Abstract
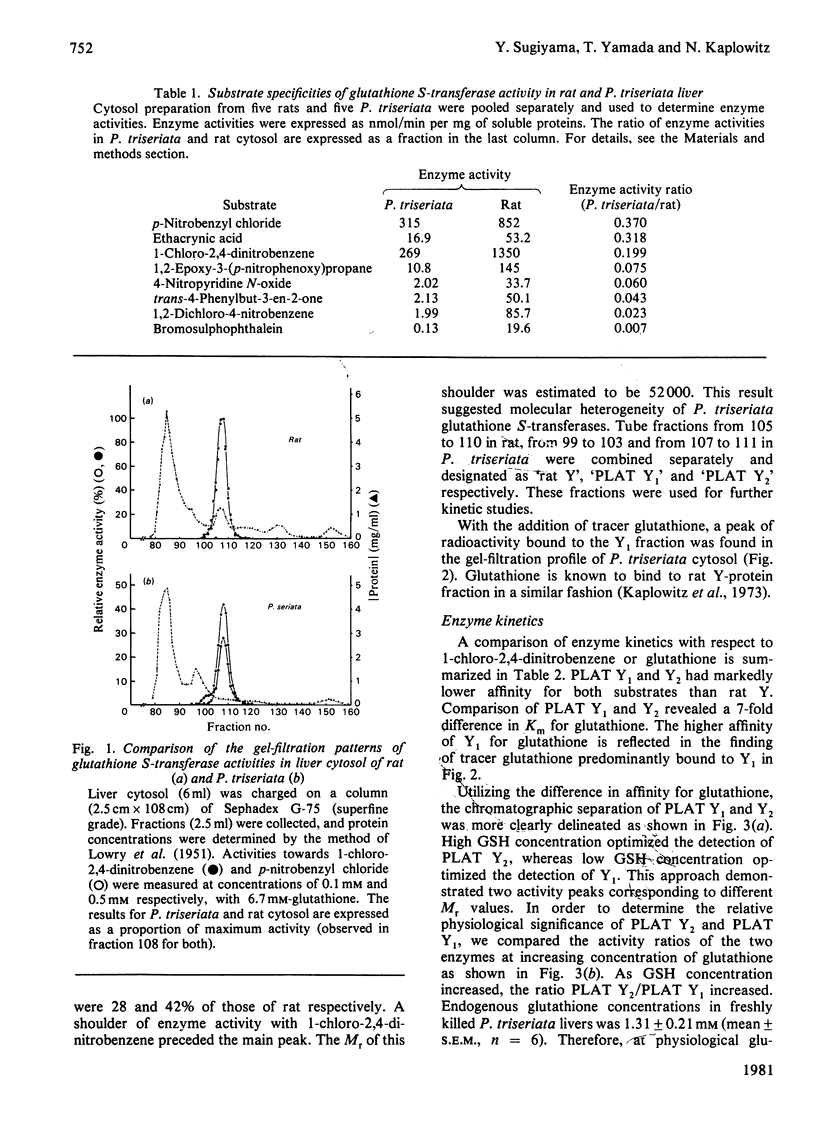
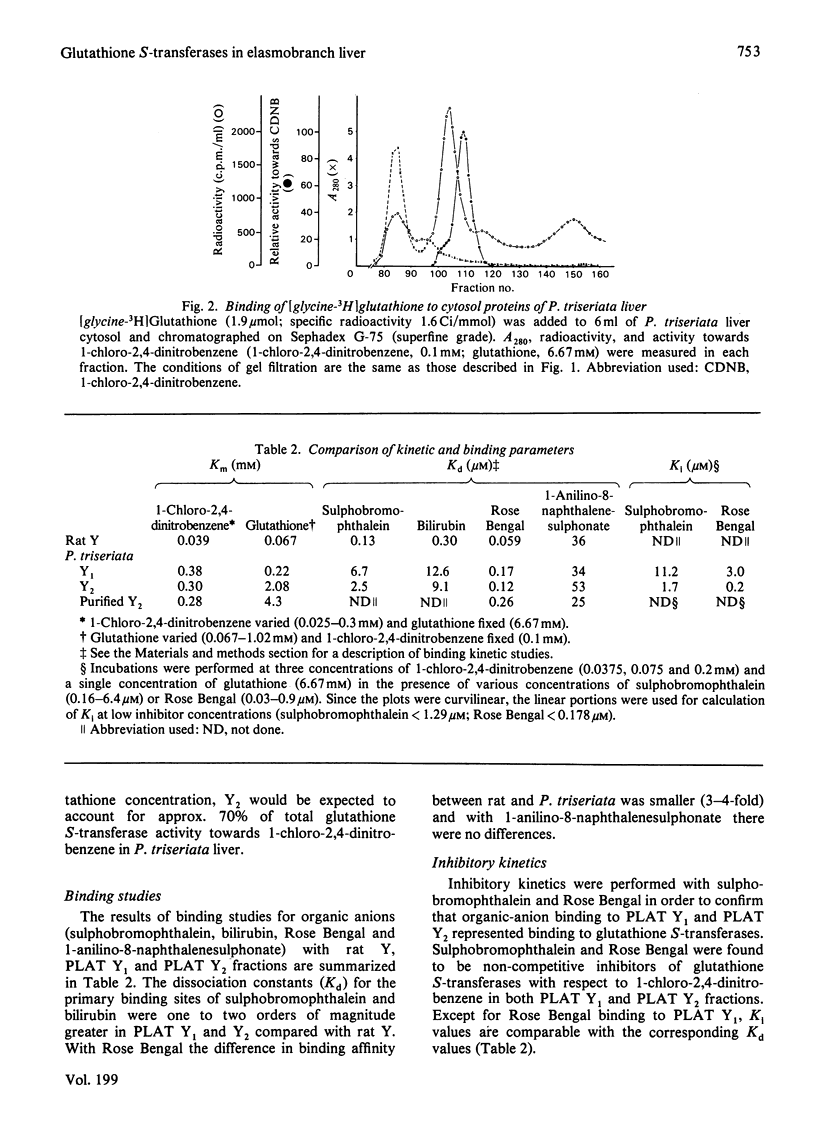
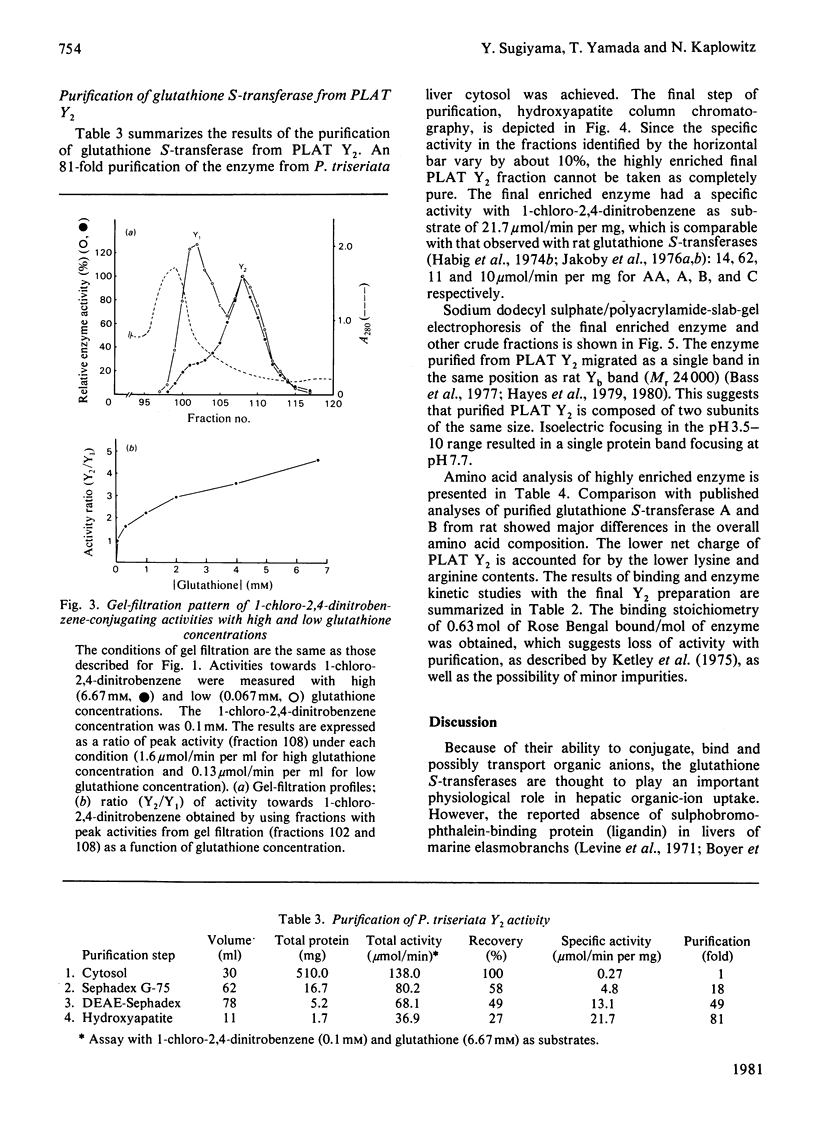
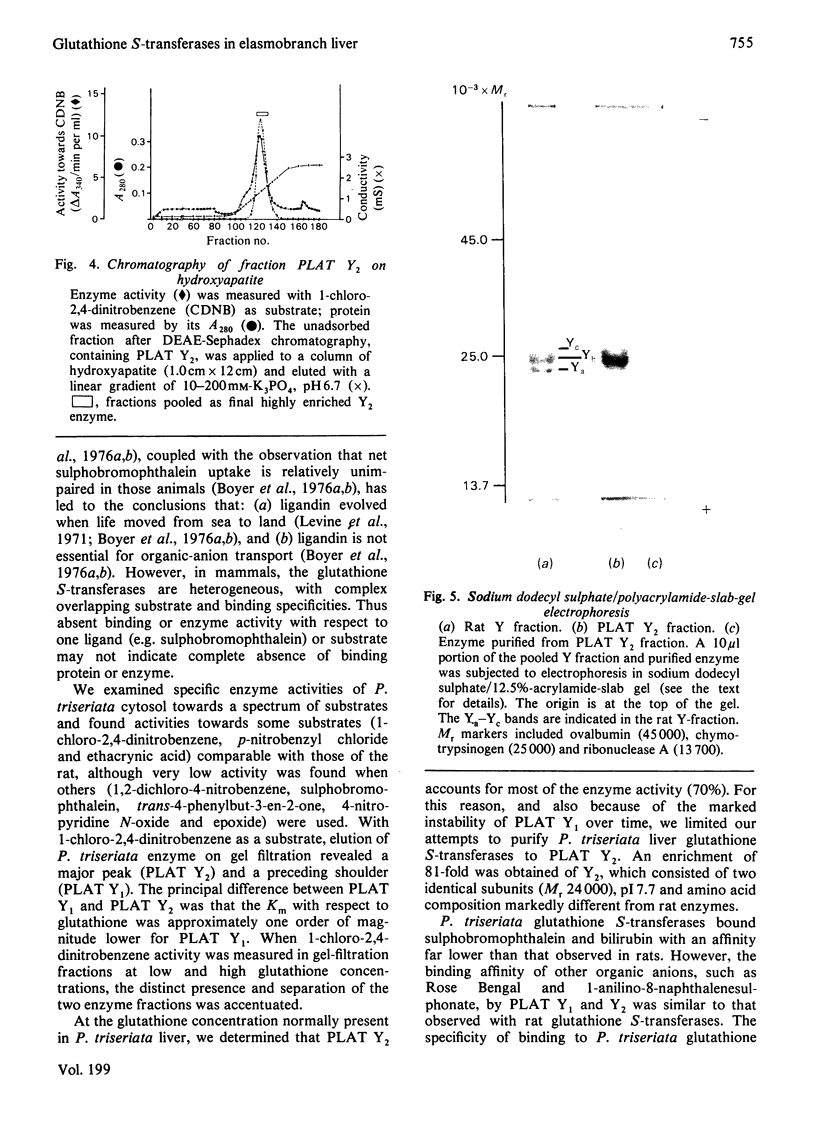
In order to gain insight into the phylogeny and physiological significance of organic-anion-binding proteins in the liver, the hepatic glutathione S-transferases of rat and a typical elasmobranch, the thorny-back shark (Platyrhinoides triseriata), were compared with respect to both glutathione S-transferase activites and organic-anion-binding properties. On gel filtration (Sephadex G-75, Superfine grade) of rat cytosol, the elution volumes of enzyme activities with 1-chloro-2,4-dinitrobenzene and p-nitrobenzyl chloride as substrates were identical (rat Y-fractions; Mr 45000). In contrast, two peaks of enzyme activity for 1-chloro-2,4-dinitrobenzene with elution volumes corresponding to Mr 52000 (PLAT Y1) and Mr 45000 (PLAT Y2) were detected on gel filtration of P. triseriata cytosol. Only fraction PLAT Y2 had enzyme activity with p-nitrobenzyl chloride. Enzyme kinetic studies showed that rat Y-fraction had higher affinities for both 1-chloro-2,4-dinitrobenzene and glutathione than PLAT Y1- and PLAT Y2-fractions. The two forms of P. triseriata glutathione S-transferases differed greatly in affinity for glutathione. At a glutathione concentration that we found to be physiological in P. triseriata, PLAT Y2 accounted for approx. 