Abstract
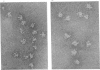
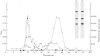
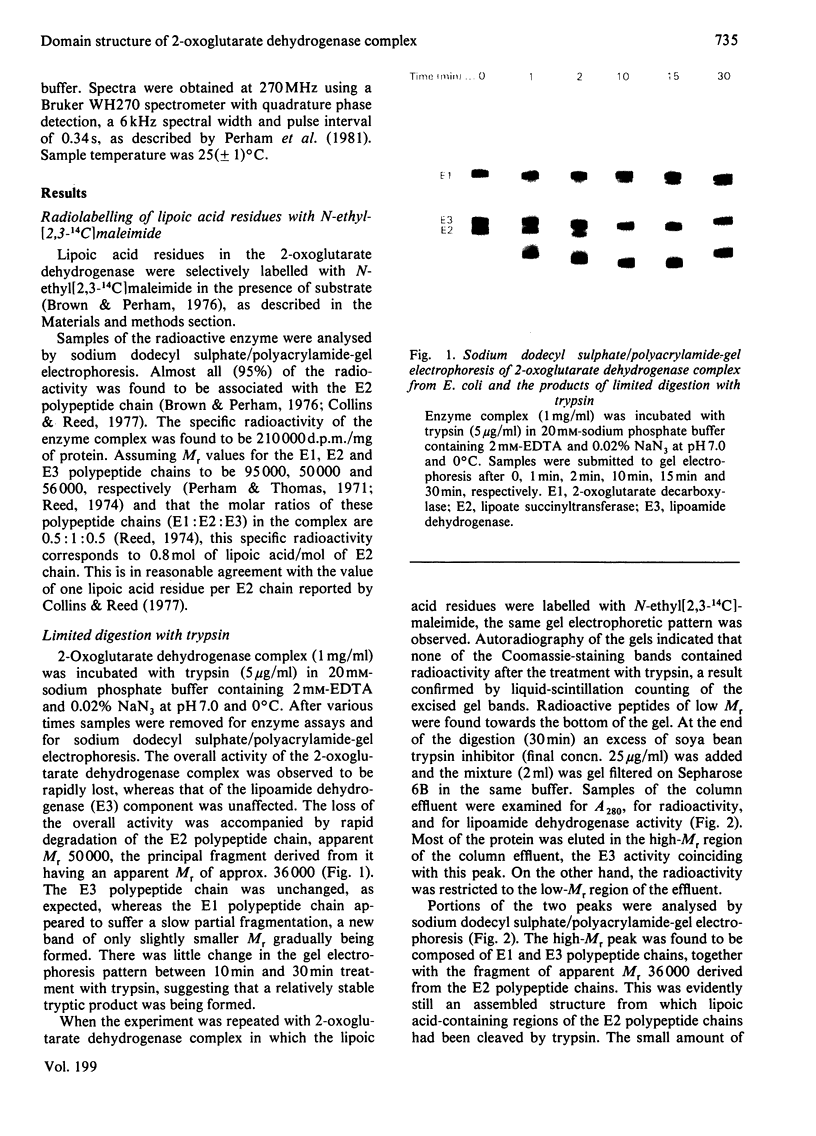
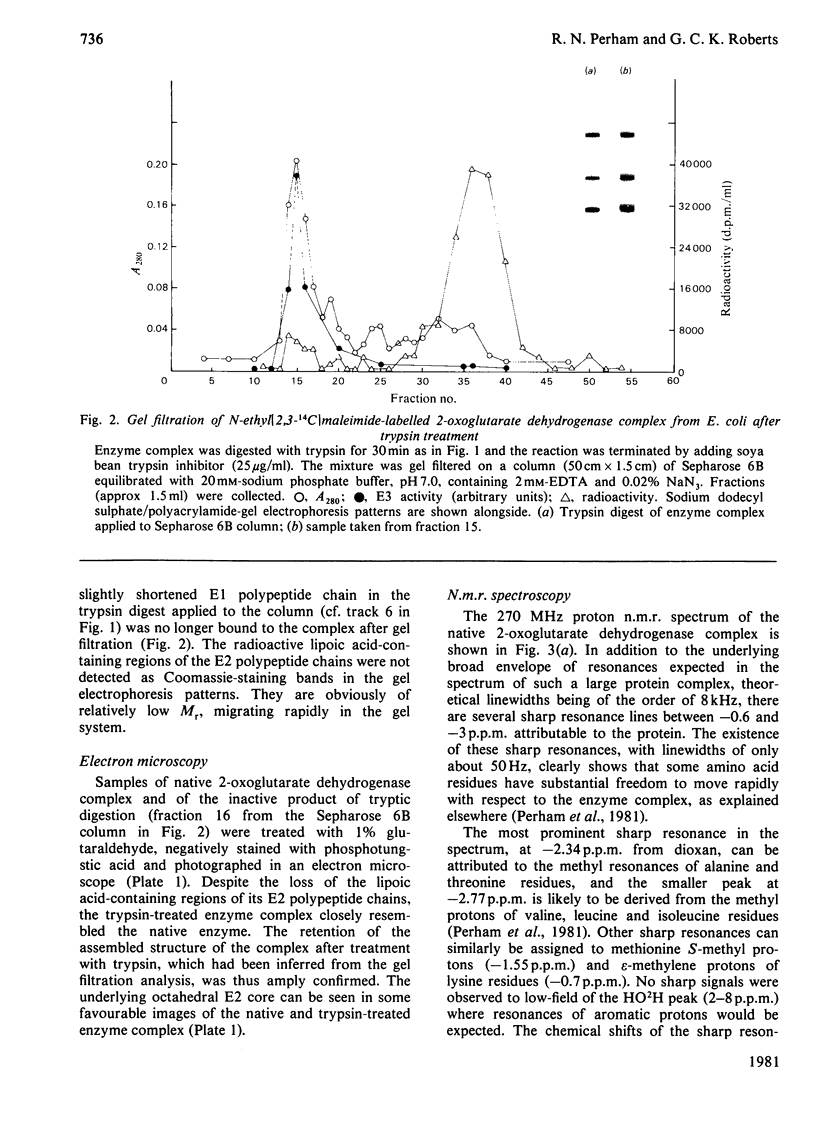
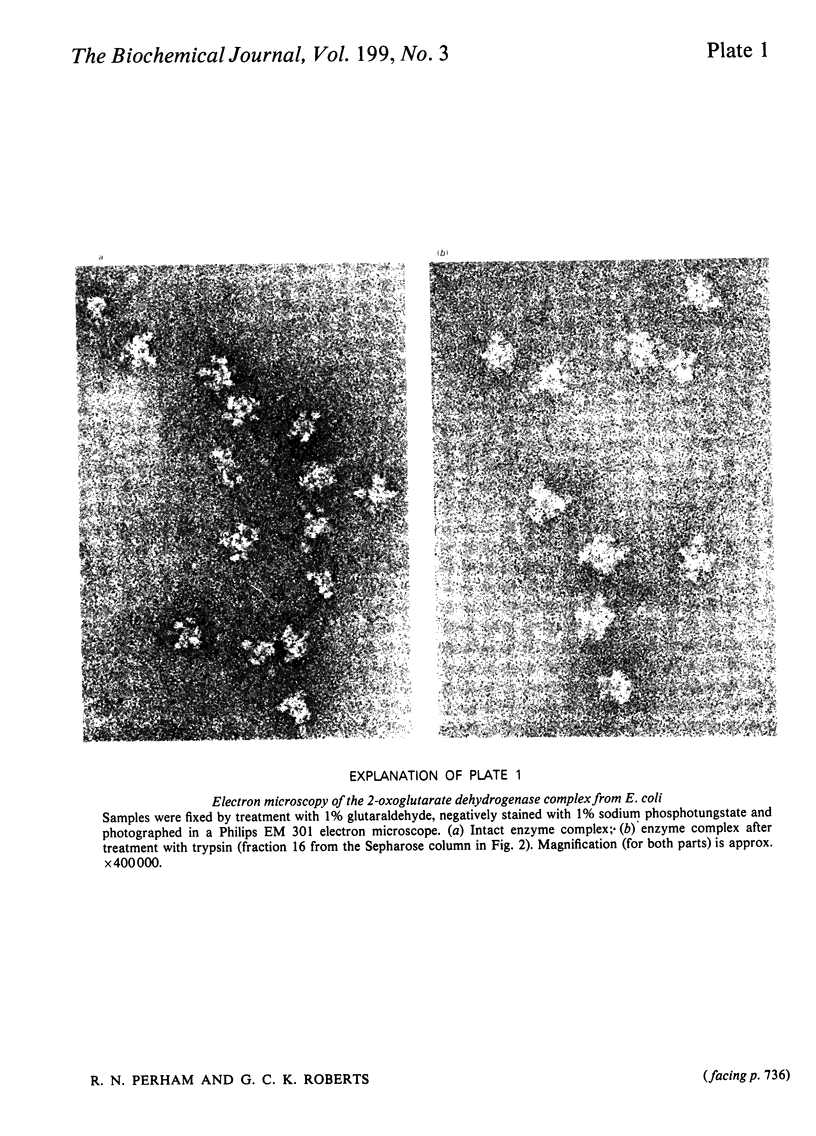
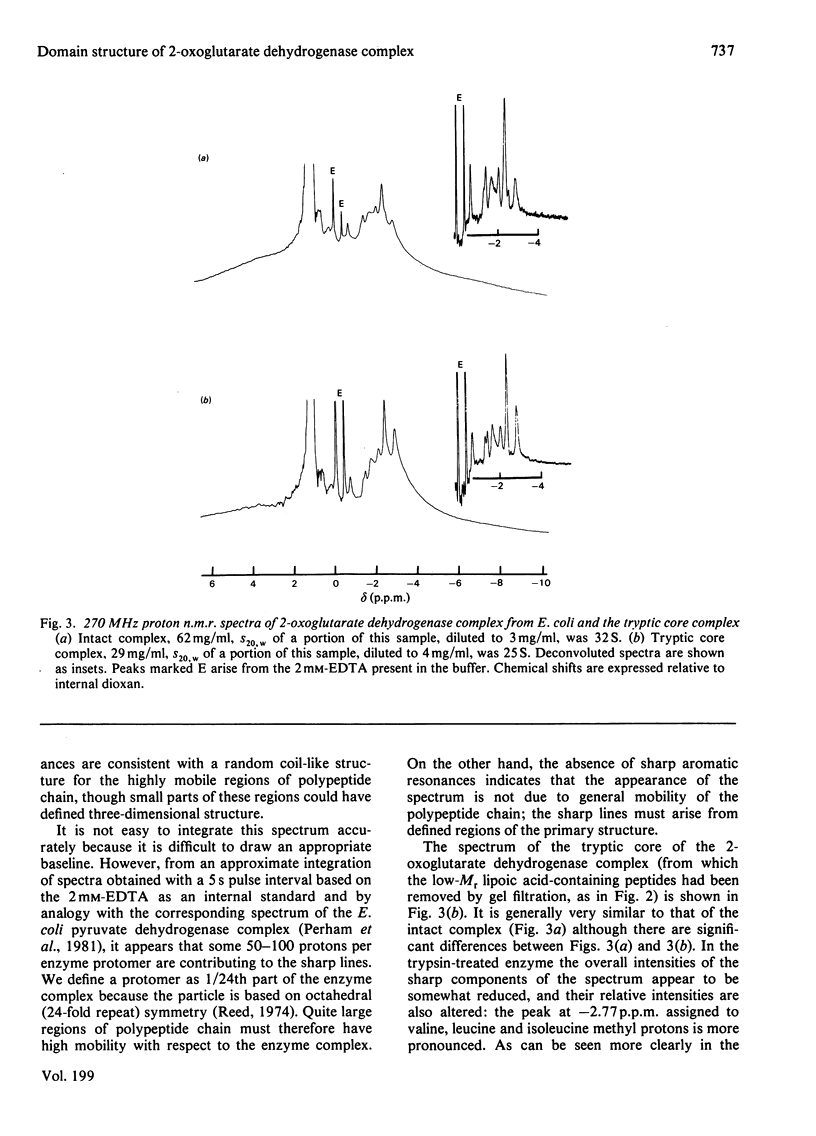
The 2-oxoglutarate dehydrogenase multienzyme complex of Escherichia coli was treated with trypsin at pH 7.0 at 0 degrees C. Loss of the overall catalytic activity was accompanied by rapid cleavage of the lipoate succinyltransferase polypeptide chains, this apparent Mr falling from 50 000 to 36 000 as judged by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. A slower shortening of the 2-oxoglutarate decarboxylase chains was also observed, whereas the lipoamide dehydrogenase chains were unaffected. The inactive trypsin-treated enzyme had lost the lipoic acid-containing regions of the lipoate succinyltransferase polypeptide chains, yet remained a highly assembled structure, as judged by gel filtration and electron microscopy. The lipoic acid-containing regions are therefore likely to be physically exposed in the complex, protruding from the structural core formed by the lipoate succinyltransferase component between the subunits of the other component enzymes. Proton nuclear magnetic resonance spectroscopy of the 2-oxoglutarate dehydrogenase complex revealed the existence of substantial regions of polypeptide chain with remarkable intramolecular mobility, most of which were retained after removal of the lipoic acid-containing regions by treatment of the complex with trypsin. By analogy with the comparably mobile regions of the pyruvate dehydrogenase complex of E. coli, it is likely that the highly mobile regions of polypeptide chain in the 2-oxoglutarate complex are in the lipoate succinyltransferase component and encompass the lipoyl-lysine residues. It is clear, however, that the mobility of this polypeptide chain is not restricted to the immediate vicinity of these residues.