Abstract
Purpose
We investigated the factors that affect the occurrence of sinusoidal obstruction syndrome (SOS) and the effect of SOS on the patient’s perioperative outcomes through histological review of liver resection specimens from patients who underwent chemotherapy.
Methods
From December 2007 to December 2020, liver specimens from patients who underwent liver resection for colorectal liver metastasis after neoadjuvant chemotherapy were analyzed regarding liver damage in the nontumorous lesion. Through pathological review, patients with grade 1–3 sinusoidal dilatation were categorized into the SOS (+) group, compared to a control group (grade 0, SOS [−]).
Results
Of 286 patients, 175 were included. Preoperative factors were similar between the groups. Although not statistically significant, the SOS (+) group had a shorter chemotherapy-free interval before resection (7.96 weeks vs. 10.0 weeks, P = 0.069). The SOS (+) group had higher intraoperative blood loss (889.1 ± 1,126.6 mL vs. 555.3 ± 566.7 mL, P = 0.012) and transfusion rates (46.6% vs. 25.3%, P = 0.003). SOS correlated with increased liver surgery-specific complications (40.9% vs. 26.4, P = 0.043). Patients with SOS experienced adverse effects on intrahepatic recurrent-free survival and overall survival (5-year survival, 46.0% vs. 33.9%; P = 0.014).
Conclusion
SOS development during liver surgery is associated with increased intraoperative blood loss, transfusion volume, and liver surgery-specific complications and has a higher risk of early recurrence and decreased overall survival. Thus, it is crucial to exercise caution during liver surgery in these patients.
Keywords: Chemical and drug induced liver injury, Colorectal neoplasms, Drug therapy, Hepatectomy, Hepatic veno-occlusive disease
INTRODUCTION
Colorectal cancer (CRC) is the third most commonly diagnosed cancer worldwide, accounting for 11% of all cancer diagnoses [1]. Approximately 50% of patients with CRC develop liver metastases at some point in their lives, and about 25% of them have liver metastases at the time of diagnosis.
Although liver resection is the best treatment for colorectal liver metastasis (CRLM), it is considered an option for hepatic resection in only 10%–30% of patients [2]. The 5-year survival of CRLM without treatment was 11% or less, while after hepatic resection, the 5-year survival was 25%–40%. Initially, patients with unresectable CRLM were treated with systemic chemotherapy, and later, if surgery is to be performed, these patients have similar survival rates to those undergoing liver resection, initially. The introduction of neoadjuvant systemic chemotherapy has resulted in downstaging liver metastases, potentially enabling future hepatic resection and prolonged survival [3,4,5]. In a previously published systematic review of neoadjuvant chemotherapy in locally advanced colon cancer, the R0 resection rate in the patient group who received neoadjuvant chemotherapy was 96.1%, which was higher than that of the control group (85.4%) [6].
For decades, 5-fluorouracil (FU) has been used as the primary treatment for CRLM. With the development of cytotoxic agents such as oxaliplatin and irinotecan, these regimens are now considered standard therapy (FOLFOX, 5-FU/leucovorin/oxaliplatin; FOLFIRI, 5-FU/leucovorin/irinotecan). Although chemotherapy can help with oncological outcomes, concerns about its toxicity persist. Previous studies have reported that chemotherapy-associated liver injury (CALI) is linked with an increase in postoperative morbidity and mortality and liver dysfunction after hepatic resection [5]. These hepatic toxicities are often reported in patients with CRLM, and it was thought to be regimen-specific. For instance, 5-FU is associated with an increase in hepatocyte steatosis, and irinotecan appears to be related to steatohepatitis. Oxaliplatin is associated with sinusoidal obstruction syndrome (SOS) [2,7].
The most common type of CALI, SOS, was also known as hepatic veno-occlusive disease. It is characterized by pathologic features such as sinusoidal dilatation, centrilobular fibrosis, and hepatocellular necrosis [8]. Reports on the perioperative outcome after liver resection of SOS are still controversial, but it is known to have negative consequences. Therefore, attempts have been made to predict SOS before surgery due to these concerns. However, it is difficult to predict compared to steatosis or steatohepatitis, and its effect on the perioperative outcome may also be greater than other types of CALI.
Previous studies have introduced factors to predict the occurrence of SOS, such as splenomegaly, platelet (PLT) count, and the aspartate AST/PLT ratio, etc. However, there are currently no dependable tools that can be used routinely [9,10]. Therefore, in this study, we investigated the factors that affect the occurrence of SOS and the effect of SOS on the patient’s perioperative outcomes through histological review of liver resection specimens from patients who underwent chemotherapy.