70% of the total glutathione S-transferase activity with 1-chloro-2,4-dinitrobenzene. Binding studies revealed that PLAT Y1 and PLAT Y2 fractions had much lower affinities for sulphobromophthalein and bilirubin than rat Y-fraction. In contrast, binding affinities of PLAT Y1 and PLAT Y2 for Rose Bengal and 1-anilino-8-naphthalenesulphonate were comparable with that of rat Y-fraction. Inhibitory kinetics suggested that sulphobromophthalein and Rose Bengal were non-competitive inhibitors of glutathione S-transferase activities when 1-chloro-2,4-dinitrobenzene was used as substrate for both PLAT Y1 and PLAT Y2. The major glutathione S-transferase from the PLAT Y2 fraction was purified 81-fold by sequential chromatography on Sephadex G-75, DEAE-Sephadex and hydroxyapatite, and consisted of two identical subunits with pI7.7. The highly enriched Y2-fraction retained high affinity binding of Rose Bengal and 1-anilino-8-naphthalenesulphonate.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass N. M., Kirsch R. E., Tuff S. A., Marks I., Saunders S. J. Ligandin heterogeneity : evidence that the two non-identical subunits are the monomers of two distinct proteins. Biochim Biophys Acta. 1977 May 27;492(1):163–175. doi: 10.1016/0005-2795(77)90223-9. [DOI] [PubMed] [Google Scholar]