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrose-Griffin M. C., Danson M. J., Griffin W. G., Hale G., Perham R. N. Kinetic analysis of the role of lipoic acid residues in the pyruvate dehydrogenase multienzyme complex of Escherichia coli. Biochem J. 1980 May 1;187(2):393–401. doi: 10.1042/bj1870393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelides K. J., Hammes G. G. Structural and mechanistic studies of the alpha-ketoglutarate dehydrogenase multienzyme complex from Escherichia coli. Biochemistry. 1979 Dec 11;18(25):5531–5537. doi: 10.1021/bi00592a001. [DOI] [PubMed] [Google Scholar]
- Bates D. L., Danson M. J., Hale G., Hooper E. A., Perham R. N. Self-assembly and catalytic activity of the pyruvate dehydrogenase multienzyme complex of Escherichia coli. Nature. 1977 Jul 28;268(5618):313–316. doi: 10.1038/268313a0. [DOI] [PubMed] [Google Scholar]
- Bates D. L., Harrison R. A., Perham R. N. The stoichiometry of polypeptide chains in the pyruvate dehydrogenase multienzyme complex of E. coli determined by a simple novel method. FEBS Lett. 1975 Dec 15;60(2):427–430. doi: 10.1016/0014-5793(75)80764-2. [DOI] [PubMed] [Google Scholar]
- Berman J. N., Chen G. X., Hale G., Perham R. N. Lipoic acid residues in a take-over mechanism for the pyruvate dehydrogenase multienzyme complex of Escherichia coli. Biochem J. 1981 Dec 1;199(3):513–520. doi: 10.1042/bj1990513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleile D. M., Hackert M. L., Pettit F. H., Reed L. J. Subunit structure of dihydrolipoyl transacetylase component of pyruvate dehydrogenase complex from bovine heart. J Biol Chem. 1981 Jan 10;256(1):514–519. [PubMed] [Google Scholar]
