCs was observed to be considerably increased after 4 h of exposure to ionizing radiation, as determined by measuring mitochondrial oxygen consumption244. Cell proliferation has been induced in many in vitro trials using a modest amount of laser therapy. Osteoblasts, lymphocytes, keratinocytes, and fibroblasts exhibit enhanced proliferation when exposed to laser irradiation245. Other types of stimulation include electrical stimulation to enhance stem-cell therapy in nerve regeneration242, electrical stimulation to promote cell differentiation and proliferation of fatal neuronal stem cells into neuronal stem cells246, and nonpeptide small molecules247, in addition to mechanical stimuli such as cyclic stretch, three forces, laminar shear stress, cyclic pressure248, and gamma radiation249.
Biotechnological advancements in stem-cell research
Stem-cell studies have been significantly promoted by cutting-edge technologies that have revolutionized our understanding and utilization of these versatile cells. This discussion focuses on some of the most impactful biotechnological advancements in stem-cell studies, with a specific focus on exosome-based therapeutics, scRNA-Seq, and the revolutionary CRISPR-Cas9 gene-editing technology250–252.
Exosome-based therapeutics and stem cells
The new frontier of exosomes produced from stem cell-based therapeutics represents a promising avenue for the field of regenerative medicine253. RNAs, signaling molecules, and proteins are bioactive substances encapsulated in exosomes and small vessels secreted by stem cells. These nanovesicles are essential for intercellular interactions and can control a number of cellular functions254. Stem cell-derived exosomes exhibit unique properties that modulate immune responses, promote tissue regeneration, and foster repair mechanisms255. Harnessing the therapeutic potential of these exosomes holds considerable promise for developing innovative treatments for diverse medical conditions, including inflammatory disorders, neurodegenerative diseases, and tissue injuries253,256–258. Stem cell-derived exosome-based therapies represent a burgeoning frontier in regenerative medicine, providing new opportunities for targeted, minimally invasive therapeutic interventions259.
Single-cell RNA sequencing and stem-cell research
Advances in scRNA-seq have allowed investigators to examine stem-cell transcriptomes individually, providing unprecedented insights into cellular heterogeneity and gene expression patterns13. This technology has played an essential role in comprehending the dynamics of stem-cell populations during differentiation and disease progression260,261.
CRISPR-Cas9 technology and gene editing in stem cells
With the advent of CRISPR-Cas9, a new era in gene editing has begun, which enables the precise modifications of stem-cell DNA14. Researchers can now edit or introduce specific genes with unprecedented accuracy, facilitating cancer and disease modeling, studying gene function, and developing potential therapeutic interventions262,263.
CRISPR-based technologies have enabled large-scale functional genomic studies and high-throughput screening of stem cells. That allows researchers to systematically interrogate gene function on a genome-wide scale, uncovering novel regulators of stem-cell fate, pluripotency, and differentiation264,265.
Beyond traditional CRISPR-Cas9, recent innovations, such as base editing and prime editing, offer enhanced precision in gene editing266. These techniques allow the modification of specific nucleotides without causing double-strand breaks, minimizing off-target effects and expanding the possibilities for therapeutic genome editing in stem cells267 (Fig. 6).
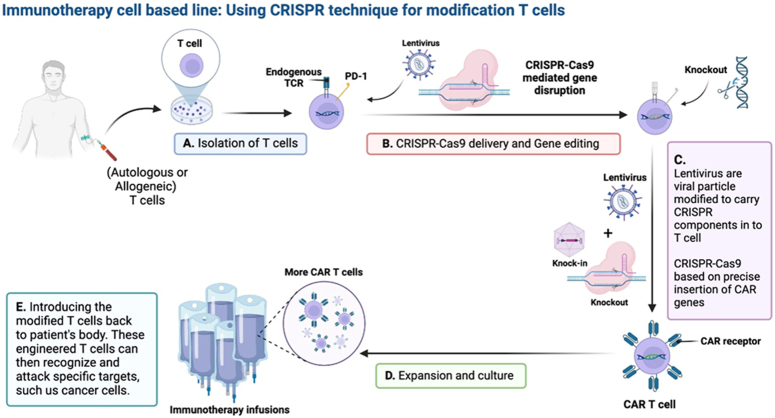
Figure 6.
Immunotherapy chimeric antigen receptor (CAR) T-cell therapy can be filled with the help of recent developments in genome editing using CRISPR-Cas9. To enable robust, accurate, and controllable genetic alteration, genome editing techniques are used, such as base and prime editing. In both hematopoietic and non-hematopoietic cancers, T-cells can be circumvented through CRISPR-Cas9-induced multiplex deletion of inhibitory molecules, which enhances CAR T-cell growth and persistence. The use of targeted knock-in techniques during CAR T-cell engineering offers the possibility of producing highly effective and potent cell products. Lentivirus is viral particles modified to carry CRISPR components in T cells, CRISPR-Cas9 based on the precise insertion of CAR genes, more and strong CAR T-cells product engineered using CRISPR-Cas9 to overcome specific histocompatibility hurdles and with improved persistence/antitumor function could greatly improve the production of cellular immunotherapies and the therapeutic durability.
Overall, CRISPR-based gene editing shows great promise for therapeutic applications in stem cell-based regenerative medicine. This opens new avenues for correcting genetic mutations underlying various diseases, generating genetically modified cells for transplantation, and developing personalized cell therapies.
Personalized medicine and stem cells
Stem cells are integral to the advancement of personalized medicine, aligned with the goal of tailoring healthcare to individual characteristics and encompassing genetic, environmental, and lifestyle factors16. From a patient’s cells, iPSCs provide a potent platform for building disease models that accurately reflect the person’s genetic background268. This capability facilitates in-depth studies of disease mechanisms at the cellular and molecular levels, enabling more precise diagnosis and the establishment of targeted therapeutic strategies60. Moreover, modern gene-editing techniques, including CRISPR-Cas9, enable accurate alterations in stem-cell genomes269.
This breakthrough allowed the correction of genetic mutations associated with diseases, laying the groundwork for personalized therapies addressing specific genetic alterations in individual cells270. In pharmacogenomics, stem cells significantly contribute to the assessment of individual drug responses. Leveraging patient-derived stem cells in pharmacogenomic studies enables researchers to understand the impact of an individual’s genetic composition on their reaction to various medications271. This knowledge serves as a guide for formulating personalized treatment plans, minimizing adverse reactions, and enhancing the overall therapeutic outcomes. Moreover, stem cells actively contribute to the identification of personalized biomarkers associated with specific diseases272. Differentiating patient-derived stem cells into cell types relevant to the disease makes it easier to identify molecular signatures that can be used as diagnostic indicators. These personalized biomarkers substantially improve the accuracy of disease detection and monitoring, marking a significant step toward more individualized and effective healthcare strategies273,274.
Stem-cell clinical trials
Stem-cell therapy is witnessing a surge in clinical trials, reflecting a growing interest in translating laboratory findings into viable treatments275. Clinical trials involving various stem-cell types are currently underway and include a wide range of health issues276. The goal of ongoing trials is to determine whether stem-cell therapies are effective in alleviating symptoms of neurological diseases such as Alzheimer’s, Parkinson’s, and spinal cord injuries277 (Table 3). Researchers are investigating how stem cells might be able to repair damaged neurons, encourage brain regeneration, and lessen the symptoms of these crippling conditions288.
Table 3.
Examples of clinical trials with results involved in neurological diseases, cancer, cardiovascular, and Orthopedics, from http://clinicaltrials.gov/.
| Conditions | Registration | Sample size | Study design | Treatment | Outcomes | Ref. |
|---|---|---|---|---|---|---|
| Alzheimer’s Disease | NCT01297218/NCT01696591 | Nine cases divided into: three cases treated with low dose and six treated with high dose | Phase 1 | hUCB-MSCs | Injecting hUCB-MSCs into the hippocampus and precuneus using stereotactic was safe, feasible, and well tolerated | 278 |
| NCT02054208/ NCT03172117 | Nine patients were divided into three who received a low dose, and six received a high dose of hUCB-MSCs | Phase 1/ 2 | hUCB-MSCs | It was conceivable, comparatively, and adequately safe to administer hUCB-MSCs three times into the lateral ventricle using an Ommaya reservoir | 279 | |
| Parkinson’s disease | NCT03550183 | — | Phase 1 | (UCMSC) | Ensuring safety and effectiveness study of Parkinson’s disease patients via transplant of hUCB-MSCs | 280 |
| NCT03309514 | — | Phase 1/2 | Autologous Hope Biosciences-ADMSCs | Used to investigate the safety and effectiveness of transplanting adult neural stem cells derived from the central nervous system in specific individuals with Parkinson’s disease | ||
| Spinal cord injury | — | Sixteen patients were classified into a group injected with a low dose and another group with a high dose | Phase 3 | Autologous MSCs | Applying a single MSC to the intramedullary and intradural region is considered safe, but its therapeutic impact is significantly weaker when compared to the injection of several MSCs | 281 |
| — | Twelve individuals with chronic and complete paraplegia | Phase 1/2 | Autologous-BMSCs | Administration of personalized MSC therapy is a safe approach that results in evident enhancements in clinical parameters and the overall well-being of individuals suffering from total and long-standing paraplegia | 282 | |
| Cardiovascular | NCT01739777 | Thirty cases divided into 15 individuals who received an injection and another 15-control group | Phase 1/2 | UCMSC | safety and effectiveness of using uCMSC in individuals diagnosed with heart failure | 283 |
| — | Forty patients | Phase 2 | Autologous CD133+ stem cells | Providing evidence that isolating and delivering autologous CD133+ cells on the same day as coronary artery bypass grafting is both safe and achievable | 284 | |
| Cancer | NCT01579812 | Thirty-eight patients were divided into 1 at the IIC stage, 25 at III stage, and 12 at the IV stage | Phase 2 | Carcinoma-associated mesenchymal stem cells (CA-MSCs) | Antitumor effect on ovarian cancer | 285 |
| NCT03087409 | Eight hundred eighty-five cases | Phase 3 | High-dose chemotherapy with hematopoietic stem cell | Cumulative survival and safety to heal breast cancer | 286 | |
| Orthopedics and musculoskeletal disorders | ACTRN12614000814673 | Thirty participants were classified into three groups: two treatment groups were administered intra-articular ADMSC therapy, either with a single injection or two injections, and the third group was control | NA | Autologous-ADMSC | Effective and safe therapy for knee osteoarthritis | 127 |
| — | Seven participants, four were received a low dose, and another three participants received a high dose | Phase 1/2 | hUCB-MSCs | hUCB-MSCs seemed to be Safe and effective | 287 |
Clinical trials in cardiovascular medicine aim to evaluate the use of stem cells, such as progenitor cells and MSCs, for treating conditions like heart failure and ischemic heart disease. These trials explored the regenerative potential of stem cells in repairing impaired cardiac tissues and improving overall cardiac function289.
