Abstract
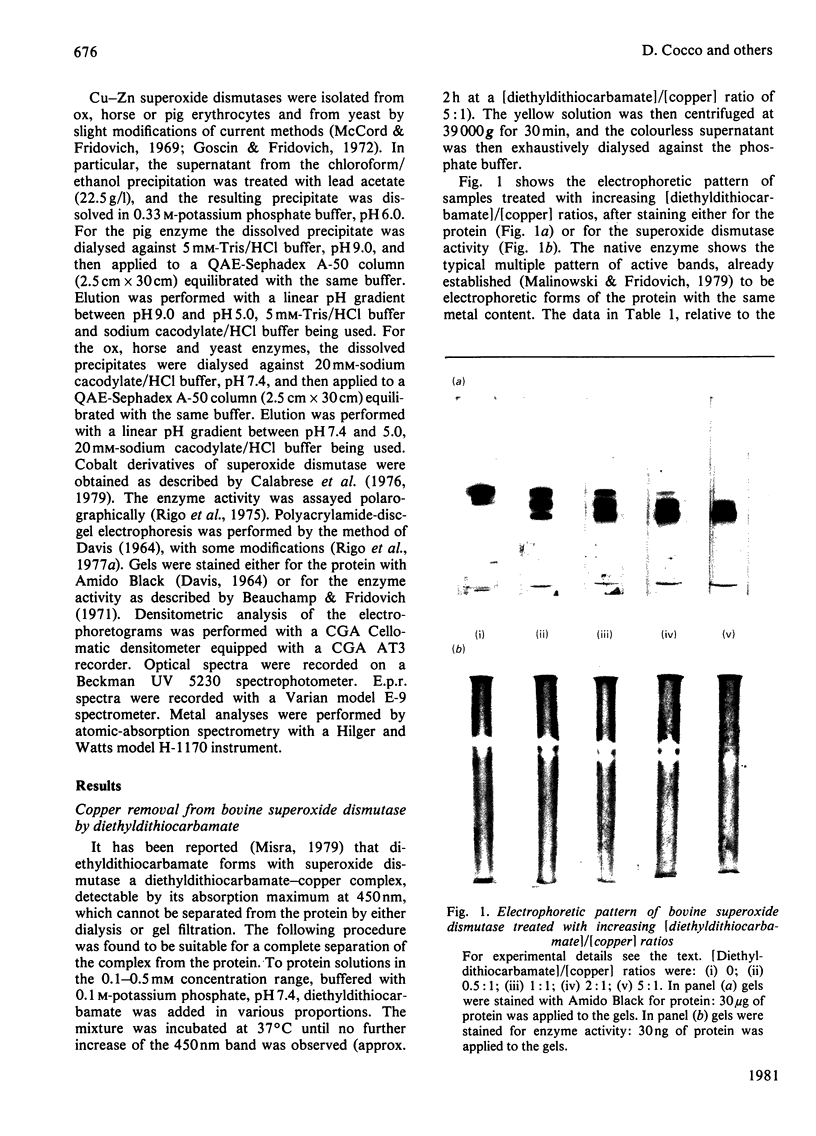
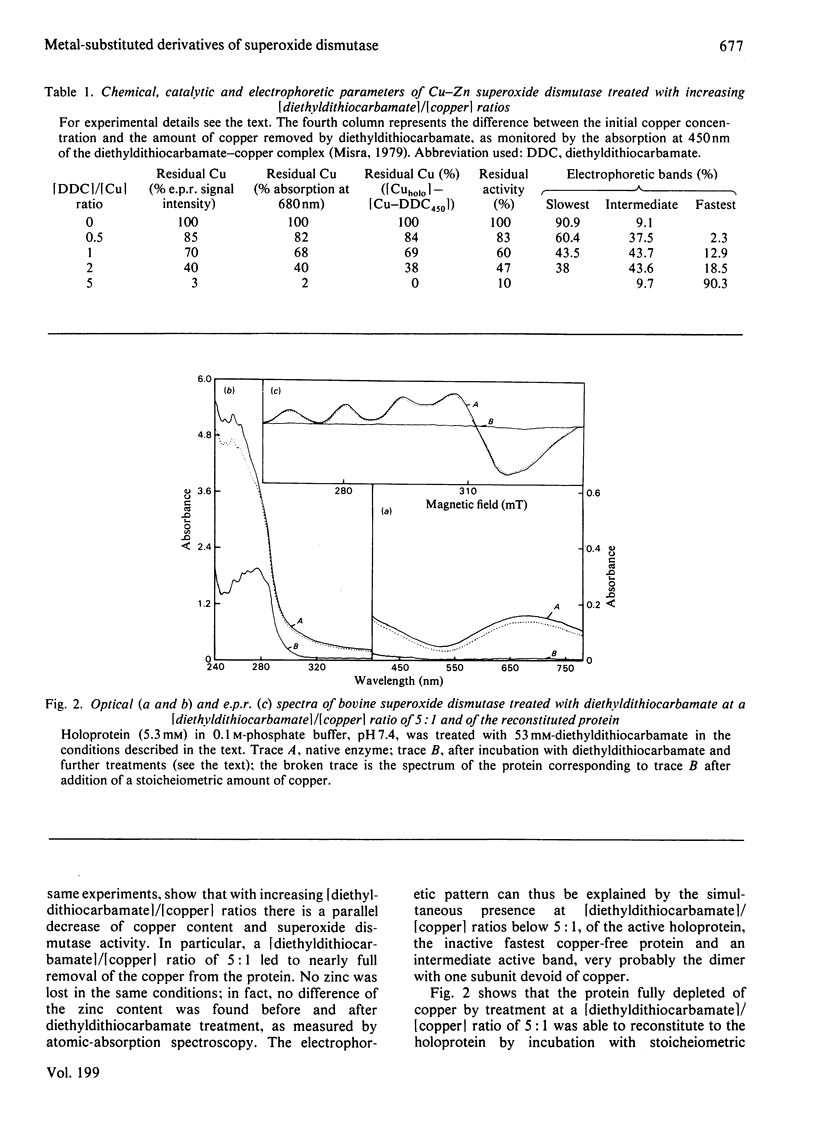
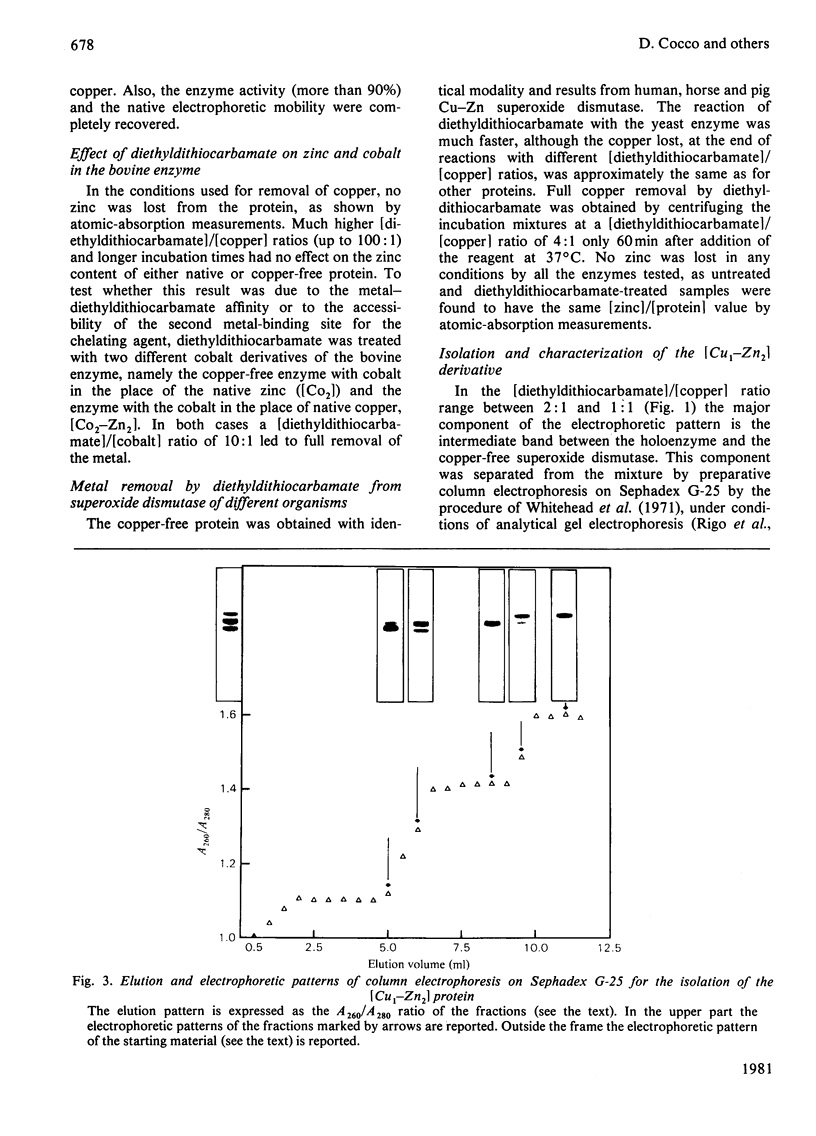
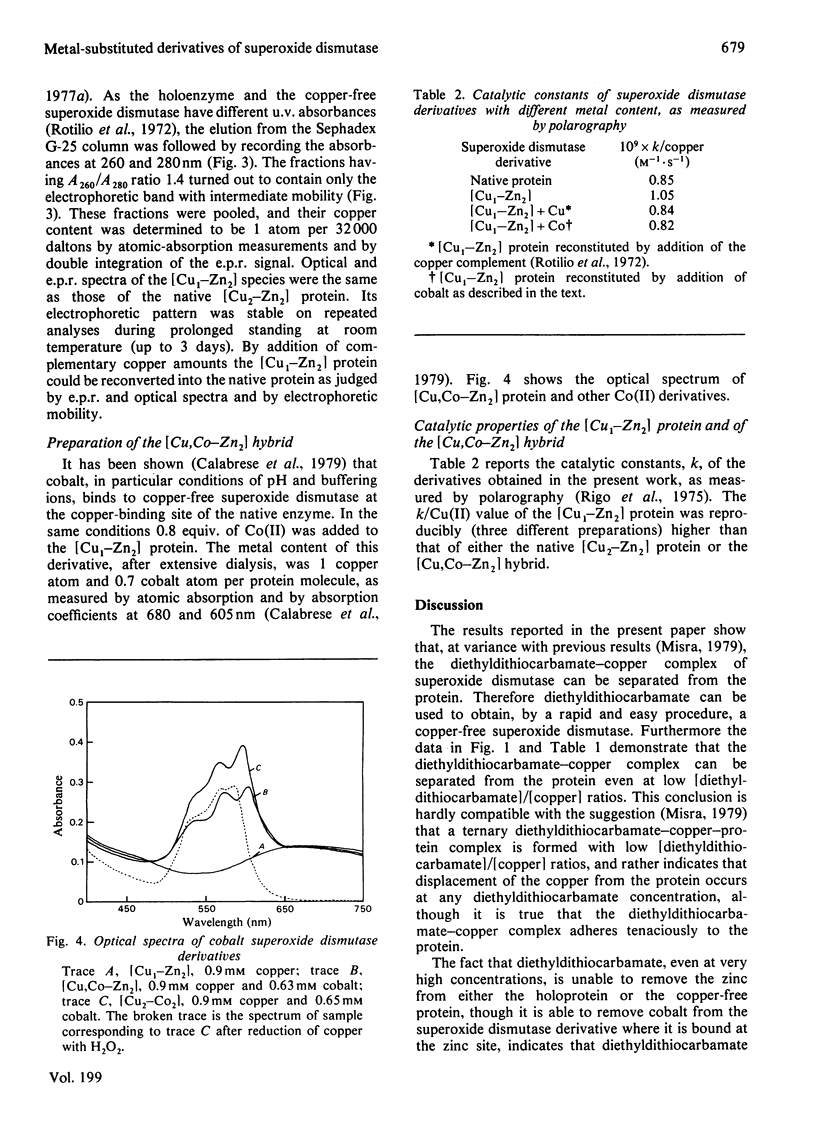
Incubation of Cu--Zn superoxide dismutase with diethyldithiocarbamate at increasing ligand/protein ratios and subsequent high-speed centrifugation led to proportional removal of copper from the protein, at variance with previous results [Misra (1979) J. Biol. Chem. 254, 11623--11628]. No zinc was lost, even at very high excesses of chelating agent. In this way a copper-free protein could be readily prepared, with avoidance of the critical pH condition and the dialysis step required in a previous method employing cyanide. The holoprotein was fully reconstituted from the copper-free protein by stoicheiometric re-addition of copper. From the mixture of metal-depleted forms originated by treatment with slight diethyldithiocarbamate excess, the protein containing copper only on one subunit, [Cu1--Zn2], could be isolated by preparative column electrophoresis. This species reproducibly showed 25% more specific activity (catalytic constant per copper) than that of the native or reconstituted [Cu2--Zn2] protein. This