- Bleile D. M., Munk P., Oliver R. M., Reed L. J. Subunit structure of dihydrolipoyl transacetylase component of pyruvate dehydrogenase complex from Escherichia coli. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4385–4389. doi: 10.1073/pnas.76.9.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. P., Perham R. N. Selective inactivation of the transacylase components of the 2-oxo acid dehydrogenase multienzyme complexes of Escherichia coli. Biochem J. 1976 May 1;155(2):419–427. doi: 10.1042/bj1550419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cate R. L., Roche T. E. Function and regulation of mammalian pyruvate dehydrogenase complex. Acetylation, interlipoyl acetyl transfer, and migration of the pyruvate dehydrogenase component. J Biol Chem. 1979 Mar 10;254(5):1659–1665. [PubMed] [Google Scholar]
- Collins J. H., Reed L. J. Acyl group and electron pair relay system: a network of interacting lipoyl moieties in the pyruvate and alpha-ketoglutarate dehydrogenase complexes from Escherichia coli. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4223–4227. doi: 10.1073/pnas.74.10.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danson M. J., Fersht A. R., Perham R. N. Rapid intramolecular coupling of active sites in the pyruvate dehydrogenase complex of Escherichia coli: mechanism for rate enhancement in a multimeric structure. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5386–5390. doi: 10.1073/pnas.75.11.