Research is now being conducted on stem cell-based therapeutics for cancer treatment, including studies focusing on HSC transplantation (SCT) to treat hematological malignancies290. In addition, researchers have explored potential applications for stem cells in conjunction with traditional cancer therapies in order to enhance therapeutic results and minimize negative consequences291. Additionally, clinical trials in orthopedics and musculoskeletal disorders involve the use of stem cells to treat conditions like osteoarthritis and bone defects. MSCs, which are known for their capacity to differentiate into bone and cartilage, are being studied for their regenerative potential in restoring joint and bone health292. Furthermore, stem-cell therapies are now under investigation for their potential applications to treat diabetes by replenishing pancreatic beta cells. Clinical trials have investigated the use of stem cell-derived insulin-producing cells as transplants to regulate blood glucose levels in patients with diabetes293.
Challenges and ethical considerations
Stem-cell therapy, although showing great promise, faces multiple obstacles and constraints that need to be carefully considered. One prominent challenge is the potential for tumorigenesis, wherein the number of transplanted stem cells may increase uncontrollably, leading to tumor formation294,295. The security of stem cells can only be ensured by thorough preclinical examinations before they can be used in clinical settings. Additionally, the immune response poses a challenge due to the recipient’s immune system perceiving the transplanted cells as alien, leading to rejection296,297. The development of strategies to mitigate immune rejection and improve engraftment remains an ongoing challenge.
Furthermore, precisely controlling stem cell development into the desired cell types is a significant challenge298. The variability in differentiation protocols and the possibility of off-target consequences raise concerns regarding the reliability and safety of the therapeutic outcomes. Additionally, scalability and cost-effectiveness in the production of sufficient quantities of quality-controlled stem cells for widespread clinical use remain logistic obstacles that must be overcome for the field to attain its full potential110,299,300.
Ethical considerations are central to the discourse surrounding stem-cell therapy, particularly the use of ESCs301. Discussions over the moral standing of the early human embryo arose because of the killing of embryos during the extraction of ESCs. Because of these concerns, scientists are looking at alternative sources of pluripotent stem cells, such as iPSCs, which are reprogrammed from adult cells and do not have the same ethical concerns as ESCs. Regulatory frameworks are essential for negotiating the moral challenges presented by different stem cell therapies302.
Countries have varying regulations governing the clinical utilization of stem cells, ranging from permissive to restrictive. Achieving a balance between promoting innovation and ensuring patient safety remains a challenge for regulatory bodies303. The evolving nature of stem-cell research and therapies necessitates dynamic regulatory frameworks that can be adapted for scientific advancement. Ongoing debates persist in this field, particularly regarding the commercialization of stem-cell therapies. Issues of accessibility, affordability, and equitable distribution of these therapies raise ethical questions.
Moreover, concerns regarding the premature marketing of unproven stem-cell therapies and the need for transparent communication regarding the state of scientific evidence contribute to the ethical complexity of this field304. In conclusion, addressing the difficulties and ethical considerations of stem-cell therapy requires a multidisciplinary approach that encompasses rigorous scientific research, transparent communication, and dynamic regulatory frameworks. Realizing the full promise of stem-cell therapies will require a careful balance between ethical responsibility and innovation as the field develops.
Future prospects
With the help of new technologies and the results of continuing research, stem-cell treatment might potentially transform many different areas of medicine. One key direction involves the integration of stem-cell therapy into precision medicine approaches, opening a new chapter in medical history, where customized care based on a person’s genetic composition promises enhanced therapeutic outcomes and reduced side effects. Advances in genomics and the application of patient-specific stem cells are expected to drive this integration. Additionally, future research should focus on refining the immune modulation strategies associated with stem-cell therapies and addressing challenges such as immune rejection and graft-versus-host responses. Innovative approaches, including engineered stem cells and immunomodulatory molecules, aim to enhance compatibility with stem-cell treatment.
The continued evolution of gene-editing tools, including CRISPR-Cas9, will perform a key function in ensuring the precision and safety of stem-cell therapies. This technology enables the modification of specific genes in stem cells, offering avenues for targeted therapeutic interventions and correction of genetic disorders at the cellular level. The synergy between stem-cell therapy and bioengineering has emerged as a significant area of exploration. The integration of stem cells with advanced biomaterials can potentially create functional tissues and organs with improved structural and functional properties. Bioengineered constructs provide innovative solutions for tissue-specific regeneration and transplantation. These key directions underscore the multidimensional nature of future advancements in stem-cell therapy, bringing together precision medicine, immune modulation, gene editing, and bioengineering to propel the field toward transformative developments.
Recent developments in stem-cell therapy have illuminated a path of immense promise and transformative potential for revolutionizing modern medicine. The exploration of stem cells across diverse medical disciplines guided by advancements in science, biotechnology, and clinical trial applications has positioned this field at the forefront of biomedical research. The historical journey from foundational concepts laid by pioneering scientists in the late 19th and early 20th centuries to groundbreaking milestones such as the isolation of ESCs and the discovery of iPSCs underscores a monumental leap in medical science.
The regenerative processes of stem cells, categorized into embryonic, adult, induced pluripotent, and perinatal stem cells, offer unprecedented opportunities for therapeutic interventions. Development, tissue repair, and regeneration are all intricately linked to stem cells due to their remarkable capacity to differentiate into different cell types and self-renew. Their diverse applications include neurodegenerative disorders, cardiovascular ailments, spinal cord injuries, diabetes, and tissue damage, opening novel avenues for treating debilitating conditions. However, as the field advances, the critical challenges and complexities must be addressed. Problems like immunological rejection, tumorigenesis, and the precise manipulation of stem-cell behavior pose hurdles that demand comprehensive exploration and innovative solutions. The landscape of stem-cell therapy is intricate and requires a nuanced understanding of its historical foundations, current realities, and future trajectories.
Conclusion
In collating recent biotechnology advancements, critical trial evaluations, and emerging technologies, this review provides a comprehensive compass for clinicians, researchers, and stakeholders navigating the intricate terrain of stem-cell therapy. Future directions, marked by precision medicine integration, immune modulation strategies, advancements in gene-editing technologies, and synergy with bioengineering, offer a roadmap for the continued evolution of stem-cell therapies.
Resonating with the revolutionary promise of stem-cell therapy not only in the realms of science and medicine but also in the lives of individuals with debilitating diseases and injuries. The journey from conceptualization to practical utilization represents a testament to human ingenuity and the relentless pursuit of improving healthcare. As stem-cell research continues, it holds the promise of reshaping the landscape of medicine, bringing forth a new era in which personalized regenerative therapies can mitigate the impact of a spectrum of medical challenges.
Ethical approval
Not applicable.
Consent
Not applicable.
Source of funding
No funding was received for this study.
Author contribution
B.M.H.: study design and data analysis; R.K.Y.: writing the paper; G.H.A.: data collection; S.R.A. and R.K.K.: data analysis and interpretation; S.A.M.: study design and writing the paper.
Conflicts of interest disclosure
The authors declare no conflicts of interest.
Research registration unique identifying number (UIN)
Not applicable.
Guarantor
Bashdar Mahmud Hussen and Suhad A. Mustafa.
Data availability statement
All the data are available in the manuscript.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 5 November 2024
Contributor Information
Bashdar M. Hussen, Email: bashdar.hussen@hmu.edu.krd.
Mohammad Taheri, Email: Mohammad.taheri@uni-jena.de.
Raya Kh. Yashooa, Email: raya.yashooa@su.edu.krd.
Gaylany H. Abdullah, Email: gaylany.abdulla@hmu.edu.krd.
Snur R. Abdullah, Email: Snur.rasool@lfu.edu.krd.
Ramiar Kamal Kheder, Email: Ramiar.kheder@uor.edu.krd.
