Keywords: Renal insufficiency, chronic; Peritoneal dialysis; Icodextrin; Glucose; Urgent start; Hypervolemia; Survival
In Brief
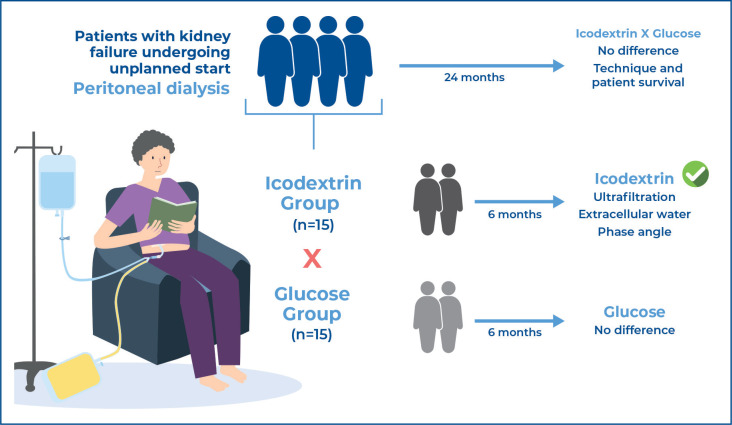
The osmotic characteristics of icodextrin are related to higher ultrafiltration than glucose-based peritoneal dialysis solutions. Consequently, it is associated with an improvement in the biomarkers of volume overload in patients undergoing automated peritoneal dialysis at an unplanned start. However, the mortality and technique survival rates did not differ with glucose use.
Highlights
-
■
No baseline differences between the groups icodextrin versus glucose.
-
■
Icodextrin significantly improved ultrafiltration, extracellular water, and phase angle.
-
■
Mortality and technique survival did not differ between the groups during follow-up.
ABSTRACT
Objective:
The efficacy of icodextrin versus glucose patients undergoing peritoneal dialysis remains unclear. The study was designed to compare the effects of once-daily long-dwell icodextrin versus glucose on markers of hypervolemia and survival among patients with kidney failure undergoing an unplanned initiation of automated peritoneal dialysis.
Methods:
This was a randomized, non-blinded, and prospective controlled study. Prevalent and stable patients undergoing automated peritoneal dialysis with a recent peritoneal equilibration test showing a dialysate/plasma creatinine of >0.50 were randomized to receive either 7.5% icodextrin or 2.5% glucose solution. Patients were evaluated at baseline (one month after the start of peritoneal dialysis), 3 months, and 6 months after inclusion. The peritoneal dialysis solution was used for at least 3 months, with a follow-up period of 24 months.
Results:
Thirty patients were enrolled. There were no baseline differences between the groups. During the study period, patients in the Icodextrin Group showed improvements in the phase angle and ultrafiltration, whereas there were no changes in the Glucose Group. Additionally, extracellular water was significantly lower in the Icodextrin Group at the end of the study than at baseline. No statistical differences between the two groups were observed in urine volume, ultrafiltration, extracellular water, phase angle, renal creatinine clearance, use of diuretics and antihypertensives, or blood pressure. During the 24-month follow-up, the number of events related to overall mortality was seven (Icodextrin Group, n=4; Glucose Group, n=3), with no difference between the groups for this outcome or technique survival.
Conclusion:
Icodextrin significantly improved ultrafiltration, extracellular water, and phase angle at the end of the study compared to baseline in patients on the urgent start of automated peritoneal dialysis.
Registry of Clinical Trials:
(www.ctri.nic.in) under the number RBR-97z4wh6.
INTRODUCTION
Icodextrin, a high molecular weight glucose polymer, has become an essential osmotic agent in peritoneal dialysis (PD) solutions over the past few decades. It plays a crucial role in PD treatment by creating an osmotic gradient that removes excess fluid and waste products from the blood. These properties have been associated with better control of volume overload, which is a common finding in patients undergoing dialysis. Given its osmotic characteristics, higher ultrafiltration is obtained compared with glucose-based PD solutions; consequently, an improvement in biomarkers associated with volume overload is anticipated.(1–5)
Despite its increasing prevalence in clinical practice, there is a lack of randomized clinical trials investigating the efficacy and safety of icodextrin compared to other fields of medicine. In particular, the main interest of most RCTs is ultrafiltration, and only a few studies provide information on other important markers of hypervolemia, such as blood pressure and bioimpedance data, including phase angle (PA) and extracellular water (ECW).
OBJECTIVE
To compare the effects of once-daily long-dwell icodextrin versus glucose on markers of hypervolemia and survival among patients with kidney failure undergoing unplanned automated peritoneal dialysis.