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danson M. J., Perham R. N. Evidence for two lipoic acid residues per lipoate acetyltransferase chain in the pyruvate dehydrogenase multienzyme complex of Escherichia coli. Biochem J. 1976 Dec 1;159(3):677–682. doi: 10.1042/bj1590677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRosier D. J., Oliver R. M. A low resolution electron-density map of lipoyl transsuccinylase, the core of the -ketoglutarate dehydrogenase complex. Cold Spring Harb Symp Quant Biol. 1972;36:199–203. doi: 10.1101/sqb.1972.036.01.027. [DOI] [PubMed] [Google Scholar]
- Hale G., Perham R. N. Amino acid sequence around lipoic acid residues in the pyruvate dehydrogenase multienzyme complex of Escherichia coli. Biochem J. 1980 Jun 1;187(3):905–908. doi: 10.1042/bj1870905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale G., Perham R. N. Limited proteolysis of the pyruvate dehydrogenase multienzyme complex of Escherichia coli. Eur J Biochem. 1979 Feb 15;94(1):119–126. doi: 10.1111/j.1432-1033.1979.tb12878.x. [DOI] [PubMed] [Google Scholar]
- Hale G., Perham R. N. Primary structure of the swinging arms of the pyruvate dehydrogenase complex of Escherichia coli. FEBS Lett. 1979 Sep 15;105(2):263–266. doi: 10.1016/0014-5793(79)80625-0. [DOI] [PubMed] [Google Scholar]
- Henderson C. E., Perham R. N., Finch J. T. Structure and symmetry of B. stearothermophilus pyruvate dehydrogenase multienzyme complex and implications for eucaryote evolution. Cell. 1979 May;17(1):85–93. doi: 10.1016/0092-8674(79)90297-6. [DOI] [PubMed] [Google Scholar]
- Henderson C. E., Perham R. N. Purificaton of the pyruvate dehydrogenase multienzyme complex of Bacillus stearothermophilus and resolution of its four component polypeptides. Biochem J. 1980 Jul 1;189(1):161–172. doi: 10.1042/bj1890161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kresze G. B., Ronft H. Bovine kidney pyruvate dehydrogenase complex. Limited proteolysis and molecular structure of the lipoate acetyltransferase component. Eur J Biochem. 1980 Dec;112(3):589–599. [PubMed] [Google Scholar]