may result from long-range conformational effects between the active sites. By adding Co2+ ions to the vacant copper site of [Cu1--Zn2] a hybrid molecule containing Cu(II) on one subunit and Co(II) in the homologous site of the other subunit was prepared. Its activity, referred to copper, was identical with that of the native protein.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Beem K. M., Rich W. E., Rajagopalan K. V. Total reconstitution of copper-zinc superoxide dismutase. J Biol Chem. 1974 Nov 25;249(22):7298–7305. [PubMed] [Google Scholar]
- Calabrese L., Cocco D., Desideri A. A novel Co(II) binding site in copper-free superoxide dismutase: evidence for binding of cobalt at the copper binding site. FEBS Lett. 1979 Oct 1;106(1):142–144. doi: 10.1016/0014-5793(79)80713-9. [DOI] [PubMed] [Google Scholar]
- Calabrese L., Cocco D., Morpurgo L., Mondovì B., Rotilio G. Cobalt bovine superoxide dismutase. Reactivity of the cobalt chromophore in the copper-containing and in the copper-free enzyme. Eur J Biochem. 1976 May 1;64(2):465–470. doi: 10.1111/j.1432-1033.1976.tb10324.x. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Fielden E. M., Roberts P. B., Bray R. C., Lowe D. J., Mautner G. N., Rotilio G., Calabrese L. Mechanism of action of superoxide dismutase from pulse radiolysis and electron paramagnetic resonance. Evidence that only half the active sites function in catalysis. Biochem J. 1974 Apr;139(1):49–60. doi: 10.1042/bj1390049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goscin S. A., Fridovich I. The purification and properties of superoxide dismutase from Saccharomyces cerevisiae. Biochim Biophys Acta. 1972 Dec 7;289(2):276–283. doi: 10.1016/0005-2744(72)90078-2. [DOI] [PubMed] [Google Scholar]
- Malinowski D. P., Fridovich I. Bovine erythrocyte superoxide dismutase: diazo coupling, subunit interactions, and electrophoretic variants. Biochemistry. 1979 Jan 9;18(1):237–244. doi: 10.1021/bi00568a037. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Misra H. P. Reaction of copper-zinc superoxide dismutase with diethyldithiocarbamate. J Biol Chem. 1979 Nov 25;254(22):11623–11628. [PubMed] [Google Scholar]
- Rigo A., Terenzi M., Viglino P., Calabrese L., Cocco D., Rotilio G. The binding of copper ions to copper-free bovine superoxide dismutase. Properties of the protein recombined with increasing amounts