- Boyer J. L., Schwarz J., Smith N. Biliary secretion in elasmobranchs. II. Hepatic uptake and biliary excretion of organic anions. Am J Physiol. 1976 Apr;230(4):974–981. doi: 10.1152/ajplegacy.1976.230.4.974. [DOI] [PubMed] [Google Scholar]
- Boyer J. L., Schwarz J., Smith N. Selective hepatic uptake and biliary excretion of 35S-sulfobromophthalein in marine elasmobranchs. Gastroenterology. 1976 Feb;70(2):254–256. [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Fleischner G., Gatmaitan Z., Arias I. M., Jakoby W. B. The identity of glutathione S-transferase B with ligandin, a major binding protein of liver. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3879–3882. doi: 10.1073/pnas.71.10.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Hayes J. D., Strange R. C., Percy-Robb I. W. Cholic acid binding by glutathione S-transferases from rat liver cytosol. Biochem J. 1980 Jan 1;185(1):83–87. doi: 10.1042/bj1850083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Strange R. C., Percy-Robb I. W. Identification of two lithocholic acid-binding proteins. Separation of ligandin from glutathione S-transferase B. Biochem J. 1979 Sep 1;181(3):699–708. doi: 10.1042/bj1810699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplowitz N., Percy-Robb I. W., Javitt N. B. Role of hepatic anion-binding protein in bromsulphthalein conjugation. J Exp Med. 1973 Aug 1;138(2):483–487. doi: 10.1084/jem.138.