Suhad A. Mustafa, Email: suhad.mustafa@su.edu.krd.
References
- 1.Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell 2015;17:11–22. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676. [DOI] [PubMed] [Google Scholar]
- 3.Hansford S, Huntsman DG. Boveri at 100: Theodor Boveri and genetic predisposition to cancer. J Pathol 2014;234:142–145. [DOI] [PubMed] [Google Scholar]
- 4.Maximow AA. Der lymphozyt als gemeinsame stammzelle der verschiedenen blutelemente in der embryonalen entwicklung und im postfetalen leben der säugetiere. Cellul Ther Transplantat 2009;1:9–13. [Google Scholar]
- 5.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981;292:154–156. [DOI] [PubMed] [Google Scholar]
- 6.Zhao T, Zhang Z-N, Rong Z, et al. Immunogenicity of induced pluripotent stem cells. Nature 2011;474:212–215. [DOI] [PubMed] [Google Scholar]
- 7.Lister R, Pelizzola M, Kida YS, et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature 2011;471:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007;318:1917–1920. [DOI] [PubMed] [Google Scholar]
- 9.Diederichs S, Shine KM, Tuan RS. The promise and challenges of stem cell-based therapies for skeletal diseases: stem cell applications in skeletal medicine: potential, cell sources and characteristics, and challenges of clinical translation. Bioessays 2013;35:220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rando TA, Chang HY. Aging, rejuvenation, and epigenetic reprogramming: resetting the aging clock. Cell 2012;148:46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blau HM, Daley GQ. Stem cells in the treatment of disease. New Engl J Med 2019;380:1748–1760. [DOI] [PubMed] [Google Scholar]
- 12.De Los Angeles A, Ferrari F, Xi R, et al. Hallmarks of pluripotency. Nature 2015;525:469–478. [DOI] [PubMed] [Google Scholar]
- 13.Chen T, Li J, Jia Y, et al. Single-cell sequencing in the field of stem cells. Curr Genomics 2020;21:576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valenti MT, Serena M, Dalle Carbonare LD, et al. CRISPR/Cas system: an emerging technology in stem cell research. World J Stem Cells 2019;11:937–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han C, Sun X, Liu L, et al. Exosomes and their therapeutic potentials of stem cells. Stem Cells Int 2016;2016:7653489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lattanzi W, Ripoli C, Greco V, et al. Basic and preclinical research for personalized medicine. J Pers Med 2021;11:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kavya ANL, Subramanian S, Ramakrishna S. Therapeutic applications of exosomes in various diseases: a review. Biomat Adv 2022;134:112579. [DOI] [PubMed] [Google Scholar]
- 18.Mousaei Ghasroldasht M, Seok J, Park H-S, et al. Stem cell therapy: from idea to clinical practice. Int J Mol Sci 2022;23:2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bacakova L, Zarubova J, Travnickova M, et al. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells–a review. Biotechnol Adv 2018;36:1111–1126. [DOI] [PubMed] [Google Scholar]
- 20.Zhao W, Ji X, Zhang F, et al. Embryonic stem cell markers. Molecules 2012;17:6196–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen G, Yin S, Zeng H, et al. Regulation of embryonic stem cell self-renewal. Life (Basel) 2022;12:1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natnl Acad Sci 1981;78:7634–7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines, derived from human blastocysts. Science 1998;282:1145–1147. [DOI] [PubMed] [Google Scholar]
- 24.Smith AG. Culture and differentiation of embryonic stem cells. J Tissue Cult Methods 1991;13:89–94. [Google Scholar]
- 25.Keller GM. In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol 1995;7:862–869. [DOI] [PubMed] [Google Scholar]
- 26.Wiles MV, Johansson BM. Embryonic stem cell development in a chemically defined medium. Exp Cell Res 1999;247:241–248. [DOI] [PubMed] [Google Scholar]
- 27.Guest D, Allen W. Expression of cell-surface antigens and embryonic stem cell pluripotency genes in equine blastocysts. Stem Cells Dev 2007;16:789–796. [DOI] [PubMed] [Google Scholar]
- 28.Saito S, Ugai H, Sawai K, et al. Isolation of embryonic stem‐like cells from equine blastocysts and their differentiation in vitro1. FEBS Lett 2002;531:389–396. [DOI] [PubMed] [Google Scholar]
- 29.Leeper NJ, Hunter AL, Cooke JP. Stem cell therapy for vascular regeneration: adult, embryonic, and induced pluripotent stem cells. Circulation 2010;122:517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dvash T, Ben-Yosef D, Eiges R. Human embryonic stem cells as a powerful tool for studying human embryogenesis. Pediatr Res 2006;60:111–117. [DOI] [PubMed] [Google Scholar]
- 31.He Q, Li J, Bettiol E, et al. Embryonic stem cells: new possible therapy for degenerative diseases that affect elderly people. J Gerontol A Biol Sci Med Sci 2003;58:M279–M287. [DOI] [PubMed] [Google Scholar]
- 32.Nichols J, Smith A. The origin and identity of embryonic stem cells. Development 2011;138:3–8. [DOI] [PubMed] [Google Scholar]
- 33.Biteau B, Hochmuth CE, Jasper H. Maintaining tissue homeostasis: dynamic control of somatic stem cell activity. Cell Stem Cell 2011;9:402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tweedell KS. The adaptability of somatic stem cells: a review. J Stem Cells Regen Med 2017;13:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vats A, Tolley N, Polak J, et al. Stem cells: sources and applications. Clin Otolaryngol All Sci 2002;27:227–232. [DOI] [PubMed] [Google Scholar]
- 36.Gurusamy N, Alsayari A, Rajasingh S, et al. Adult stem cells for regenerative therapy. Prog Mol Biol Transl Sci 2018;160:1–22. [DOI] [PubMed] [Google Scholar]
- 37.Marion NW, Mao JJ. Mesenchymal stem cells and tissue engineering. Methods Enzymol 2006;420:339–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernández-Susavila H, Bugallo-Casal A, Castillo J, et al. Adult stem cells and induced pluripotent stem cells for stroke treatment. Front Neurol 2019;10:908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahla RS. Stem cells applications in regenerative medicine and disease therapeutics. Int J Cell Biol 2016;2016:6940283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001;105:369–377. [DOI] [PubMed] [Google Scholar]
- 41.Franceschetti T, De Bari C. The potential role of adult stem cells in the management of the rheumatic diseases. Ther Adv Musculoskelet Dis 2017;9:165–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cable J, Fuchs E, Weissman I, et al. Adult stem cells and regenerative medicine—a symposium report. Ann N Y Acad Sci 2020;1462:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mariano ED, Teixeira MJ, Marie SKN, et al. Adult stem cells in neural repair: current options, limitations and perspectives. World J Stem Cells 2015;7:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan KY, Kim FS, Wagers AJ, et al. Hematopoietic stem cells and somatic stem cells. Hematopoietic Stem Cell Biol 2010:57–92. [Google Scholar]
- 45.Lee JY, Hong SH. Hematopoietic stem cells and their roles in tissue regeneration. Int J Stem Cells 2020;13:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dessie G, Derbew Molla M, Shibabaw T, et al. Role of stem-cell transplantation in leukemia treatment. Stem Cells Clon Adv Appl 2020;13:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swart JF, Delemarre EM, Van Wijk F, et al. Haematopoietic stem cell transplantation for autoimmune diseases. Nat Rev Rheumatol 2017;13:244–256. [DOI] [PubMed] [Google Scholar]
- 48.Hatzimichael E, Tuthill M. Hematopoietic stem cell transplantation. Stem Cells Cloning 2010;3:105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torre Pdl, Flores AI. Current status and future prospects of perinatal stem cells. Genes (Basel) 2020;12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen P-M, Yen M-L, Liu K-J, et al. Immunomodulatory properties of human adult and fetal multipotent mesenchymal stem cells. J Biomed Sci 2011;18:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deus IA, Mano JF, Custodio CA. Perinatal tissues and cells in tissue engineering and regenerative medicine. Acta Biomater 2020;110:1–14. [DOI] [PubMed] [Google Scholar]
- 52.Brown PT, Handorf AM, Jeon WB, et al. Stem cell-based tissue engineering approaches for musculoskeletal regeneration. Curr Pharm Des 2013;19:3429–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paris F, Marrazzo P, Pizzuti V, et al. Characterization of perinatal stem cell spheroids for the development of cell therapy strategy. Bioengineering 2023;10:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Z, Han ZC. Introduction of perinatal tissue-derived stem cells. Perinatal Stem Cells Biol Manufacturing Transl Med 2019:1–7. [Google Scholar]
- 55.Omole AE, Fakoya AOJ. Ten years of progress and promise of induced pluripotent stem cells: historical origins, characteristics, mechanisms, limitations, and potential applications. PeerJ 2018;6:e4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim JB, Zaehres H, Wu G, et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature 2008;454:646–650. [DOI] [PubMed] [Google Scholar]
- 57.Kunisato A, Wakatsuki M, Kodama Y, et al. Generation of induced pluripotent stem cells by efficient reprogramming of adult bone marrow cells. Stem Cells Dev 2010;19:229–238. [DOI] [PubMed] [Google Scholar]
- 58.Ye L, Swingen C, Zhang J. Induced pluripotent stem cells and their potential for basic and clinical sciences. Curr Cardiol Rev 2013;9:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bilic J, Belmonte JCI. Concise review: induced pluripotent stem cells versus embryonic stem cells: close enough or yet too far apart? Stem Cells 2011;30:33–41. [DOI] [PubMed] [Google Scholar]