METHODS
Patients
This prospective randomized controlled clinical trial was conducted at the Hospital das Clínicas of Faculdade de Medicina de Botucatu, São Paulo, Brazil. Adult patients were eligible and included in the study if they were prevalent patients with stage 5 chronic kidney disease (CKD) on automated PD, started urgent PD, and had a recent peritoneal equilibration test (PET) showing a dialysate/plasma creatinine level >0.50. The exclusion criteria were age <18 or >80 years, urine volume <400ml/d, urinary tract obstruction due to neoplasm, neurogenic bladder, pregnancy, and previous renal replacement therapies, including PD, HD, and kidney transplantation.
Study protocol
A total of 30 patients with prevalent PDs from the Faculdade de Medicina de Botucatu were enrolled from September 2021 to March 2022. They were randomly assigned to a Glusose Group (n=15) treated with a maximum of 10L of 1.5% or 2.5% daily (Dianeal, Baxter) or an Icodextrin Group (n=15) treated with a maximum of 8L of 1.5% or 2.5% Dianeal in association with a daytime dwell of 2 or 1.5L of 7.5% icodextrin-containing solution (Extraneal, Baxter) for at least 3 months. The patients were evaluated at baseline (one month after the start of PD), 3 months, and 6 months after inclusion, and the follow-up period was 24 months.
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and was approved by the Faculdade de Medicina de Botucatu Research Ethics Committee (CAAE: 28325020.5.0000.5411; #3.936.169). All the patients provided written informed consent to participate in the study.
Clinical efficacy and outcomes
The primary outcome was the improvement in markers of hypervolemia such as ultrafiltration, ECW, PA, and blood pressure of patients undergoing CCPD with an unplanned start after 3 and 6 months. The secondary objectives were the rates of technical and patient survival at 6 months after PD. Technical survival was defined as the discontinuation of PD therapy owing to volume overload and infectious or mechanical complications. Clinical indices, including markers of body fluid status and RRF, were measured using daily urine volume, renal creatinine clearance, and weekly Kt/V, which were measured at one (baseline), three, and six months after the initiation of urgent PD.
A PET was performed at 1 and 6 months to measure the peritoneal transport status(3) with a 4-hour dwell period using a 2.5% glucose concentration and 2L volume exchange. The D/P creatinine ratio was measured after the 4-hour dwell period.
Weekly Kt/V urea was calculated from a 24-hour collection of dialysate and urine samples. RRF was calculated as the average residual renal creatinine and urea clearance. Markers of fluid status were evaluated using single-frequency bioimpedance analysis (Biodynamics model 450), which measures resistance, reactance, and PA and estimates total body water and intra-and extracellular water.
Statistical analyses
Continuous variables were expressed as either mean and standard deviation or median and interquartile range, depending on the distribution. Categorical variables were expressed as frequencies and percentages. Comparison of continuous variable values between the Icodextrin and Glucose Groups was performed using Student's t-test and Mann-Whitney U test. Comparison of ordinal variables, such as PET, use of diuretics, and antihypertensives was evaluated using the χ2 test.
We used repeated-measures ANOVA to assess changes within the group for clinical variables and examined the sphericity assumption using Mauchly's test (F ratio). If the sphericity assumption was violated, which was the case only for diastolic blood pressure, we used the recommended Greenhouse-Geisser epsilon value to test for significance. To compare the impact of the intervention on different clinical outcomes, we calculated the Z-score (individual value- mean/standard deviation) for each outcome and assessed the delta between Time 6 and Time 0 using ANOVA.
For survival analysis, we used the log-rank test to compare outcomes and plotted a Kaplan- Meier curve for overall mortality, technique failure, and dropout of any cause. We defined censoring for overall mortality and technique failure as any event that was not the primary outcome of interest as well as patients who remained active at the end of the study. Censoring for the outcome of dropout was restricted to patients who remained active at the end of the study. Finally, owing to the sample size of our study, and to facilitate the visualization of individual variations, we created scatter plots with the T0 and T6 values for each subgroup.