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketley J. N., Habig W. H., Jakoby W. B. Binding of nonsubstrate ligands to the glutathione S-transferases. J Biol Chem. 1975 Nov 25;250(22):8670–8673. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levi A. J., Gatmaitan Z., Arias I. M. Two hepatic cytoplasmic protein fractions, Y and Z, and their possible role in the hepatic uptake of bilirubin, sulfobromophthalein, and other anions. J Clin Invest. 1969 Nov;48(11):2156–2167. doi: 10.1172/JCI106182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R. I., Reyes H., Levi A. J., Gatmaitan Z., Arias I. M. Phylogenetic study of organic anion transfer from plasma into the liver. Nat New Biol. 1971 Jun 30;231(26):277–279. doi: 10.1038/newbio231277a0. [DOI] [PubMed] [Google Scholar]
- Litwack G., Ketterer B., Arias I. M. Ligandin: a hepatic protein which binds steroids, bilirubin, carcinogens and a number of exogenous organic anions. Nature. 1971 Dec 24;234(5330):466–467. doi: 10.1038/234466a0. [DOI] [PubMed] [Google Scholar]
- Radola B. J. Thin-layer isoelectric focusing of proteins. Biochim Biophys Acta. 1969 Nov 11;194(1):335–338. doi: 10.1016/0005-2795(69)90214-1. [DOI] [PubMed] [Google Scholar]
- Reeve J., Kuhlenkamp J., Kaplowitz N. Estimation of glutathione in rat liver by reversed-phase high-performance liquid chromatography: separation from cysteine and gamma-glutamylcysteine. J Chromatogr. 1980 Jun 27;194(3):424–428. doi: 10.1016/s0021-9673(00)81435-1. [DOI] [PubMed] [Google Scholar]