- 60.Park S, Gwon Y, Khan SA, et al. Engineering considerations of iPSC-based personalized medicine. Biomater Res 2023;27:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shi Y, Inoue H, Wu JC, et al. Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov 2017;16:115–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doss MX, Sachinidis A. Current challenges of iPSC-based disease modeling and therapeutic implications. Cells 2019;8:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karami Z, Moradi S, Eidi A, et al. Induced pluripotent stem cells: generation methods and a new perspective in COVID-19 research. Front Cell Dev Biol 2023;10:1050856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moradi S, Mahdizadeh H, Šarić T, et al. Research and therapy with induced pluripotent stem cells (iPSCs): social, legal, and ethical considerations. Stem Cell Res Ther 2019;10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao F, Chiu S, Motan D, et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis 2016;7:e2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mili B, Choudhary OP. Advancements and mechanisms of stem cell-based therapies for spinal cord injury in animals. Int J Surg 2024;110:6182–6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kode JA, Mukherjee S, Joglekar MV, et al. Mesenchymal stem cells: immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy 2009;11:377–391. [DOI] [PubMed] [Google Scholar]
- 68.Jiang W, Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif 2020;53:e12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang H, Ma S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am J Emerg Med 2008;26:711–715. [DOI] [PubMed] [Google Scholar]
- 70.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer 2018;6:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee K-H, Tseng W-C, Yang C-Y, et al. The anti-inflammatory, anti-oxidative, and anti-apoptotic benefits of stem cells in acute ischemic kidney injury. Int J Mol Sci 2019;20:3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kristensen DM, Kalisz M, Nielsen JH. Cytokine signalling in embryonic stem cells. APMIS 2005;113(11‐12):756–772. [DOI] [PubMed] [Google Scholar]
- 73.Schnabel LV, Abratte CM, Schimenti JC, et al. Induced pluripotent stem cells have similar immunogenic and more potent immunomodulatory properties compared with bone marrow-derived stromal cells in vitro. Regen Med 2014;9:621–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bernard O, Jeny F, Uzunhan Y, et al. Mesenchymal stem cells reduce hypoxia-induced apoptosis in alveolar epithelial cells by modulating HIF and ROS hypoxic signaling. Am J Physiol Lung Cell Mol Physiol 2018;314:L360–L371. [DOI] [PubMed] [Google Scholar]
- 75.Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. New Engl J Med 2007;357:1450–1451. [DOI] [PubMed] [Google Scholar]
- 76.Pan S, Zhao X, Wang X, et al. Sfrp1 attenuates TAC-induced cardiac dysfunction by inhibiting Wnt signaling pathway-mediated myocardial apoptosis in mice. Lipids Health Dis 2018;17:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Najar M, Martel-Pelletier J, Pelletier J-P, et al. Mesenchymal stromal cell immunology for efficient and safe treatment of osteoarthritis. Front Cell Dev Biol 2020;8:567813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chow L, Johnson V, Impastato R, et al. Antibacterial activity of human mesenchymal stem cells mediated directly by constitutively secreted factors and indirectly by activation of innate immune effector cells. Stem Cells Transl Med 2020;9:235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krasnodembskaya A, Song Y, Fang X, et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells 2010;28:2229–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han Y, Li X, Zhang Y, et al. Mesenchymal stem cells for regenerative medicine. Cells 2019;8:886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang K, Cheng K. Stem cell-derived exosome versus stem cell therapy. Nat Rev Bioeng 2023;1:608–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.DiMarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol 2013;4:52511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shafiee A, Patel J, Lee JS, et al. Mesenchymal stem/stromal cells enhance engraftment, vasculogenic and pro-angiogenic activities of endothelial colony forming cells in immunocompetent hosts. Sci Rep 2017;7:13558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matthay MA, Thompson BT, Read EJ, et al. Therapeutic potential of mesenchymal stem cells for severe acute lung injury. Chest 2010;138:965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sadeghi S, Soudi S, Shafiee A, et al. Mesenchymal stem cell therapies for COVID-19: current status and mechanism of action. Life Sci 2020;262:118493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fleifel D, Rahmoon MA, AlOkda A, et al. Recent advances in stem cells therapy: a focus on cancer, Parkinson’s and Alzheimer’s. J Genetic Engineer Biotechnol 2018;16:427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hwang NS, Varghese S, Elisseeff J. Controlled differentiation of stem cells. Adv Drug Deliv Rev 2008;60:199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aly RM. Current state of stem cell-based therapies: an overview. Stem Cell Investig 2020;7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xing W, Yang J, Zheng Y, et al. The role of the notch signaling pathway in the differentiation of human umbilical cord-derived mesenchymal stem cells. Front Biosc 2024;29:74. [DOI] [PubMed] [Google Scholar]
- 90.Fox V, Gokhale PJ, Walsh JR, et al. Cell-cell signaling through NOTCH regulates human embryonic stem cell proliferation. Stem Cells 2008;26:715–723. [DOI] [PubMed] [Google Scholar]
- 91.Noisa P, Lund C, Kanduri K, et al. Notch signaling regulates the differentiation of neural crest from human pluripotent stem cells. J Cell Sci 2014;127:2083–2094. [DOI] [PubMed] [Google Scholar]
- 92.Benveniste P, Serra P, Dervovic D, et al. Notch signals are required for in vitro but not in vivo maintenance of human hematopoietic stem cells and delay the appearance of multipotent progenitors. Blood 2014;123:1167–1177. [DOI] [PubMed] [Google Scholar]
- 93.Dong C, Wang X, Sun L, et al. ATM modulates subventricular zone neural stem cell maintenance and senescence through Notch signaling pathway. Stem Cell Res 2022;58:102618. [DOI] [PubMed] [Google Scholar]
- 94.Sancho R, Cremona CA, Behrens A. Stem cell and progenitor fate in the mammalian intestine: Notch and lateral inhibition in homeostasis and disease. EMBO Rep 2015;16:571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jafari M, Ghadami E, Dadkhah T, et al. PI3k/AKT signaling pathway: erythropoiesis and beyond. J Cell Physiol 2019;234:2373–2385. [DOI] [PubMed] [Google Scholar]
- 96.Liu C, Peng G, Jing N. TGF-β signaling pathway in early mouse development and embryonic stem cells. Acta Biochim Biophys Sin (Shanghai) 2018;50:68–73. [DOI] [PubMed] [Google Scholar]
- 97.Kaltschmidt C, Greiner JF, Kaltschmidt B. The transcription factor NF-κB in stem cells and development. Cells 2021;10:2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lyssiotis CA, Lairson LL, Boitano AE, et al. Chemical control of stem cell fate and developmental potential. Angewandte Chemie Int Ed 2011;50:200–242. [DOI] [PubMed] [Google Scholar]
- 99.Eom Y-S, Park J-H, Kim T-H. Recent advances in stem cell differentiation control using drug delivery systems based on porous functional materials. J Funct Biomater 2023;14:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Donowitz M, Turner JR, Verkman AS, et al. Current and potential future applications of human stem cell models in drug development. J Clin Invest 2020;130:3342–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meissner A. Epigenetic modifications in pluripotent and differentiated cells. Nat Biotechnol 2010;28:1079–1088. [DOI] [PubMed] [Google Scholar]
- 102.Ankam S, Rovini A, Baheti S, et al. DNA methylation patterns in human iPSC-derived sensory neuronal differentiation. Epigenetics 2019;14:927–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ratajczak MZ, Jadczyk T, Pedziwiatr D, et al. New advances in stem cell research: practical implications for regenerative medicine. Pol Arch Med Wewn 2014;124:417–426. [DOI] [PubMed] [Google Scholar]
- 104.Rodríguez-Fuentes DE, Fernández-Garza LE, Samia-Meza JA, et al. Mesenchymal stem cells current clinical applications: a systematic review. Arch Med Res 2021;52:93–101. [DOI] [PubMed] [Google Scholar]
- 105.Labusca L, Herea DD, Mashayekhi K. Stem cells as delivery vehicles for regenerative medicine-challenges and perspectives. World J Stem Cells 2018;10:43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Anderson JD. Advances in stem cell immunotherapy. Stem Cells 2023;41:307–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sharma SK. Stem cell transplant for autoimmune diseases. Basics of Hematopoietic Stem Cell Transplant. Springer; 2023:247–258. [Google Scholar]
- 108.Sakowski SA, Chen KS. Stem cell therapy for central nervous system disorders: metabolic interactions between transplanted cells and local microenvironments. Neurobiol Dis 2022;173:105842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rahman MM, Islam MR, Islam MT, et al. Stem cell transplantation therapy and neurological disorders: current status and future perspectives. Biology 2022;11:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mahmud S, Alam S, Emon NU, et al. Opportunities and challenges in stem cell therapy in cardiovascular diseases: position standing in 2022. Saudi Pharmaceut J 2022;30:1360–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Povsic TJ, Gersh BJ. Stem cells in cardiovascular diseases: 30,000-foot view. Cells 2021;10:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maniar H, Tawari A, Suk M, et al. The current role of stem cells in orthopaedic surgery. Malays Orthop J 2015;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Akpancar S, Tatar O, Turgut H, et al. The current perspectives of stem cell therapy in orthopedic surgery. Arch Trauma Res 2016;5:e37976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Malekpour K, Hazrati A, Zahar M, et al. The potential use of mesenchymal stem cells and their derived exosomes for orthopedic diseases treatment. Stem Cell Rev Rep 2022;18:933–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xie X, Yao H, Han X, et al. Therapeutic use of red blood cells and platelets derived from human cord blood stem cells. Stem Cells Transl Med 2021;10(S2):S48–S53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hamad FRB, Rahat N, Shankar K, et al. Efficacy of stem cell application in diabetes mellitus: promising future therapy for diabetes and its complications. Cureus 2021;13:e13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mathur A, Taurin S, Alshammary S. The safety and efficacy of mesenchymal stem cells in the treatment of type 2 diabetes-a literature review. Diab Metabol Syndr Obes 2023;16:769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bagher Z, Azami M, Ebrahimi-Barough S, et al. Differentiation of Wharton’s jelly-derived mesenchymal stem cells into motor neuron-like cells on three-dimensional collagen-grafted nanofibers. Mol Neurobiol 2016;53:2397–2408. [DOI] [PubMed] [Google Scholar]