RESULTS
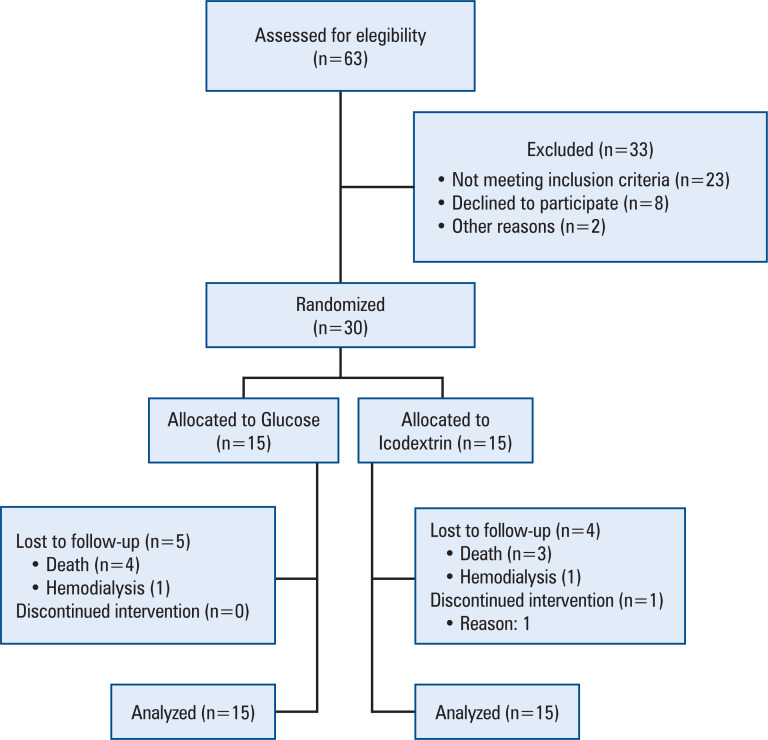
Thirty patients were enrolled in the study; 15 were randomized to the Icodextrin Group and 15 to the Glucose Group (Figure 1). At baseline, the Icodextrin and Glucose Groups were similar in all analyzed variables, including clinical characteristics, etiology of CKD, time on automated peritoneal dialysis (APD), characteristics of peritoneal transport, laboratory measurements, blood pressure, dialysis efficacy, use of diuretics and antihypertensives, PA, and ECW (Table 1).
Figure 1. Flow-chart of patient inclusion.
Table 1. Clinical and demographic characteristics of the study population at the baseline.
| Variable | Glucose Group n=15 |
Icodextrin Group n=15 |
Overall | p value | ||
|---|---|---|---|---|---|---|
| Demographic | ||||||
| Age (years) | 56.5±16.1 | 56.1±15.3 | 56.3±15.4 | 0.94 | ||
| Sex (male) | 7 (46.7) | 3 (20) | 10 (33.3) | |||
| Etiology of chronic Kidney disease [n (%)] | 0.58 | |||||
| Diabetes | 6 (40) | 3 (20) | 9 (30) | |||
| Hypertension | 2 (13.3) | 5 (33.3) | 7 (23.3) | |||
| Glomerulonephritis | 1 (6.7) | 2 (13.3) | 3 (10) | |||
| Others | 2 (13.3) | 2 (13.3) | 4 (12.3) | |||
| Unknown | 4 (26.7) | 3 (20) | 7 (23.3) | |||
| Clinical | ||||||
| Dialisate/Plasma creatinine [n (%)] | 0.18 | |||||
| High | 3 (23.1) | 7 (58.3) | 10 (40) | |||
| High average | 8 (61.5) | 3 (25) | 11 (44) | |||
| Low average | 2 (15.4) | 2 (16.7) | 4 (16) | |||
| Kt/V (total) | 1.80±0.53 | 2.28±0.82 | 2.0±0.7 | 0.07 | ||
| Kt/V (renal) | 0.43(0-0.84) | 0.75(0.14-1.01) | 0.54 (0-0.98) | 0.16 | ||
| Systolic blood pressure (mmHg) | 138.5±22.8 | 148.4±25.5 | 143.5±24.3 | 0.27 | ||
| Diastolic blood pressure (mmHg) | 77.1±16.3 | 85.3±16.8 | 81.2±16.8 | 0.19 | ||
| Extracellular water (L) | 20.5±5.0 | 23.4±11.5 | 22.0±8.8 | 0.36 | ||
| Ultrafiltration (L) | 1134±611 | 1083±530 | 1112±565 | 0.82 | ||
| Residual kidney function (ml) | 805 (0–1500) | 352(0–500) | 762 (0–1000) | 0.10 | ||
| Use of medications [n(%)] | 0.08 | |||||
| Loop diuretics (furosemide)* | 15 (100) | 15 (100) | 30 (100) | |||
| Diuretics | 3 (20) | 3 (20) | 6 (20) | |||
| ACEI + Diuretics | 7 (46) | 6 (40) | 13 (43.3) | |||
| ACEI + Diuretics + CCB | 5 (33) | 4 (36) | 9 (30) | |||
| ACEI + Diuretics + BB | - | 2 (13.3) | 2 (6.7) | |||
| Phase angle (°) | 5.08 ± 1.36 | 5.04 ±1.12 | 5.51±1.34 | 0.83 | ||
Dose of 160 to 320mg/day.