- Reyes H., Levi A. J., Gatmaitan Z., Arias I. M. Studies of Y and Z, two hepatic cytoplasmic organic anion-binding proteins: effect of drugs, chemicals, hormones, and cholestasis. J Clin Invest. 1971 Nov;50(11):2242–2252. doi: 10.1172/JCI106721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y., Iga T., Awazu S., Hanano M. Binding protein for 1-anilino-8-naphthalenesulfonate in rat liver cytoplasm. Biochem Pharmacol. 1980 Jul 15;29(14):2063–2069. doi: 10.1016/0006-2952(80)90492-x. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y., Iga T., Awazu S., Hanano M. Studies on ligand binding properties of Z-fraction from rat liver cytosol using 1-anilino-8-naphthalenesulfonate. Chem Pharm Bull (Tokyo) 1978 Jan;26(1):199–208. doi: 10.1248/cpb.26.199. [DOI] [PubMed] [Google Scholar]
- Tipping E., Ketterer B., Christodoulides L., Enderby G. Spectroscopic studies of the binding of bilirubin by ligandin and aminoazo-dye-binding protein A. Biochem J. 1976 Jul 1;157(1):211–216. doi: 10.1042/bj1570211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkoff A. W., Goresky C. A., Sellin J., Gatmaitan Z., Arias I. M. Role of ligandin in transfer of bilirubin from plasma into liver. Am J Physiol. 1979 Jun;236(6):E638–E648. doi: 10.1152/ajpendo.1979.236.6.E638. [DOI] [PubMed] [Google Scholar]



