Abstract
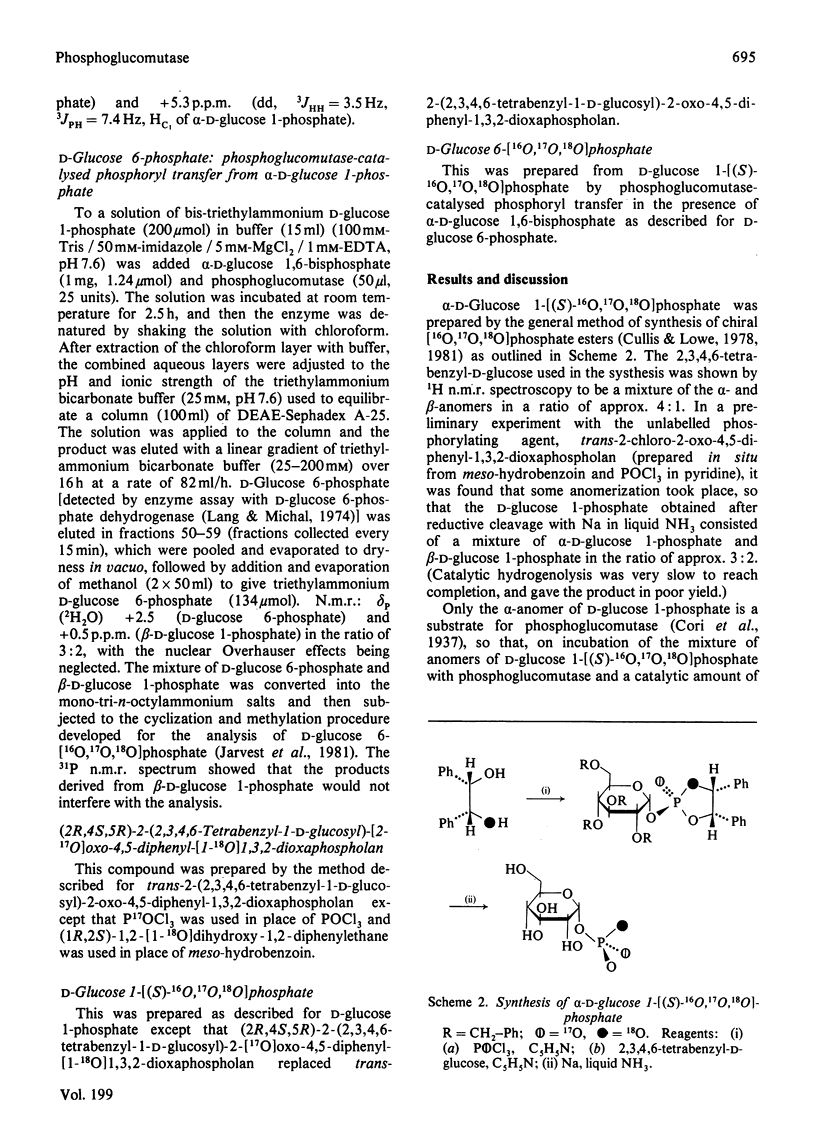
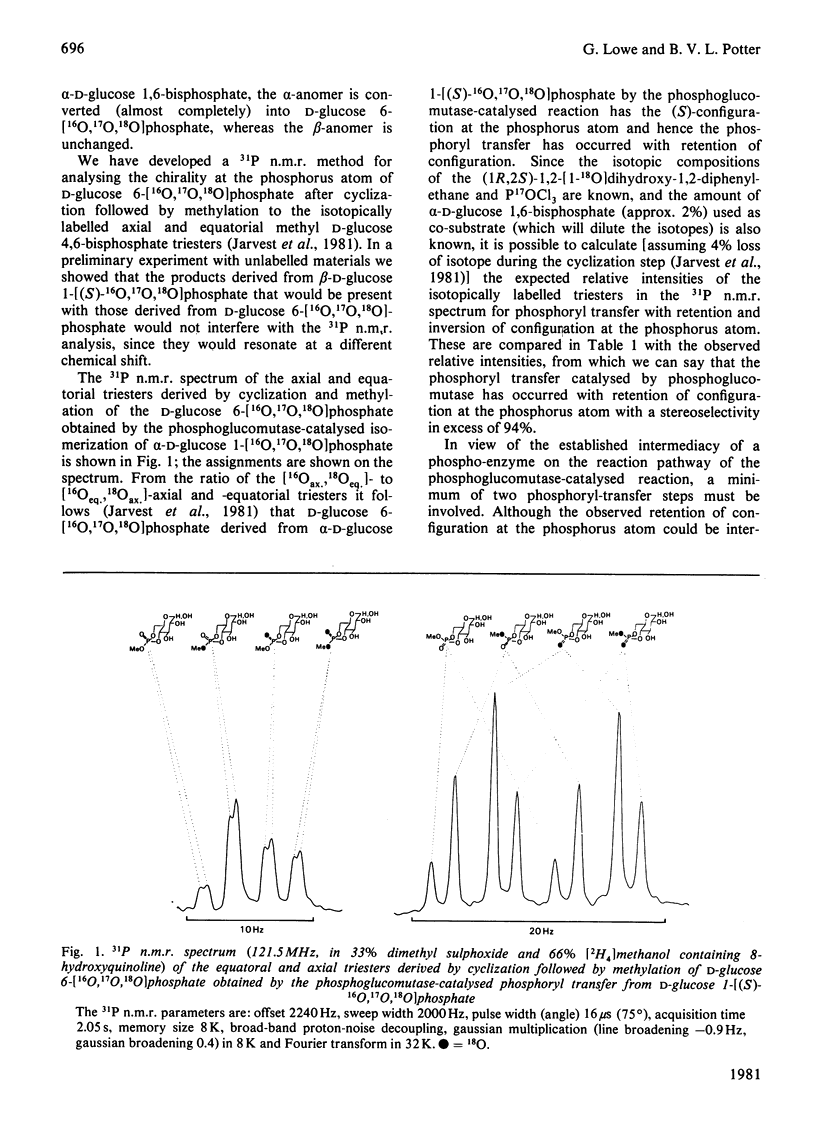
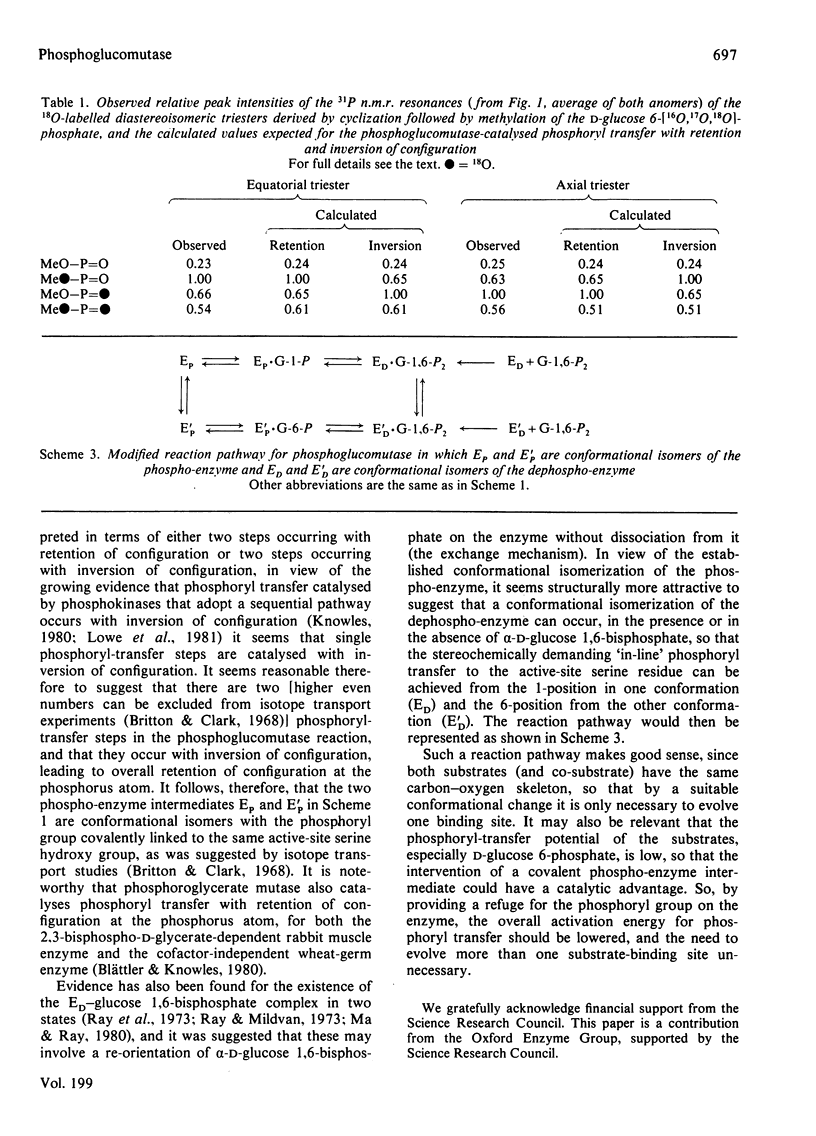
Rabbit muscle phosphoglucomutase converts alpha-D-glucose 1-[(S)-16O,17O,18O]phosphate into D-glucose 6-[16O,17O,18O]phosphate, which is shown by 31P n.m.r. spectroscopy, after cyclization and methylation, to have the (S)-configuration at the phosphorus atom. Since phosphoglucomutase is known to catalyse phosphoryl transfer by way of a phospho-enzyme intermediate, and since individual phosphoryl-transfer steps appear in general to occur with inversion of configuration, this observation is most simply interpreted in terms of a double-displacement mechanism with two phosphoryl-transfer steps.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINSON M. R., JOHNSON E., MORTON R. K. Equilibrium constants of phosphoryl transfer from C-1 to C-6 of alpha-D-glucose 1-phosphate and from glucose 6-phosphate to water. Biochem J. 1961 Apr;79:12–15. doi: 10.1042/bj0790012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blättler W. A., Knowles J. R. Phosphoglycerate mutases: stereochemical course of the phosphoryl group transfers catalyzed by the cofactor-dependent enzyme from rabbit muscle and the cofactor-independent enzyme from wheat germ. Biochemistry. 