- Kresze G. B., Ronft H., Dietl B., Steber L. Limited proteolysis of 2-oxoglutarate dehydrogenase multienzyme complex from bovine kidney. FEBS Lett. 1981 May 5;127(1):157–160. doi: 10.1016/0014-5793(81)80364-x. [DOI] [PubMed] [Google Scholar]
- Perham R. N., Duckworth H. W., Roberts G. C. Mobility of polypeptide chain in the pyruvate dehydrogenase complex revealed by proton NMR. Nature. 1981 Jul 30;292(5822):474–477. doi: 10.1038/292474a0. [DOI] [PubMed] [Google Scholar]
- Perham R. N., Thomas J. O. The subunit molecular weights of the alpha-ketoacid dehydrogenase multienzyme complexes from E. coli. FEBS Lett. 1971 Jun 2;15(1):8–12. doi: 10.1016/0014-5793(71)80066-2. [DOI] [PubMed] [Google Scholar]
- REED L. J., KOIKE M., LEVITCH M. E., LEACH F. R. Studies on the nature and reactions of protein-bound lipoic acid. J Biol Chem. 1958 May;232(1):143–158. [PubMed] [Google Scholar]
- Stanley C. J., Packman L. C., Danson M. J., Henderson C. E., Perham R. N. Intramolecular coupling of active sites in the pyruvate dehydrogenase multienzyme complexes from bacterial and mammalian sources. Biochem J. 1981 Jun 1;195(3):715–721. doi: 10.1042/bj1950715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrzynczak E. J., Perham R. N., Roberts G. C. Conformational mobility of polypeptide chains in the 2-oxo acid dehydrogenase complexes from ox heart revealed by proton NMR spectroscopy. FEBS Lett. 1981 Aug 17;131(1):151–154. doi: 10.1016/0014-5793(81)80908-8. [DOI] [PubMed] [Google Scholar]