ACEI: angiotensin-converting enzyme inhibitor; CCB: calcium channel blocker; BB: beta-blocker.
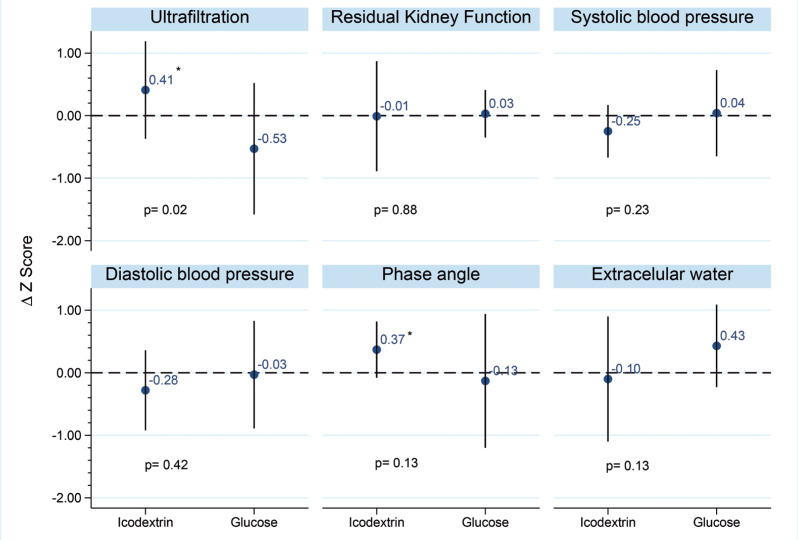
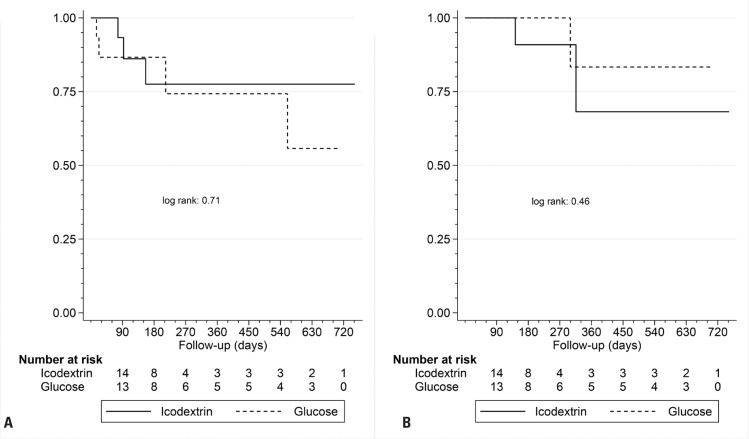
During the study period, patients in the Icodextrin Group showed improvements in the PA and UF, whereas there were no changes in the Glucose Group (Tables 2 and 3). Additionally, ECW was significantly lower in the Icodextrin Group at the end of the study than at baseline (Tables 2 and 3). No statistical differences between the two groups were observed in urine volume, UF, ECW, PA, renal creatinine clearance, or BP (Figure 2). During the follow-up period of 24 months, the number of events related to overall mortality was seven (Icodextrin Group, n=4; Glucose Group, n=3), and two events were reported as failure of technique (Icodextrin Group n=1, Glucose Group n=1) (Tables 1S and 2S, Supplementary Material). No differences were observed in the technique or patient survival (Figures 3A and 3B).
Table 2. Clinical outcomes at baseline, T3 and T6.
| Baseline | T3 (after 3 months) | T6 (after 6 months) | ||||
|---|---|---|---|---|---|---|
| Icodextrin Group | Glucose Group | Icodextrin Group | Glucose Group | Icodextrin Group | Glucose Group | |
| Phase angle (°) | 5.04±1.12* | 5.08±1.36 | 4.5 (4.1-6.4) | 5.7 (5.4-7.0) | 5.8±1.0* | 5.6±1.3 |
| Extracellular water (L) | 23.4±11.5* | 20.5±5.0 | 19.1±3.9 | 19.4±3.6 | 16.4±7.0* | 20.2±5.3 |
| Diastolic blood pressure (mmHg) | 85.3±16.8 | 77.1±16.3 | 87.3±18.0 | 85.3±18.6 | 82.6±15.0 | 81.2±18.4 |
| Systolic blood pressure (mmHg) | 148.4±25.5 | 138.5±22.8 | 137.5±18.8 | 146.6±29.3 | 141.1±19.3 | 138.4±23.2 |
| Residual diuresis | 352 (0–500) | 805 (0–1500) | 325 (0–1500) | 50 (0–350) | 0 (0–830) | 0 (200–1200) |
| Kidney Kt/V | 0.75 (0.14–1.01) | 0.43 (0–0.84) | 0.63 (0–0.98) | 0.66 (0.42–0.94) | 0.21 (0–0.98) | 0.66 (0.42–0.94) |
| Total Kt/V | 2.28±0.82 | 1.80±0.53 | 2.3±0.92 | 1.9±0.50 | 2.35±0.94 | 1.97±0.50 |
| Ultrafiltration (L) | 1083±530* | 1134±611 | 1200 (515–1300) | 615 (195–1218) | 1296 (1050–1621) | 807 (345–1058) |
p<0.05.