1980 Feb 19;19(4):738–743. doi: 10.1021/bi00545a020. [DOI] [PubMed] [Google Scholar]
- Britton H. G., Clarke J. B. The mechanism of the phosphoglucomutase reaction. Studies on rabbit muscle phosphoglucomutase with flux techniques. Biochem J. 1968 Nov;110(2):161–180. doi: 10.1042/bj1100161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles J. R. Enzyme-catalyzed phosphoryl transfer reactions. Annu Rev Biochem. 1980;49:877–919. doi: 10.1146/annurev.bi.49.070180.004305. [DOI] [PubMed] [Google Scholar]
- Lowe G., Cullis P. M., Jarvest R. L., Potter B. V., Sproat B. S. Stereochemistry of phosphoryl transfer. Philos Trans R Soc Lond B Biol Sci. 1981 Jun 26;293(1063):75–92. doi: 10.1098/rstb.1981.0062. [DOI] [PubMed] [Google Scholar]
- MILSTEIN C., SANGER F. An amino acid sequence in the active centre of phosphoglucomutase. Biochem J. 1961 Jun;79:456–469. doi: 10.1042/bj0790456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Ray W. J., Jr Structural comparisons among the central complexes in the phosphoglucomutase system by means of spectral techniques. Biochemistry. 1980 Feb 19;19(4):751–759. doi: 10.1021/bi00545a022. [DOI] [PubMed] [Google Scholar]
- NAJJAR V. A., PULLMAN M. E. The occurrence of a group transfer involving enzyme (phosphoglucomutase) and substrate. Science. 1954 May 7;119(3097):631–634. doi: 10.1126/science.119.3097.631. [DOI] [PubMed] [Google Scholar]
- RAY W. J., Jr, ROSCELLI G. A. A KINETIC STUDY OF THE PHOSPHOGLUCOMUTASE PATHWAY. J Biol Chem. 1964 Apr;239:1228–1236. [PubMed] [Google Scholar]
- Ray W. J., Jr, Mildvan A. S. Arrangement of the phosphate-and metal-binding subsites of phosphoglucomutase. Intersubsite distance by means of nuclear magnetic resonance measurements. Biochemistry. 1973 Sep 11;12(19):3733–3743. doi: 10.1021/bi00743a024. [DOI] [PubMed] [Google Scholar]
- Ray W. J., Jr, Mildvan A. S., Long J. W. Arrangement of the phosphate-and metal-binding subsites of phosphoglucomutase. Intersubsite relationships by means of inhibition patterns. Biochemistry. 1973 Sep 11;12(19):3724–3732. doi: 10.1021/bi00743a023. [DOI] [PubMed] [Google Scholar]