- 119.Rincon‐Benavides MA, Mendonca NC, Cuellar‐Gaviria TZ, et al. Engineered vasculogenic extracellular vesicles drive nonviral direct conversions of human dermal fibroblasts into induced endothelial cells and improve wound closure. Adv Therap 2023;6:2200197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang Z, Yang H, Xu X, et al. Ion elemental-optimized layered double hydroxide nanoparticles promote chondrogenic differentiation and intervertebral disc regeneration of mesenchymal stem cells through focal adhesion signaling pathway. Bioactive Materials 2023;22:75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mo H, Kim J, Kim JY, et al. Intranasal administration of induced pluripotent stem cell-derived cortical neural stem cell-secretome as a treatment option for Alzheimer’s disease. Transl Neurodegener 2023;12:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou Z, Shi B, Xu Y, et al. Neural stem/progenitor cell therapy for Alzheimer disease in preclinical rodent models: a systematic review and meta-analysis. Stem Cell Res Ther 2023;14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hamedi H, Ghorbanian SH, Mirzaeian L, et al. Intravenous transplantation of adipose-derived mesenchymal stem cells promoted the production of dopaminergic neurons and improved spatial memory in a rat model of Parkinson’s disease. Cell J 2023;25:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mendes-Pinheiro B, Campos J, Marote A, et al. Treating Parkinson’s disease with human bone marrow mesenchymal stem cell secretome: a translational investigation using human brain organoids and different routes of in vivo administration. Cells 2023;12:2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vo QD, Saito Y, Nakamura K, et al. Induced pluripotent stem cell-derived cardiomyocytes therapy for ischemic heart disease in animal model: a meta-analysis. Int J Mol Sci 2024;25:987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang J, Li J, Qu X, et al. Development of a thick and functional human adipose-derived stem cell tissue sheet for myocardial infarction repair in rat hearts. Stem Cell Res Ther 2023;14:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Freitag J, Bates D, Wickham J, et al. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regen Med 2019;14:213–230. [DOI] [PubMed] [Google Scholar]
- 128.Shima T, Takigawa K, Utsumi S, et al. Outcomes of allogeneic stem cell transplantation for patients with hematologic diseases ≥60 years old. Blood Cell Ther 2023;6:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ding L, Han D-M, Zheng X-L, et al. A study of human leukocyte antigen-haploidentical hematopoietic stem cells transplantation combined with allogenic mesenchymal stem cell infusion for treatment of severe aplastic anemia in pediatric and adolescent patients. Stem Cells Transl Med 2020;10:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zang L, Li Y, Hao H, et al. Efficacy of umbilical cord-derived mesenchymal stem cells in the treatment of type 2 diabetes assessed by retrospective continuous glucose monitoring. Stem Cells Transl Med 2023;12:775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rezaeian A, Khatami F, Heidari Keshel S, et al. The effect of mesenchymal stem cells-derived exosomes on the prostate, bladder, and renal cancer cell lines. Sci Rep 2022;12:20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sun L, Xu Y, Zhang X, et al. Mesenchymal stem cells functionalized sonodynamic treatment for improving therapeutic efficacy and compliance of orthotopic oral cancer. Adv Mat 2020;32:2005295. [DOI] [PubMed] [Google Scholar]
- 133.Hoseinzadeh A, Mahmoudi M, Rafatpanah H, et al. A new generation of mesenchymal stromal/stem cells differentially trained by immunoregulatory probiotics in a lupus microenvironment. Stem Cell Res Ther 2023;14:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gong Z, He Y, Zhou M, et al. Ultrasound imaging tracking of mesenchymal stem cells intracellularly labeled with biosynthetic gas vesicles for treatment of rheumatoid arthritis. Theranostics 2022;12:2370–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Feng G, Shi L, Huang T, et al. Human umbilical cord mesenchymal stromal cell treatment of severe COVID-19 patients: a 3-month follow-up study following hospital discharge. Stem Cells Dev 2021;30:773–781. [DOI] [PubMed] [Google Scholar]
- 136.Dilogo IH, Aditianingsih D, Sugiarto A, et al. Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: a randomized controlled trial. Stem Cells Transl Med 2021;10:1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Marfe G, Perna S, Shukla AK. Effectiveness of COVID‑19 vaccines and their challenges. Exp Ther Med 2021;22:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen J, Hu C, Chen L, et al. Clinical study of mesenchymal stem cell treatment for acute respiratory distress syndrome induced by epidemic influenza A (H7N9) infection: a hint for COVID-19 treatment. Engineering 2020;6:1153–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kheder RK, Darweesh O, Hussen BM, et al. Mesenchymal stromal cells (MSCs) as a therapeutic agent of inflammatory disease and infectious COVID-19 virus: live or dead mesenchymal? Mol Biol Rep 2024;51:295. [DOI] [PubMed] [Google Scholar]
- 140.Shu L, Niu C, Li R, et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther 2020;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xie Q, Liu R, Jiang J, et al. What is the impact of human umbilical cord mesenchymal stem cell transplantation on clinical treatment? Stem Cell Res Ther 2020;11:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li S, Zhu H, Zhao M, et al. When stem cells meet COVID-19: recent advances, challenges and future perspectives. Stem Cell Res Ther 2022;13:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zakrzewski W, Dobrzyński M, Szymonowicz M, et al. Stem cells: past, present, and future. Stem Cell Res Ther 2019;10:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zangi L, Margalit R, Reich-Zeliger S, et al. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells 2009;27:2865–2874. [DOI] [PubMed] [Google Scholar]
- 145.Nauta AJ, Westerhuis G, Kruisselbrink AB, et al. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood 2006;108:2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schu S, Nosov M, O’Flynn L, et al. Immunogenicity of allogeneic mesenchymal stem cells. J Cell Mol Med 2012;16:2094–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kwon SG, Kwon YW, Lee TW, et al. Recent advances in stem cell therapeutics and tissue engineering strategies. Biomater Res 2018;22:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Trounson A, DeWitt ND. Pluripotent stem cells progressing to the clinic. Nat Rev Mol Cell Biol 2016;17:194–200. [DOI] [PubMed] [Google Scholar]
- 149.Mason C, Dunnill P. A brief definition of regenerative medicine. Regen Med 2008;3:1–5. [DOI] [PubMed] [Google Scholar]
- 150.Atala A. Regenerative medicine strategies. J Pediatr Surg 2012;47:17–28. [DOI] [PubMed] [Google Scholar]
- 151.Mansouri V, Beheshtizadeh N, Gharibshahian M, et al. Recent advances in regenerative medicine strategies for cancer treatment. Biomed Pharmacother 2021;141:111875. [DOI] [PubMed] [Google Scholar]
- 152.Derks LL, van Boxtel R. Stem cell mutations, associated cancer risk, and consequences for regenerative medicine. Cell Stem Cell 2023;30:1421–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natnl Acad Sci 2000;97:12846–12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Aboody KS, Najbauer J, Metz MZ, et al. Neural stem cell–mediated enzyme/prodrug therapy for glioma: preclinical studies. Sci Transl Med 2013;5:184ra59–ra59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang CL, Huang T, Wu BL, et al. Stem cells in cancer therapy: opportunities and challenges. Oncotarget 2017;8:75756–75766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Evans CH. Advances in regenerative orthopedics. Mayo Clin Proc 2013;88:1323–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhao J, Meng H, Liao S, et al. Therapeutic effect of human umbilical cord mesenchymal stem cells in early traumatic osteonecrosis of the femoral head. J Orthop Transl 2022;37:126–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Masoudi EA, Ribas J, Kaushik G, et al. Platelet-rich blood derivatives for stem cell-based tissue engineering and regeneration. Curr Stem Cell Rep 2016;2:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sundelacruz S, Kaplan DL. Stem cell-and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Semin Cell Dev Biol. Elsevier; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Andia I, Maffulli N. New biotechnologies for musculoskeletal injuries. Surgeon 2019;17:244–255. [DOI] [PubMed] [Google Scholar]
- 161.Dzobo K, Thomford NE, Senthebane DA, et al. Advances in regenerative medicine and tissue engineering: innovation and transformation of medicine. Stem Cells Int 2018;2018:2495848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Madonna R, Van Laake LW, Botker HE, et al. ESC working group on cellular biology of the heart: tissue engineering and cell-based therapies for cardiac repair in ischemic heart disease and heart failure. Cardiovasc Res 2019;115:488–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Arjmand B, Abedi M, Arabi M, et al. Regenerative medicine for the treatment of ischemic heart disease; status and future perspectives. Front Cell Dev Biol 2021;9:704903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kim SU, Lee HJ, Kim YB. Neural stem cell‐based treatment for neurodegenerative diseases. Neuropathology 2013;33:491–504. [DOI] [PubMed] [Google Scholar]
