Abstract
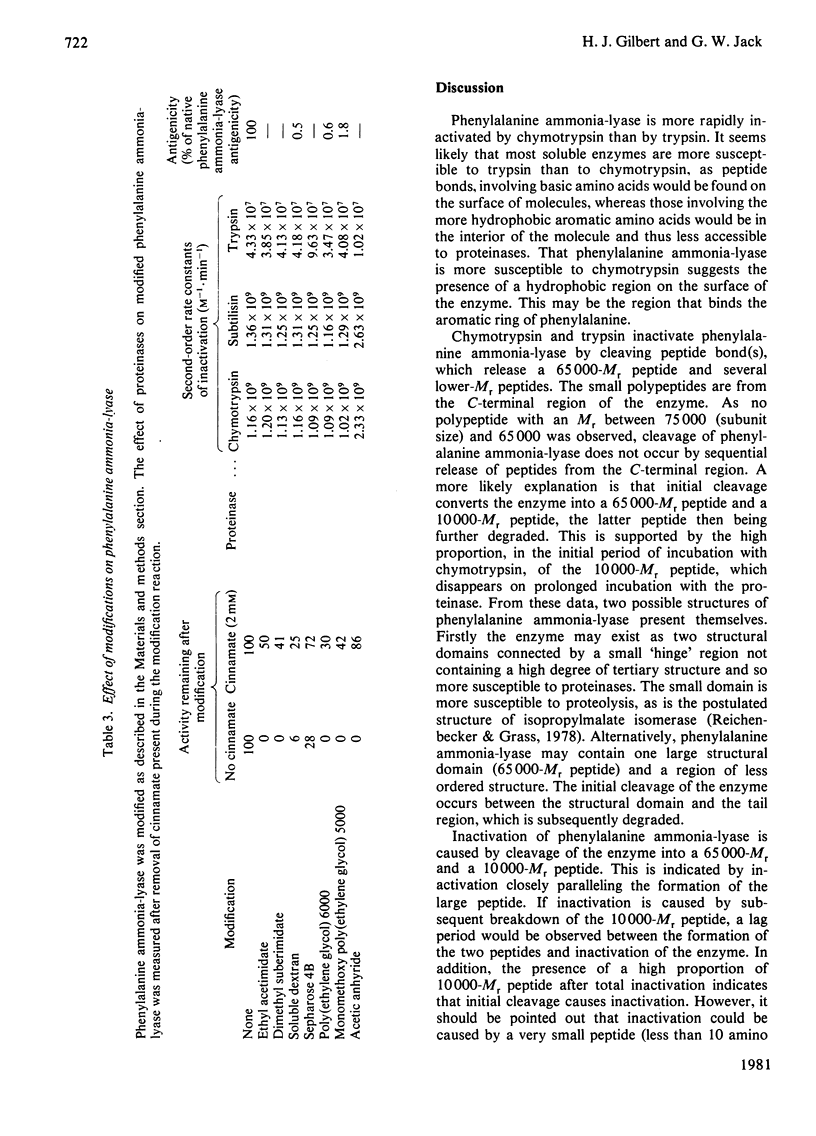
Phenylalanine ammonia-lyase (EC 4.3.1.5) of the yeast Rhodotorula glutinis was rapidly inactivated by duodenal juice. It was susceptible to chymotrypsin and subtilisin and to a lesser extent trypsin. Initial proteolysis of the enzyme by chymotrypsin and trypsin resulted in cleavage of the monomeric subunit (75 000 Mr) into a large (65 000 Mr) and a small (10 000 Mr) peptide. The small peptide was rapidly degraded. The 65 000-Mr fragment was resistant to prolonged incubation with chymotrypsin, but was degraded by trypsin under the same conditions. Phenylalanine ammonia-lyase was cleaved into several polypeptides by subtilisin, the 65 000-Mr peptide being totally absent. The N-terminal region of the enzyme was contained in the 65 000-Mr fragment, as was the dehydroalanine moiety, the prosthetic group. Active-site-binding ligands protect the enzyme from inactivation by the three proteinases, and peptide-bond cleavage by trypsin and chymotrypsin. Several chemical modifications were performed on phenylalanine ammonia-lyase. Some decreased its antigenicity, and ethyl acetimidate decreased the rate of degradation of the 65 000-Mr peptide by trypsin. The modification did not protect the enzyme from proteolytic inactivation of the enzymic activity. These observations are discussed in terms of the structure of phenylalanine ammonia-lyase and site of action of the proteinases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abuchowski A., van Es T., Palczuk N. C., Davis F. F. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J Biol Chem. 1977 Jun 10;252(11):3578–3581. [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloxham D. P., Ericsson L. H., Titani K., Walsh K. A., Neurath H. Limited proteolysis of pig heart citrate synthase by subtilisin, chymotrypsin, and trypsin. Biochemistry. 1980 Aug 19;19(17):3979–3985. doi: 10.1021/bi00558a014. [DOI] [PubMed] [Google Scholar]
- Bond J. S. A comparison of the proteolytic susceptibility of several rat liver enzymes. Biochem Biophys Res Commun. 1971 Apr 16;43(2):333–339. doi: 10.1016/0006-291x(71)90757-1. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Emes A. V., Vining L. C. Partial purification and properties of L-phenylalanine ammonia-lyase from Streptomyces verticillatus. Can J Biochem. 1970 May;48(5):613–622. doi: 10.1139/o70-099. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gilbert H. J., Lowe C. R., Drabble W. T. Inosine 5'-monophosphate dehydrogenase of Escherichia coli. Purification by affinity chromatography, subunit structure and inhibition by guanosine 5'-monophosphate. Biochem J. 1979 Dec 1;183(3):481–494. doi: 10.1042/bj1830481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeeb A. F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966 Mar;14(3):328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- Hodgins D. S. The presence of a carbonyl group at the active site of L-phenylalanine ammonia-lyase. Biochem Biophys Res Commun. 1968 Jul 26;32(2):246–253. doi: 10.1016/0006-291x(68)90376-8. [DOI] [PubMed] [Google Scholar]
- Hoskins J. A., Jack G., Wade H. E., Peiris R. J., Wright E. C., Starr D. J., Stern J. Enzymatic control of phenylalanine intake in phenylketonuria. Lancet. 1980 Feb 23;1(8165):392–394. doi: 10.1016/s0140-6736(80)90944-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marshall J. J., Rabinowitz M. L. Preparation and characterization of a dextran-trypsin conjugate. J Biol Chem. 1976 Feb 25;251(4):1081–1087. [PubMed] [Google Scholar]
- Wade H. E., Phillips B. P. Automated determination of bacterial asparaginase and glutaminase. Anal Biochem. 1971 Nov;44(1):189–199. doi: 10.1016/0003-2697(71)90360-5. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weiner A. M., Platt T., Weber K. Amino-terminal sequence analysis of proteins purified on a nanomole scale by gel electrophoresis. J Biol Chem. 1972 May 25;247(10):3242–3251. [PubMed] [Google Scholar]