Table 3. Changes within groups in different clinical parameters along the study.
| F ratio | Greenhouse-Geisser épsilon (p value)* | |||
|---|---|---|---|---|
| Icodextrin Group |
Glucose Group |
Icodextrin Group |
Glucose Group |
|
| Phase angle (°) | 0.82 | 0.68 | 0.04 | 0.39 |
| Extracellular water (L) | 2.37 | 0.57 | 0.05 | 0.56 |
| Diastolic blood pressure (mmHg) | 0.87 | 1.80 | 0.41 | 0.19 |
| Systolic blood pressure (mmHg) | 3.31 | 0.84 | 0.07 | 0.40 |
| Residual diuresis | 2.95 | 1.80 | 0.08 | 0.20 |
| Kidney KtV | 0.73 | 0.76 | 0.42 | 0.48 |
| Total KtV | 0.77 | 0.17 | 0.41 | 0.71 |
| Ultrafiltration (L) | 5.41 | 2.58 | 0.02 | 0.12 |
Repeated-measures analysis of variance (ANOVA).
Figure 2. Comparison of changes in Z-scores of different clinical outcomes from baseline to 6 months between groups.
Figure 3. Patient (3A) and Technique Survival (3B) - Kaplan-Meier.
DISCUSSION
In this RCT, we showed that the Icodextrin Group presented with increased UF, higher PA, and reduced ECW at the end of the study compared with baseline, whereas these parameters were unaltered in the Glucose Group. There were no differences in the Icodextrin Group and Glucose Group in terms of urine volume, UF, ECW, PA, renal creatinine clearance, use of diuretics and antihypertensives, BP, technique, or patient survival.
A previous study with a large cohort of incident patients on PD in different countries showed that the majority of patients started PD with volume overload (33% moderate and 24% severe), and the use of hypertonic PD solutions (defined as at least one exchange with a dextrose concentration >1.5%) at baseline was 31%, which increased to 51% after three years.(6) In this context, the use of icodextrin should be discussed. Our findings are consistent with those of previous studies and with a recent systematic review showing that icodextrin resulted in increased UF and fewer episodes of fluid overload.(4,5,7–15)
There was no benefit of icodextrin in terms of the PD technique and patient survival. However, these outcomes are confounded by the small number of patients included and by interventions or contamination between groups. Our findings are in contrast with those of observational studies, which generally showed improved technique and patient survival with icodextrin treatment.(16–19)
Icodextrin reduces the daily exposure of the peritoneum to glucose compared to conventional glucose solutions, with a greater peritoneal biocompatibility. Despite this, we observed no difference in peritoneal solute transport, peritoneal small-solute clearance, and technique survival between the ICO- and GLU-only PD regimens. A single exchange of icodextrin per day instead of glucose may be clinically insufficient as a PD regimen with enhanced biocompatibility.(20,21)
This study had a few limitations. Firstly, it was performed at a single center and included a small number of patients. Secondly, carbohydrate metabolism was not evaluated in this study. Despite these limitations, this was the first study to evaluate the role of the ICO on markers of hypervolemia and survival of patients undergoing APD with an unplanned start.
Therefore, there is an urgent need to improve PD outcomes and reduce associated costs. Icodextrin provides an opportunity to decrease important complications such as fluid overload. Based on our updated results, increased access to icodextrin is likely to benefit patients worldwide, and multicenter RCT should be implemented.
CONCLUSION
In conclusion, our study demonstrated the clinical benefits of icodextrin due to improved ultrafiltration, extracellular water, and phase angle at the end of the study compared with the baseline in patients on urgent initiation of automated peritoneal dialysis.