- 165.Yashooa RK, Nabi AQ. The miR-146a-5p and miR-125b-5p levels as biomarkers for early prediction of Alzheimer’s disease. Human Gene 2022;34:201129. [Google Scholar]
- 166.Sun Y, Feng L, Liang L, et al. Neuronal cell-based medicines from pluripotent stem cells: development, production, and preclinical assessment. Stem Cells Transl Med 2021;10(s2):S31–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Isaković J, Šerer K, Barišić B, et al. Mesenchymal stem cell therapy for neurological disorders: the light or the dark side of the force? Front Bioeng Biotechnol 2023;11:1139359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Inoue M, Yamaguchi R, He CCJ, et al. Current status and prospects of regenerative medicine for spinal cord injury using human induced pluripotent stem cells: a review. Stem Cell Investig 2023;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dieckmann C, Renner R, Milkova L, et al. Regenerative medicine in dermatology: biomaterials, tissue engineering, stem cells, gene transfer and beyond. Exp Dermatol 2010;19:697–706. [DOI] [PubMed] [Google Scholar]
- 170.Ojeh N, Pastar I, Tomic-Canic M, et al. Stem cells in skin regeneration, wound healing, and their clinical applications. Int J Mol Sci 2015;16:25476–25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bellei B, Papaccio F, Picardo M. Regenerative medicine-based treatment for vitiligo: an overview. Biomedicines 2022;10:2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Paganelli A, Tarentini E, Benassi L, et al. Mesenchymal stem cells for the treatment of psoriasis: a comprehensive review. Clin Exp Dermatol 2020;45:824–830. [DOI] [PubMed] [Google Scholar]
- 173.Chou Y, Alfarafisa NM, Ikezawa M, et al. Progress in the development of stem cell-derived cell-free therapies for skin aging. Clin Cosmet Investig Dermatol 2023;16:3383–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Davies O, Williams S, Goldie K. The therapeutic and commercial landscape of stem cell vesicles in regenerative dermatology. J Controlled Release 2023;353:1096–1106. [DOI] [PubMed] [Google Scholar]
- 175.Nilforoushzadeh MA, Amirkhani MA, Seirafianpour F, et al. Regenerative medicine in dermatology. J Skin Stem Cell 2022;9. [Google Scholar]
- 176.Golchin A. Stem cell technology and skin disorders: from stem cell biology to clinical applications. Stem Cell Rev Rep 2022;18:1881–1882. [DOI] [PubMed] [Google Scholar]
- 177.Khandpur S, Gupta S, Gunaabalaji D. Stem cell therapy in dermatology. Indian J Dermatol Venereol Leprol 2021;87:753–767. [DOI] [PubMed] [Google Scholar]
- 178.Dhamodaran K, Subramani M, Ponnalagu M, et al. Ocular stem cells: a status update!. Stem Cell Res Ther 2014;5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tomczak W, Winkler-Lach W, Tomczyk-Socha M, et al. Advancements in ocular regenerative therapies. Biology 2023;12:737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sahle FF, Kim S, Niloy KK, et al. Nanotechnology in regenerative ophthalmology. Adv Drug Del Rev 2019;148:290–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fortress AM, Miyagishima KJ, Reed AA, et al. Stem cell sources and characterization in the development of cell-based products for treating retinal disease: an NEI Town Hall report. BioMed Central 2023;14:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Van Gelder RN, Chiang MF, Dyer MA, et al. Regenerative and restorative medicine for eye disease. Nat Med 2022;28:1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zimmerlin L, Park TS, Zambidis ET, et al. Mesenchymal stem cell secretome and regenerative therapy after cancer. Biochimie 2013;95:2235–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Patriarca F, Skert C, Sperotto A, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol 2006;34:389–396. [DOI] [PubMed] [Google Scholar]
- 185.Petrus-Reurer S, Romano M, Howlett S, et al. Immunological considerations and challenges for regenerative cellular therapies. Communications Biol 2021;4:798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Preynat-Seauve O, Krause K-H. Stem cell sources for regenerative medicine: the immunological point of view. Seminars in immunopathology. Springer; 2011. [DOI] [PubMed] [Google Scholar]
- 187.Eskew W. Regenerative medicine: an analysis of origins, trends and potential therapeutic applications, with a focus on hematopoietic stem cells. 2018.
- 188.Skulimowska I, Sosniak J, Gonka M, et al. The biology of hematopoietic stem cells and its clinical implications. FEBS J 2022;289:7740–7759. [DOI] [PubMed] [Google Scholar]
- 189.Montserrat-Vazquez S, Ali NJ, Matteini F, et al. Transplanting rejuvenated blood stem cells extends lifespan of aged immunocompromised mice. NPJ Regen Med 2022;7:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Imran SA, M. Hamizul MHA, Khairul Bariah AAN, et al. Regenerative medicine therapy in Malaysia: an update. Front Bioeng Biotechnol 2022;10:789644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Shimizu H, Suzuki K, Watanabe M, et al. Stem cell-based therapy for inflammatory bowel disease. Intest Res 2019;17:311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Okamoto R, Mizutani T, Shimizu H. Development and application of regenerative medicine in inflammatory bowel disease. Digestion 2023;104:24–29. [DOI] [PubMed] [Google Scholar]
- 193.Yan L, Cai C, Li J, et al. Present status and perspectives of stem cell-based therapies for gastrointestinal diseases. Stem Cell Rev Rep 2009;5:278–282. [DOI] [PubMed] [Google Scholar]
- 194.Peterson J, Pasricha PJ. Regenerative medicine and the gut. Gastroenterology 2011;141:1162–6.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kanetaka K, Eguchi S. Regenerative medicine for the upper gastrointestinal tract. Regen Ther 2020;15:129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Collier CA, Mendiondo C, Raghavan S. Tissue engineering of the gastrointestinal tract: the historic path to translation. J Biol Eng 2022;16:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kokturk N, Yildirim F, Gülhan PY, et al. Stem cell therapy in chronic obstructive pulmonary disease. How far is it to the clinic? Am J Stem Cells 2018;7:56. [PMC free article] [PubMed] [Google Scholar]
- 198.Adamič N, Vengust M. Regenerative medicine in lung diseases: a systematic review. Front Vet Sci 2023;10:1115708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sun Z, Li F, Zhou X, et al. Stem cell therapies for chronic obstructive pulmonary disease: current status of pre-clinical studies and clinical trials. J Thorac Dis 2018;10:1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Singh PV, Singh PV, Anjankar A, et al. Harnessing the therapeutic potential of stem cells in the management of chronic obstructive pulmonary disease: a comprehensive review. Cureus 2023;15:e44498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wu J-X, Xia T, She L-P, et al. Stem cell therapies for human infertility: advantages and challenges. Cell Transplant 2022;31:09636897221083252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wang J, Liu C, Fujino M, et al. Stem cells as a resource for treatment of infertility-related diseases. Curr Mol Med 2019;19:539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Vassena R, Eguizabal C, Heindryckx B, et al. Stem cells in reproductive medicine: ready for the patient? Hum Reprod 2015;30:2014–2021. [DOI] [PubMed] [Google Scholar]
- 204.Rahim F, Arjmand B, Shirbandi K, et al. Stem cell therapy for patients with diabetes: a systematic review and meta-analysis of metabolomics-based risks and benefits. Stem Cell Investig 2018;5:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Brovkina O, Dashinimaev E. Advances and complications of regenerative medicine in diabetes therapy. PeerJ 2020;8:e9746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bouwens L, Houbracken I, Mfopou JK. The use of stem cells for pancreatic regeneration in diabetes mellitus. Nat Rev Endocrinol 2013;9:598–606. [DOI] [PubMed] [Google Scholar]
- 207.Li Y, He C, Liu R, et al. Stem cells therapy for diabetes: from past to future. Cytotherapy 2023;25:1125–1138. [DOI] [PubMed] [Google Scholar]
- 208.Soudi A, Yazdanian M, Ranjbar R, et al. Role and application of stem cells in dental regeneration: a comprehensive overview. EXCLI J 2021;20:454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Thalakiriyawa DS, Dissanayaka WL. Advances in regenerative dentistry approaches: an update. Int Dent J 2023;74:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Smojver I, Katalinić I, Bjelica R, et al. Mesenchymal stem cells based treatment in dental medicine: a narrative review. Int J Mol Sci 2022;23:1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Song W-P, Jin L-Y, Zhu M-D, et al. Clinical trials using dental stem cells: 2022 update. World J Stem Cells 2023;15:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Xiao L, Nasu M. From regenerative dentistry to regenerative medicine: progress, challenges, and potential applications of oral stem cells. Stem Cells Clon Adv Applicat 2014;7:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kolimi P, Narala S, Nyavanandi D, et al. Innovative treatment strategies to accelerate wound healing: trajectory and recent advancements. Cells 2022;11:2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.El-Sayed ME, Atwa A, Sofy AR, et al. Mesenchymal stem cell transplantation in burn wound healing: uncovering the mechanisms of local regeneration and tissue repair. Histochem Cell Biol 2024;161:165–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Abusalah MAH, Priyanka, Abd Rahman ENSE, et al. Evolving trends in stem cell therapy: an emerging and promising approach against various diseases. Int J Surg. 9900:10.1097/JS9.0000000000001948.