METHODS
Ethics statements
This study protocol was approved by the Institutional Review Board of The Catholic University of Korea, Seoul St. Mary’s Hospital (No. KC22RISI0282). This study was performed in accordance with the Declaration of Helsinki and written informed consent was waived due to its retrospective nature.
Patient selection
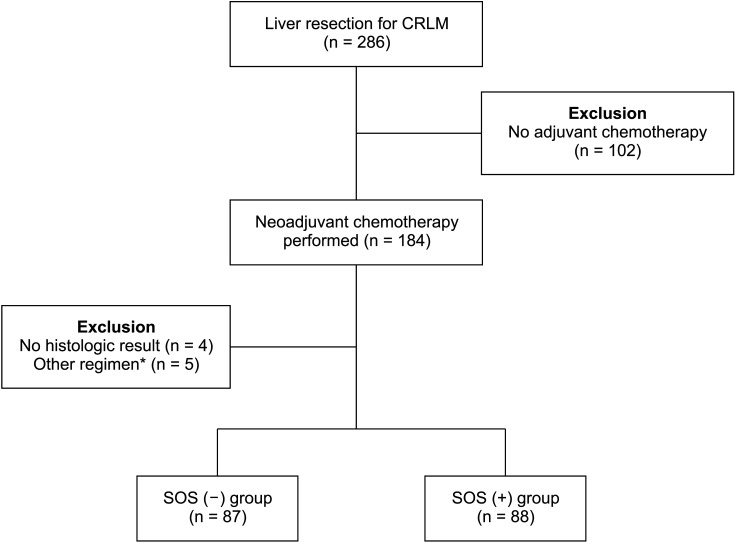
From December 1, 2007, to December 30, 2020, among the patients who had undergone liver resection in The Catholic University of Korea, Seoul St. Mary’s Hospital, patients who met the following criteria were included; (1) Diagnosed with CRC and liver metastasis, (2) received chemotherapy before liver resection, (3) could be evaluated via the histopathologic findings of non-tumor lesion from the liver specimen. In 286 patients who underwent liver resection for CLRM in our institution, 184 patients (64.3%) underwent systemic chemotherapy before hepatectomy. Among these patients, 4 patients whose histologic results could not be confirmed in the liver specimen were excluded. Also, 5 patients were excluded who underwent other regimens for chemotherapy except FOLFOX and FOLFIRI. A total of 175 patients were included (Fig. 1).
Fig. 1. The summary of the study selection process. CRLM, colorectal liver metastasis; SOS, sinusoidal obstruction syndrome. *5-fluorouracil only, 5-fluorouracil and leucovorin only.
All patients were assessed prior to liver resection, using pre-chemotherapy abdominal CT scan as well as post-chemotherapy CT. Hepatectomy was considered after identifying the response of neoadjuvant chemotherapy. Data for all recruited cases are retrospectively reviewed.
It has been reported that the effects of thrombocytopenia and liver dysfunction induced by chemotherapy last up to 1–2 years after oxaliplatin-based chemotherapy [11]. Therefore, when several regimens were serially used as neoadjuvant chemotherapy in some patients, only the regimen used within 1-year prior to hepatectomy was considered. Also, some patients received irinotecan and oxaliplatin sequentially because of the therapy failure of the other respective drugs. In these cases, analysis was based on the regimen used immediately before liver resection.
Spleen size was measured in CT studies and expressed as a splenic index (SI). SI was defined as splenic length (maximum longitudinal image) × splenic width (transversely across the hilum image) × splenic height (cephalocaudal image) [12].
Postoperative complications were classified by the Clavien-Dindo classification. Also, post-hepatectomy liver failure (PHLF) was defined according to the ‘50-50 criteria.’ A negative resection margin was defined as a surgical margin of more than 1 mm. This was confirmed to have absent tumor cells on the margin from pathologic results.