ACKNOWLEDGMENT
This research was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) grant number 2019/102116-2 and by the Baxter Health Corporation. Daniela Ponce: funding acquisition
SUPPLEMENTARY MATERIAL. Icodextrin versus Glucose 2.5% on markers of hypervolemia and survival of patients undergoing automated peritoneal dialysis with an unplanned start: a randomized controlled trial
Leonardo Sotello Azevedo, Vanessa Burgugi Banin, Dayana Bitencourt Dias, Marcela Lara Mendes, Camila Albuquerque Alves, Maryanne Zilli Canedo Silva, Thyago Proença de Moraes, Daniela Ponce
Table 1S. Number of events related to the overall mortality.
| Interval | Beg | Deaths | Lost | Survival | Standard error | [95%CI] |
|---|---|---|---|---|---|---|
| Total | ||||||
| 16-17 | 30 | 1 | 0 | 0.9667 | 0.0328 | 0.7861-0.9952 |
| 23-24 | 29 | 1 | 0 | 0.9333 | 0.0455 | 0.7589-0.9829 |
| 77-78 | 28 | 1 | 0 | 0.9000 | 0.0548 | 0.7212-0.9666 |
| 90-91 | 27 | 0 | 1 | 0.9000 | 0.0548 | 0.7212-0.9666 |
| 93-94 | 26 | 1 | 0 | 0.8654 | 0.0627 | 0.6799-0.9473 |
| 111-112 | 25 | 0 | 1 | 0.8654 | 0.0627 | 0.6799-0.9473 |
| 112-113 | 24 | 0 | 2 | 0.8654 | 0.0627 | 0.6799-0.9473 |
| 132-133 | 22 | 0 | 1 | 0.8654 | 0.0627 | 0.6799-0.9473 |
| 143-144 | 21 | 0 | 1 | 0.8654 | 0.0627 | 0.6799-0.9473 |
| 156-157 | 20 | 1 | 0 | 0.8221 | 0.0729 | 0.6213-0.9226 |
| 168-169 | 19 | 0 | 1 | 0.8221 | 0.0729 | 0.6213-0.9226 |
| 171-172 | 18 | 0 | 2 | 0.8221 | 0.0729 | 0.6213-0.9226 |
| 196-197 | 16 | 0 | 1 | 0.8221 | 0.0729 | 0.6213-0.9226 |
| 202-203 | 15 | 0 | 1 | 0.8221 | 0.0729 | 0.6213-0.9226 |
| 213-214 | 14 | 1 | 0 | 0.7634 | 0.0883 | 0.5353-0.8899 |
| 242-243 | 13 | 0 | 3 | 0.7634 | 0.0883 | 0.5353-0.8899 |
| 300-301 | 10 | 0 | 1 | 0.7634 | 0.0883 | 0.5353-0.8899 |
| 316-317 | 9 | 0 | 1 | 0.7634 | 0.0883 | 0.5353-0.8899 |
| 529-530 | 8 | 0 | 1 | 0.7634 | 0.0883 | 0.5353-0.8899 |
| 560-561 | 7 | 1 | 0 | 0.6543 | 0.1262 | 0.3556-0.8403 |
| 569-570 | 6 | 0 | 1 | 0.6543 | 0.1262 | 0.3556-0.8403 |
| 689-690 | 5 | 0 | 1 | 0.6543 | 0.1262 | 0.3556-0.8403 |
| 690-691 | 4 | 0 | 1 | 0.6543 | 0.1262 | 0.3556-0.8403 |
| 703-704 | 3 | 0 | 1 | 0.6543 | 0.1262 | 0.3556-0.8403 |
| 717-718 | 2 | 0 | 1 | 0.6543 | 0.1262 | 0.3556-0.8403 |
| 752-753 | 1 | 0 | 1 | 0.6543 | 0.1262 | 0.3556-0.8403 |
Table 2S. Number of events related to the technique survival.