- 216.Fadilah NIM, Jailani MSMAK, Hisham MAIB, et al. Cell secretomes for wound healing and tissue regeneration: Next generation acellular based tissue engineered products. J Tissue Eng 2022;13:20417314221114273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zeng N, Chen H, Wu Y, et al. Adipose stem cell-based treatments for wound healing. Front Cell Dev Biol 2022;9:821652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Urao N, Liu J, Takahashi K, et al. Hematopoietic stem cells in wound healing response. Adv Wound Care 2022;11:598–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kanji S, Das H. Advances of stem cell therapeutics in cutaneous wound healing and regeneration. Mediators Inflamm 2017;2017:5217967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Shi Y, Wang S, Zhang W, et al. Bone marrow mesenchymal stem cells facilitate diabetic wound healing through the restoration of epidermal cell autophagy via the HIF-1α/TGF-β1/SMAD pathway. Stem Cell Res Ther 2022;13:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lee YI, Kim S, Kim J, et al. Randomized controlled study for the anti‐aging effect of human adipocyte‐derived mesenchymal stem cell media combined with niacinamide after laser therapy. J Cosmet Dermatol 2021;20:1774–1781. [DOI] [PubMed] [Google Scholar]
- 222.Ma Y, Hao X, Zhang S, et al. The in vitro and in vivo effects of human umbilical cord mesenchymal stem cells on the growth of breast cancer cells. Breast Cancer Res Treat 2012;133:473–485. [DOI] [PubMed] [Google Scholar]
- 223.Li X, Wu F. Mesenchymal stem cell-derived extracellular vesicles transfer miR-598 to inhibit the growth and metastasis of non-small-cell lung cancer by targeting THBS2. Cell Death Discov 2023;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Qiao B, Shui W, Cai L, et al. Human mesenchymal stem cells as delivery of osteoprotegerin gene: homing and therapeutic effect for osteosarcoma. Drug Des Dev Ther 2015;9:969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bastos R, Mathias M, Andrade R, et al. Intra-articular injection of culture-expanded mesenchymal stem cells with or without addition of platelet-rich plasma is effective in decreasing pain and symptoms in knee osteoarthritis: a controlled, double-blind clinical trial. Knee Surg Sports Traumatol Arthrosc 2020;28:1989–1999. [DOI] [PubMed] [Google Scholar]
- 226.Marfia G, Navone SE, Di Vito C, et al. Gene expression profile analysis of human mesenchymal stem cells from herniated and degenerated intervertebral discs reveals different expression of osteopontin. Stem Cells Dev 2015;24:320–328. [DOI] [PubMed] [Google Scholar]
- 227.Jiang M, Wang X, Liu H, et al. Bone formation in adipose‐derived stem cells isolated from elderly patients with osteoporosis: a preliminary study. Cell Biol Int 2014;38:97–105. [DOI] [PubMed] [Google Scholar]
- 228.Li N, Huang R, Zhang X, et al. Stem cells cardiac patch from decellularized umbilical artery improved heart function after myocardium infarction. Bio-Med Mater Eng 2017;28(s1):S87–S94. [DOI] [PubMed] [Google Scholar]
- 229.Zhang Q, Wu Hh, Wang Y, et al. Neural stem cell transplantation decreases neuroinflammation in a transgenic mouse model of Alzheimer’s disease. J Neurochem 2016;136:815–825. [DOI] [PubMed] [Google Scholar]
- 230.Kim C-H, Lim C-Y, Lee J-H, et al. Human embryonic stem cells-derived mesenchymal stem cells reduce the symptom of psoriasis in imiquimod-induced skin model. Tissue Eng Regenerative Med 2019;16:93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhu L, Lin X, Zhi L, et al. Mesenchymal stem cells promote human melanocytes proliferation and resistance to apoptosis through PTEN pathway in vitiligo. Stem Cell Res Ther 2020;11:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Møller‐Hansen M. Mesenchymal stem cell therapy in aqueous deficient dry eye disease. Wiley Online Library; 2023. [DOI] [PubMed] [Google Scholar]
- 233.Macías-Sánchez MdM, Morata-Tarifa C, Cuende N, et al. Mesenchymal stromal cells for treating steroid-resistant acute and chronic graft versus host disease: A multicenter compassionate use experience. Stem Cells Transl Med 2022;11:343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Lu D, Gong X, Guo X, et al. Therapeutic effects of hematopoietic stem cell derived from gene-edited mice on β654-thalassemia. Stem Cells 2023;42:278–289. [DOI] [PubMed] [Google Scholar]
- 235.Lightner AL, Reese JS, Ream J, et al. A phase IB/IIA study of ex vivo expanded allogeneic bone marrow–derived mesenchymal stem cells for the treatment of rectovaginal fistulizing Crohn’s disease. Surgery 2024;175:242–249. [DOI] [PubMed] [Google Scholar]
- 236.Zhang X, Hu T, Yu X, et al. Human umbilical cord mesenchymal stem cells improve lung function in chronic obstructive pulmonary disease rat model through regulating lung microbiota. Stem Cells 2024;42:346–359; sxae007. [DOI] [PubMed] [Google Scholar]
- 237.Liao W, Tang X, Li X, et al. Therapeutic effect of human umbilical cord mesenchymal stem cells on tubal factor infertility using a chronic salpingitis murine model. Arch Gynecol Obstet 2019;300:421–429. [DOI] [PubMed] [Google Scholar]
- 238.Edessy M, Hosni HN, Shady Y, et al. Autologous stem cells therapy, the first baby of idiopathic premature ovarian failure. Acta Medica Int 2016;3:19–23. [Google Scholar]
- 239.Aierken A, Li B, Liu P, et al. Melatonin treatment improves human umbilical cord mesenchymal stem cell therapy in a mouse model of type II diabetes mellitus via the PI3K/AKT signaling pathway. Stem Cell Res Ther 2022;13:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Qiao X, Tang J, Dou L, et al. Dental pulp stem cell-derived exosomes regulate anti-inflammatory and osteogenesis in periodontal ligament stem cells and promote the repair of experimental periodontitis in rats. Int J Nanomedicine 2023;18:4683–4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zhou H, Yi Z, Le D, et al. Intravenous administration of human chorionic membrane mesenchymal stem cells promotes functional recovery in a rat traumatic brain injury model. Neuroreport 2024;35:81–89. [DOI] [PubMed] [Google Scholar]
- 242.Du J, Zhen G, Chen H, et al. Optimal electrical stimulation boosts stem cell therapy in nerve regeneration. Biomaterials 2018;181:347–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhang H, Li Z-L, Yang F, et al. Radial shockwave treatment promotes human mesenchymal stem cell self-renewal and enhances cartilage healing. Stem Cell Res Ther 2018;9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Patten DA, Ouellet M, Allan DS, et al. Mitochondrial adaptation in human mesenchymal stem cells following ionizing radiation. FASEB J 2019;33:9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ginani F, Soares DM, Barreto MPEV, et al. Effect of low-level laser therapy on mesenchymal stem cell proliferation: a systematic review. Lasers Med Sci 2015;30:2189–2194. [DOI] [PubMed] [Google Scholar]
- 246.Chang K-A, Kim JW, Kim JA, et al. Biphasic electrical currents stimulation promotes both proliferation and differentiation of fetal neural stem cells. PLoS One 2011;6:e18738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Di L, Shi Y-N, Yan Y-M, et al. Nonpeptide small molecules from the insect Aspongopus chinensis and their neural stem cell proliferation stimulating properties. RSC Adv 2015;5:70985–70991. [Google Scholar]
- 248.Maul TM, Chew DW, Nieponice A, et al. Mechanical stimuli differentially control stem cell behavior: morphology, proliferation, and differentiation. Biomech Model Mechanobiol 2011;10:939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Cmielova J, Havelek R, Soukup T, et al. Gamma radiation induces senescence in human adult mesenchymal stem cells from bone marrow and periodontal ligaments. Int J Radiat Biol 2012;88:393–404. [DOI] [PubMed] [Google Scholar]
- 250.Wani S, Dar T, Koli S, et al. Stem cell technology in medical biotechnology. Fundamentals and Advances in Medical Biotechnology. Springer; 2022:233–267. [Google Scholar]
- 251.Tan D, Huang Z, Zhao Z, et al. Single‑cell sequencing, genetics, and epigenetics reveal mesenchymal stem cell senescence in osteoarthritis. Int J Mol Med 2024;53:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wang J, Zheng Y, Zhao M. Exosome-based cancer therapy: implication for targeting cancer stem cells. Front Pharmacol 2017;7:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zhao T, Sun F, Liu J, et al. Emerging role of mesenchymal stem cell-derived exosomes in regenerative medicine. Curr Stem Cell Res Ther 2019;14:482–494. [DOI] [PubMed] [Google Scholar]