of copper ions. Biochem J. 1977 Jan 1;161(1):31–35. doi: 10.1042/bj1610031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigo A., Viglino P., Calabrese L., Cocco D., Rotilio G. The binding of copper ions to copper-free bovine superoxide dismutase. Copper distribution in protein samples recombined with less than stoicheiometric copper ion/protein ratios. Biochem J. 1977 Jan 1;161(1):27–30. doi: 10.1042/bj1610027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigo A., Viglino P., Rotilio G. Polarographic determination of superoxide dismutase. Anal Biochem. 1975 Sep;68(1):1–8. doi: 10.1016/0003-2697(75)90672-7. [DOI] [PubMed] [Google Scholar]
- Rotilio G., Calabrese L., Bossa F., Barra D., Agrò A. F., Mondovì B. Properties of the apoprotein and role of copper and zinc in protein conformation and enzyme activity of bovine superoxide dismutase. Biochemistry. 1972 May 23;11(11):2182–2187. doi: 10.1021/bi00761a027. [DOI] [PubMed] [Google Scholar]
- Whitehead J. S., Kay E., Lew J. Y., Shannon L. M. A preparative column electrophoresis apparatus using Sephadex G-25. Anal Biochem. 1971 Apr;40(2):287–291. doi: 10.1016/0003-2697(71)90387-3. [DOI] [PubMed] [Google Scholar]