| Interval | Beg | Deaths | Lost | Survival | Standard error | [95%CI] | |
|---|---|---|---|---|---|---|---|
| Total | |||||||
| 16-17 | 30 | 0 | 1 | 10.000 | 0.0000 | . | . |
| 23-24 | 29 | 0 | 1 | 10.000 | 0.0000 | . | . |
| 77-78 | 28 | 0 | 1 | 10.000 | 0.0000 | . | . |
| 90-91 | 27 | 0 | 1 | 10.000 | 0.0000 | . | . |
| 93-94 | 26 | 0 | 1 | 10.000 | 0.0000 | . | . |
| 111-112 | 25 | 0 | 1 | 10.000 | 0.0000 | . | . |
| 112-113 | 24 | 0 | 2 | 10.000 | 0.0000 | . | . |
| 132-133 | 22 | 0 | 1 | 10.000 | 0.0000 | . | . |
| 143-144 | 21 | 1 | 0 | 0.9524 | 0.0465 | 0.7072-0.9932 | |
| 156-157 | 20 | 0 | 1 | 0.9524 | 0.0465 | 0.7072-0.9932 | |
| 168-169 | 19 | 0 | 1 | 0.9524 | 0.0465 | 0.7072-0.9932 | |
| 171-172 | 18 | 0 | 2 | 0.9524 | 0.0465 | 0.7072-0.9932 | |
| 196-197 | 16 | 0 | 1 | 0.9524 | 0.0465 | 0.7072-0.9932 | |
| 202-203 | 15 | 0 | 1 | 0.9524 | 0.0465 | 0.7072-0.9932 | |
| 213-214 | 14 | 0 | 1 | 0.9524 | 0.0465 | 0.7072-0.9932 | |
| 242-243 | 13 | 0 | 3 | 0.9524 | 0.0465 | 0.7072-0.9932 | |
| 300-301 | 10 | 1 | 0 | 0.8571 | 0.0996 | 0.5091-0.9654 | |
| 316-317 | 9 | 1 | 0 | 0.7619 | 0.1261 | 0.4081-0.9208 | |
| 529-530 | 8 | 0 | 1 | 0.7619 | 0.1261 | 0.4081-0.9208 | |
| 560-561 | 7 | 0 | 1 | 0.7619 | 0.1261 | 0.4081-0.9208 | |
| 569-570 | 6 | 0 | 1 | 0.7619 | 0.1261 | 0.4081-0.9208 | |
| 689-690 | 5 | 0 | 1 | 0.7619 | 0.1261 | 0.4081-0.9208 | |
| 690-691 | 4 | 0 | 1 | 0.7619 | 0.1261 | 0.4081-0.9208 | |
| 703-704 | 3 | 0 | 1 | 0.7619 | 0.1261 | 0.4081-0.9208 | |
| 717-718 | 2 | 0 | 1 | 0.7619 | 0.1261 | 0.4081-0.9208 | |
| 752-753 | 1 | 0 | 1 | 0.7619 | 0.1261 | 0.4081-0.9208 | |
Funding Statement
This research was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) grant number 2019/102116-2 and by the Baxter Health Corporation. Daniela Ponce: funding acquisition
AVAILABILITY OF DATA AND MATERIAL.
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Davies SJ, Phillips L, Naish PF, Russell GI. Peritoneal glucose exposure and changes in membrane solute transport with time on peritoneal dialysis. J Am Soc Nephrol. 2001;12(5):1046–1051. doi: 10.1681/ASN.V1251046. [DOI] [PubMed] [Google Scholar]
- 2.Guo A, Wolfson M, Holt R. Early quality of life benefits of icodextrin in peritoneal dialysis. Kidney Int Suppl. 2002;81(81):S72–S79. doi: 10.1046/j.1523-1755.62.s81.10.x. [DOI] [PubMed] [Google Scholar]
- 3.Twardowski ZJ, Prowant BF, Moore HL, Lou LC, White E, Farris K. Short peritoneal equilibration test: impact of preceding dwell time. Adv Perit Dial. 2003;19:53–58. [PubMed] [Google Scholar]
- 4.Goossen K, Becker M, Marshall MR, Bühn S, Breuing J, Firanek CA, et al. Icodextrin Versus Glucose Solutions for the Once-Daily Long Dwell in Peritoneal Dialysis: An Enriched Systematic Review and Meta-analysis of Randomized Controlled Trials. Am J Kidney Dis. 2020;75(6):830–846. doi: 10.1053/j.ajkd.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Konings CJ, Kooman JP, Schonck M, Gladziwa U, Wirtz J, van den Wall Bake AW, et al. Effect of icodextrin on volume status, blood pressure and echocardiographic parameters: a randomized study. Kidney Int. 2003;63(4):1556–1563. doi: 10.1046/j.1523-1755.2003.00887.x. [DOI] [PubMed] [Google Scholar]
- 6.Van Biesen W, Verger C, Heaf J, Vrtovsnik F, Britto ZM, Do JY, Prieto-Velasco M, Martínez JP, Crepaldi C, De Los Ríos T, Gauly A, Ihle K, Ronco C, IPOD-PD Study Group Evolution Over Time of Volume Status and PD-Related Practice Patterns in an Incident Peritoneal Dialysis Cohort. Clin J Am Soc Nephrol. 2019;14(6):882–893. doi: 10.2215/CJN.11590918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin A, Qian J, Li X, Yu X, Liu W, Sun Y, Chen N, Mei C, Icodextrin National Multi-center Cooperation Group Randomized controlled trial of icodextrin versus glucose containing peritoneal dialysis fluid. Clin J Am Soc Nephrol. 2009;4(11):1799–1804. doi: 10.2215/CJN.02950509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mistry CD, Gokal R, Peers E, Brown CB, Smith S, Edwards DL, et al. A randomized multicenter clinical trial comparing isosmolar icodextrin with hyperosmolar glucose solutions in CAPD. MIDAS Study Group. Multicenter Investigation of Icodextrin in Ambulatory Peritoneal Dialysis. Kidney Int. 1994;46(2):496–503. doi: 10.1038/ki.1994.300. [DOI] [PubMed] [Google Scholar]