- 254.Shao H, Im H, Castro CM, et al. New technologies for analysis of extracellular vesicles. Chem Rev 2018;118:1917–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Muthu S, Bapat A, Jain R, et al. Exosomal therapy—a new frontier in regenerative medicine. Stem Cell Investig 2021;8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Rezaie J, Feghhi M, Etemadi T. A review on exosomes application in clinical trials: perspective, questions, and challenges. Cell Comm Signal 2022;20:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Fayazi N, Sheykhhasan M, Soleimani Asl S, et al. Stem cell-derived exosomes: a new strategy of neurodegenerative disease treatment. Mol Neurobiol 2021;58:3494–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Harrell CR, Jovicic N, Djonov V, et al. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells 2019;8:1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Zhang K, Cheng K. Stem cell-derived exosome versus stem cell therapy. Nat RevBioengineer 2023:608–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Zhang X, Liu L. Applications of single cell RNA sequencing to research of stem cells. World J Stem Cells 2019;11:722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Yao F, Zhan Y, Li C, et al. Single-cell rna sequencing reveals the role of phosphorylation-related genes in hepatocellular carcinoma stem cells. Front Cell Dev Biol 2022;9:734287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Mollashahi B, Latifi-Navid H, Owliaee I, et al. Research and therapeutic approaches in stem cell genome editing by CRISPR toolkit. Molecules 2023;28:1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Hussen BM, Rasul MF, Abdullah SR, et al. Targeting miRNA by CRISPR/Cas in cancer: advantages and challenges. Mil Med Res 2023;10:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Kim G, Jeon JH, Park K, et al. High throughput screening of mesenchymal stem cell lines using deep learning. Sci Rep 2022;12:17507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Brooks IR, Garrone CM, Kerins C, et al. Functional genomics and the future of iPSCs in disease modeling. Stem Cell Reports 2022;17:1033–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kantor A, McClements ME, MacLaren RE. CRISPR-Cas9 DNA base-editing and prime-editing. Int J Mol Sci 2020;21:6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Fiumara M, Ferrari S, Omer-Javed A, et al. Genotoxic effects of base and prime editing in human hematopoietic stem cells. Nat Biotechnol 2023;42:877–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Hamazaki T, El Rouby N, Fredette NC, et al. Concise review: induced pluripotent stem cell research in the era of precision medicine. Stem Cells 2017;35:545–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Chandrasekaran AP, Song M, Ramakrishna S. Genome editing: a robust technology for human stem cells. Cell Mol Life Sci 2017;74:3335–3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Sankar A, YS RK, Singh A, et al. Next-generation therapeutics for rare genetic disorders. Mutagenesis 2024;39:157–171; geae002. [DOI] [PubMed] [Google Scholar]
- 271.Liu M, Yang F, Xu Y. Global trends of stem cell precision medicine research (2018–2022): a bibliometric analysis. Front Surg 2022;9:888956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Patsalias A, Kozovska Z. Personalized medicine: stem cells in colorectal cancer treatment. Biomed Pharmacother 2021;141:111821. [DOI] [PubMed] [Google Scholar]
- 273.Basiri A, Mansouri F, Azari A, et al. Stem cell therapy potency in personalizing severe COVID-19 treatment. Stem Cell Rev Rep 2021;17:193–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Li H, Yang Y, Hong W, et al. Applications of genome editing technology in the targeted therapy of human diseases: mechanisms, advances and prospects. Signal Transduct Target Ther 2020;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Li MD, Atkins H, Bubela T. The global landscape of stem cell clinical trials. Regen Med 2014;9:27–39. [DOI] [PubMed] [Google Scholar]
- 276.Trounson A, Thakar RG, Lomax G, et al. Clinical trials for stem cell therapies. BMC Med 2011;9:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Hernández R, Jiménez-Luna C, Perales-Adán J, et al. Differentiation of human mesenchymal stem cells towards neuronal lineage: clinical trials in nervous system disorders. Biomol Ther 2020;28:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kim HJ, Seo SW, Chang JW, et al. Stereotactic brain injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: a phase 1 clinical trial. Alzheimer’s Dement 2015;1:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Kim HJ, Cho KR, Jang H, et al. Intracerebroventricular injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: a phase I clinical trial. Alzheimer’s Res Ther 2021;13:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Liu Z, Cheung H-H. Stem cell-based therapies for Parkinson disease. Int J Mol Sci 2020;21:8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Oh SK, Choi KH, Yoo JY, et al. A phase III clinical trial showing limited efficacy of autologous mesenchymal stem cell therapy for spinal cord injury. Neurosurgery 2016;78:436–447. [DOI] [PubMed] [Google Scholar]
- 282.Vaquero J, Zurita M, Rico MA, et al. An approach to personalized cell therapy in chronic complete paraplegia: the Puerta de Hierro phase I/II clinical trial. Cytotherapy 2016;18:1025–1036. [DOI] [PubMed] [Google Scholar]
- 283.Bartolucci J, Verdugo FJ, González PL, et al. Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: a phase 1/2 randomized controlled trial (RIMECARD trial [randomized clinical trial of intravenous infusion umbilical cord mesenchymal stem cells on cardiopathy]). Circul Res 2017;121:1192–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Noiseux N, Mansour S, Weisel R, et al. The IMPACT-CABG trial: a multicenter, randomized clinical trial of CD133+ stem cell therapy during coronary artery bypass grafting for ischemic cardiomyopathy. J Thorac Cardiovasc Surg 2016;152:1582–8.e2. [DOI] [PubMed] [Google Scholar]
- 285.Brown JR, Chan DK, Shank JJ, et al. Phase II clinical trial of metformin as a cancer stem cell-targeting agent in ovarian cancer. JCI Insight 2020;5:e133247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Steenbruggen TG, Steggink LC, Seynaeve CM, et al. High-dose chemotherapy with hematopoietic stem cell transplant in patients with high-risk breast cancer and 4 or more involved axillary lymph nodes: 20-year follow-up of a phase 3 randomized clinical trial. JAMA Oncol 2020;6:528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Park Y-B, Ha C-W, Lee C-H, et al. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl Med 2017;6:613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Namiot ED, Niemi JVL, Chubarev VN, et al. Stem cells in clinical trials on neurological disorders: trends in stem cells origins, indications, and status of the clinical trials. Int J Mol Sci 2022;23:11453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Bolli R, Tang X-L. Clinical trials of cell therapy for heart failure: recent results warrant continued research. Curr Opin Cardiol 2022;37:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Chivu-Economescu M, Rubach M. Hematopoietic stem cells therapies. Curr Stem Cell Res Ther 2017;12:124–133. [DOI] [PubMed] [Google Scholar]
- 291.Landeros N, Castillo I, Pérez-Castro R. Preclinical and clinical trials of new treatment strategies targeting cancer stem cells in subtypes of breast cancer. Cells 2023;12:720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Lee S, Chae D-S, Song B-W, et al. ADSC-based cell therapies for musculoskeletal disorders: a review of recent clinical trials. Int J Mol Sci 2021;22:10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.De Klerk E, Hebrok M. Stem cell-based clinical trials for diabetes mellitus. Front Endocrinol 2021;12:631463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Dragu DL, Necula LG, Bleotu C, et al. Therapies targeting cancer stem cells: current trends and future challenges. World J Stem Cells 2015;7:1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Abusalah MAH, Chopra H, Sharma A, et al. Nanovaccines: a game changing approach in the fight against infectious diseases. Biomed Pharmacother 2023;167:115597. [DOI] [PubMed] [Google Scholar]
- 296.Apostolou E, Blau H, Chien K, et al. Progress and challenges in stem cell biology. Nat Cell Biol 2023;25:203–206. [DOI] [PubMed] [Google Scholar]
- 297.Choudhary OP, Saini J, Challana A, et al. ChatGPT for veterinary anatomy education: an overview of the prospects and drawbacks. Int J Morphol 2023;41:1198–1202. [Google Scholar]
- 298.Bongso A, Fong CY, Gauthaman K. Taking stem cells to the clinic: major challenges. J Cell Biochem 2008;105:1352–1360. [DOI] [PubMed] [Google Scholar]
- 299.Lukomska B, Stanaszek L, Zuba-Surma E, et al. Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells Int 2019;2019:9628536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Chopra H, Choudhary OP. mRNA vaccines as an armor to combat the infectious diseases. Travel Med Infect Dis 2023;52:102550. [DOI] [PubMed] [Google Scholar]
- 301.Ghosh D, Mehta N, Patil A, et al. Ethical issues in biomedical use of human embryonic stem cells (hESCs). J Reproduct Health Med 2016;2:S37–S47. [Google Scholar]
- 302.King NM, Perrin J. Ethical issues in stem cell research and therapy. Stem Cell Res Ther 2014;5:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Harris AR, Walker MJ, Gilbert F. Ethical and regulatory issues of stem cell-derived 3-dimensional organoid and tissue therapy for personalised regenerative medicine. BMC Med 2022;20:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Assen LS, Jongsma KR, Isasi R, et al. Recognizing the ethical implications of stem cell research: a call for broadening the scope. Stem Cell Reports 2021;16:1656–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data are available in the manuscript.