Histopathology
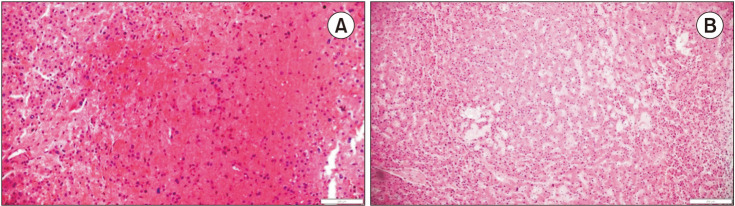
The pathologic assessment of the liver injury pattern was rereviewed by 1 pathologist; and it was performed in the representative non-tumor tissues which are at least 1.0 cm away from the tumor lesions to minimize the tissue effect caused by the inflammatory reaction shown at the invasive fronts. The degrees of steatosis were estimated using a nonalcoholic fatty liver disease activity scoring system: 0, absent; 1, minimal (steatosis in <5% of the hepatocytes); 2, mild (5%–33%); 3, moderate (34%–66%); 4, severe (>66%) [13]. Sinusoidal dilatation was graded semi-quantitatively as proposed by Rubbia-Brandt et al. [14]. The details of the tissue analysis process are as follows: 0, absent; 1, mild (centrilobular involvement limited to 1/3 of the lobular surface); 2, moderate (centrilobular involvement extending in 2/3 of the lobular surface); and 3, severe (complete lobular involvement). SOS was defined as grade 1 to grade 3 sinusoidal dilatation; and these patients were classified as SOS (+) group. The other, grade 0 patients were classified as the SOS (−) group [14] (Fig. 2).
Fig. 2. Histological staining of liver tissue in sinusoidal obstruction syndrome (H&E). (A) Microscopic finding reveals a focal area of hepatocellular plate atrophy associated with mild sinusoidal dilation (×40). (B) Hemorrhage into markedly dilated sinusoids with hepatocyte necrosis is shown (×100).
Statistical analysis
Data analyses were performed with IBM SPSS Statistics ver. 25.0 (IBM Corp.). For categorical variables, the Pearson chi-square test with continuity correction was applied, or the Fisher exact test when any of the expected values was smaller than 5; and those were expressed as frequency distribution and percentages. Continuous variables were compared using the Student t-test and analysis of variance t-test, the Mann-Whitney U-test and were expressed as the average standard ± deviation.
Overall and recurrence-free survival probabilities were estimated using the Kaplan-Meier method. Recurrent-free survival was calculated from the date of liver resection to the first intrahepatic recurrence diagnosed or the last follow-up contact. Overall survival (OS) was estimated from the date of hepatectomy to the last follow-up date. The 95% confidence interval of the difference in proportions was calculated. A P-value of <0.05 was considered statistically significant.
RESULTS
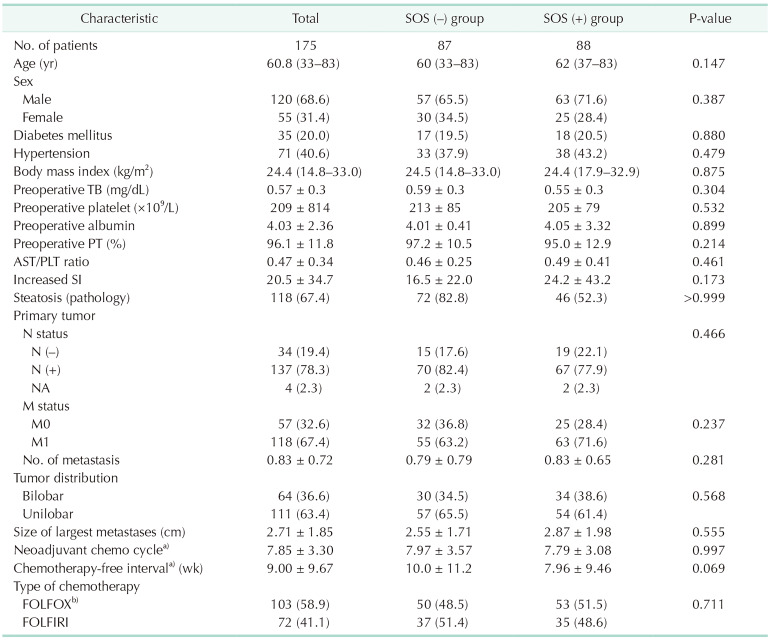
The mean age of all patients who underwent curative surgery was 60.8 years, and there were 120 male (68.6%) and 55 female patients (31.4%). SOS and steatosis developed in 88 and 118 of 175 enrolled patients (50.3% and 67.4%, respectively). There were no statistically significant differences between the 2 groups in the distributions of patients’ sex and age, the blood test results such as bilirubin and PLT, AST/PLT ratio as well as the underlying diseases of the patients before surgery. Concerning the stage at the time of diagnosis of primary tumor, there was no difference including M stage status or the number of metastases.