- 9.Gokal R, Mistry CD, Peers EM. Peritonitis occurrence in a multicenter study of icodextrin and glucose in CAPD. MIDAS Study Group. Multicenter Investigation of Icodextrin in Ambulatory Dialysis. Perit Dial Int. 1995;15(6):226–230. [PubMed] [Google Scholar]
- 10.Ota K, Akiba T, Maeba T. Clinical results of icodextrin dialysis solution in Japan: results of a double-blind comparative study using glucose dialysate as a control drug. Kidney Dial. 2003;55(1):211–219. [Google Scholar]
- 11.Paniagua R, Ventura MD, Avila-Díaz M, Cisneros A, Vicenté-Martínez M, Furlong MD, et al. Icodextrin improves metabolic and fluid management in high and high-average transport diabetic patients. Perit Dial Int. 2009;29(4):422–432. [PubMed] [Google Scholar]
- 12.Plum J, Gentile S, Verger C, Brunkhorst R, Bahner U, Faller B, et al. Efficacy and safety of a 7.5% icodextrin peritoneal dialysis solution in patients treated with automated peritoneal dialysis. Am J Kidney Dis. 2002;39(4):862–871. doi: 10.1053/ajkd.2002.32009. [DOI] [PubMed] [Google Scholar]
- 13.Rodríguez-Carmona A, Pérez Fontán M, García López E, García Falcón T, Díaz Cambre H. Use of icodextrin during nocturnal automated peritoneal dialysis allows sustained ultrafiltration while reducing the peritoneal glucose load: a randomized crossover study. Perit Dial Int. 2007;27(3):260–266. [PubMed] [Google Scholar]
- 14.Takatori Y, Akagi S, Sugiyama H, Inoue J, Kojo S, Morinaga H, et al. Icodextrin increases technique survival rate in peritoneal dialysis patients with diabetic nephropathy by improving body fluid management: a randomized controlled trial. Clin J Am Soc Nephrol. 2011;6(6):1337–1344. doi: 10.2215/CJN.10041110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfson M, Piraino B, Hamburger RJ, Morton AR, Icodextrin Study Group A randomized controlled trial to evaluate the efficacy and safety of icodextrin in peritoneal dialysis. Am J Kidney Dis. 2002;40(5):1055–1065. doi: 10.1053/ajkd.2002.36344. [DOI] [PubMed] [Google Scholar]
- 16.Han SH, Ahn SV, Yun JY, Tranaeus A, Han DS. Effects of icodextrin on patient survival and technique success in patients undergoing peritoneal dialysis. Nephrol Dial Transplant. 2012;27(5):2044–2050. doi: 10.1093/ndt/gfr580. [DOI] [PubMed] [Google Scholar]
- 17.Han SH, Ahn SV, Yun JY, Tranaeus A, Han DS. Mortality and technique failure in peritoneal dialysis patients using advanced peritoneal dialysis solutions. Am J Kidney Dis. 2009;54(4):711–720. doi: 10.1053/j.ajkd.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Wang IK, Li YF, Chen JH, Liang CC, Liu YL, Lin HH, et al. Icodextrin decreases technique failure and improves patient survival in peritoneal dialysis patients. Nephrology (Carlton) 2015;20(3):161–167. doi: 10.1111/nep.12375. [DOI] [PubMed] [Google Scholar]
- 19.See EJ, Johnson DW, Cho Y. In reply to ‘the importance of icodextrin use for technique and patient survival in peritoneal dialysis.’. Am J Kidney Dis. 2018;72(2):309–310. doi: 10.1053/j.ajkd.2018.02.357. [DOI] [PubMed] [Google Scholar]
- 20.Dai H, Lin A, Qian J. Improved angiogenesis of peritoneal membrane in continuous ambulatory peritoneal dialysis patients with icodextrin (translated) Zhonghua Yi Xue Za Zhi. 2010;90(40):2843–2845. [PubMed] [Google Scholar]
- 21.Elphick EH, Teece L, Chess JA, Do JY, Kim YL, Lee HB, et al. Biocompatible solutions and long-term changes in peritoneal solute transport. Clin J Am Soc Nephrol. 2018;13(10):1526–1533. doi: 10.2215/CJN.02380218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.