The number of applied cycles of chemotherapy was 7.85 in total patients. Also, there were no differences between the 2 groups. The mean chemotherapy-free interval prior to resection was found to be 9.00 weeks in all patients. In the SOS (+) group, the interval was shorter, but there was no statistical significance (10.0 weeks vs. 7.96 weeks, P = 0.069). In terms of the type of chemotherapy regimen, 103 (58.9%) received oxaliplatin-based chemotherapy including FOLFOX (n = 97) and XELOX (capecitabine/oxaliplatin; n = 7), 72 (41.1%) received irinotecan-based chemotherapy including FOLFIRI. There was no difference in the effect of the chemotherapy regimen on the incidence of SOS. Also, the degree of increase in SI, before and after neoadjuvant chemotherapy of the SOS (−) and SOS (+) groups, was 16.5 ± 22.0, 24.2 ± 43.2, respectively. It was found to be increased in the SOS (+) group, but there was no statistical significance (P = 0.173) (Table 1).
Table 1. Patient characteristics and preoperative factors.
Values are presented as median (range), number (%), or mean ± standard deviation.
SOS, sinusoidal obstructive syndrome; TB, total bilirubin; AST, aspartate aminotransferase; PLT, platelet; SI, splenic index; NA, not available; XELOX, capecitabine/oxaliplatin; FOLFOX, 5-FU/leucovorin/oxaliplatin; FOLFIRI, 5-FU/leucovorin/irinotecan.
a)Except for more than 1 year. b)Including XELOX.
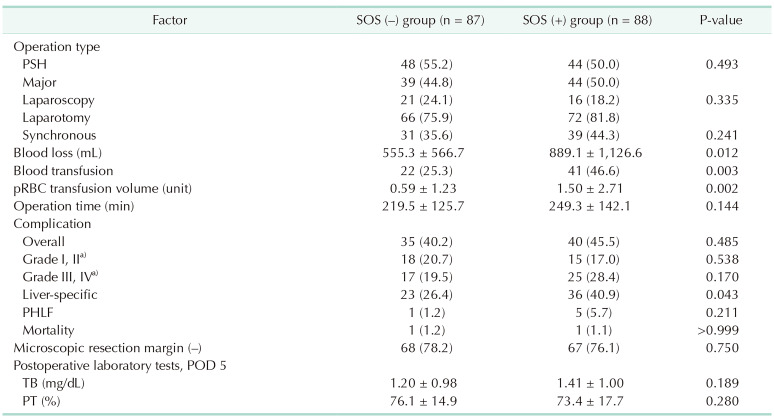
In intraoperative factors, the SOS (+) group had a larger amount of intraoperative blood loss than the SOS (−) group (889.1 ± 1,126.6 mL vs. 555.3 ± 566.7 mL, P = 0.012). Also, the former group had more packed RBC transfusion (1.50 ± 2.71 units vs. 0.59 ± 1.23 units, P = 0.002) and the transfusion rate was higher than the latter group (46.6% vs. 25.3%, P = 0.003). The SOS (+) group had a longer operation time; however, there was no significant difference.
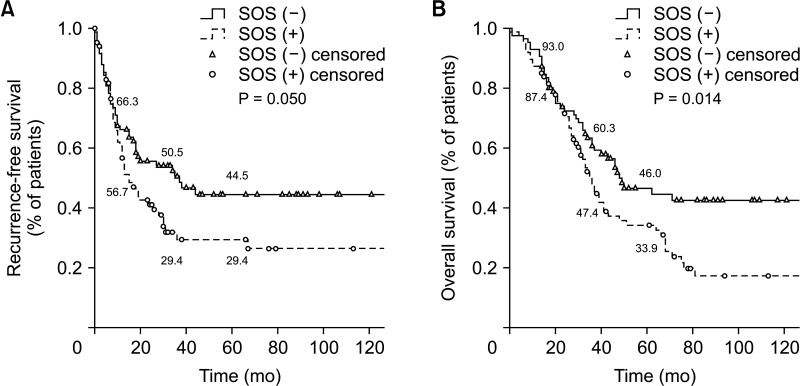
During postoperative care, the prevalence of complications was similar between the 2 groups (40.2% vs. 45.5% cases, respectively; P = 0.485) overall or regardless of classification. However, the incidence of liver surgery-specific complications such as ascites, pleural effusion, bile leakage, and hepatic failure was higher in the SOS (+) group than in the SOS (−) group (40.9% vs. 26.4%, P = 0.043). Frequencies of microscopic negative hepatic resection margin and post-hepatectomy liver failure, postoperative day (POD) 5 total bilirubin or PT were not significantly different. Intrahepatic recurrence of CRLM occurred earlier in the SOS (+) group, and the survival rate of this group was lower than that of the SOS (−) group (33.9 vs. 46.0%, P = 0.014) (Table 2, Fig. 3).
Table 2. Comparison of perioperative factors.
Values are presented as number (%) or mean ± standard deviation.
SOS, sinusoidal obstructive syndrome; PSH, parenchymal sparing hepatectomy; pRBC, packed RBC; PHLF, post-hepatectomy liver failure, POD, postoperative day; TB, total bilirubin.
a)Clavien-Dindo classification.
Fig. 3. Kaplan-Mayer curve. (A) Proportional recurrence-free survival and (B) overall survival. SOS, sinusoidal obstruction syndrome.
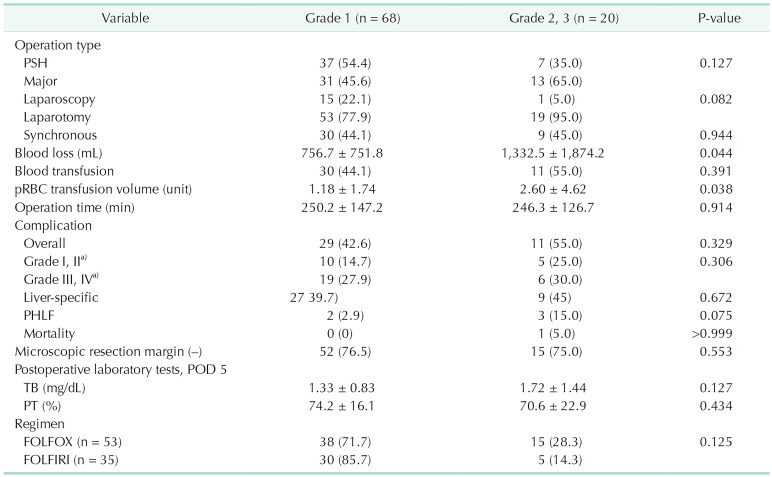
According to the severity of SOS, patients with SOS were divided into 2 groups: moderate SOS (grade 0, 1) and severe SOS (grades 2, 3), and subgroup analyses were performed. In the results, there were no significant differences among preoperative factors including primary tumor characters or preoperative lab results. However, in the perioperative factors, there were significant differences in intraoperative blood loss and blood transfusion volume. With severe SOS, more blood loss occurred and, accordingly, more transfusion was performed (Table 3).
Table 3. Perioperative outcomes according to the grade of sinusoidal obstructive syndrome.
Values are presented as number (%) or mean ± standard deviation.
PSH, parenchymal sparing hepatectomy; pRBC, packed RBC; PHLF, post-hepatectomy liver failure, POD, postoperative day; TB, total bilirubin; FOLFOX, 5-FU/leucovorin/oxaliplatin; FOLFIRI, 5-FU/leucovorin/irinotecan.
a)Clavien-Dindo classification
There were 2 mortality cases during hospitalization after liver resection; 1 patient with SOS and the other without SOS. One was the patient who previously underwent colectomy for colon cancer. Neoadjuvant chemotherapy was performed by FOLFOX regimen for 7 cycles. Three weeks following, as a staged approach to advanced metastasis, he underwent a right hepatectomy and left partial hepatectomy with splenectomy, and distal pancreatectomy. It was confirmed as a sinusoidal injury by a pathologic review of non-tumor lesion from the liver specimen. During postoperative care, PHLF occurred. It progressed to multiorgan failure accompanied by respiratory failure and renal failure. Eventually, he expired after POD 45. The other underwent synchronous surgery for colon cancer and liver metastasis 3 weeks after neoadjuvant chemotherapy. For liver metastasis, he underwent a right hepatectomy. Chemotherapy was performed for 4 cycles using the FOLFOX regimen.
There was no sinusoidal injury on the pathologic review. On the second day after surgery, bleeding occurred in the colon anastomosis site; thus, bleeding control was performed through angiography. However, he could not recover his general condition. Renal failure occurred and progressed to multiorgan failure. He expired after POD 23.
DISCUSSION
Liver injury induced by chemotherapy regimens for CRLM typically includes SOS and steatosis. The occurrence of steatosis is known to be influenced by patient characteristics such as obesity, diabetes, and metabolic syndrome in addition to chemotherapy. In contrast, SOS is mainly related to the drugs used in chemotherapy regimens. SOS induced by chemotherapy in CRLM has been reported to have negative effects on post-hepatectomy outcomes. However, the exact mechanism of this process is still poorly understood, and the perioperative factors that contribute to SOS are still controversial.
When severe SOS occurs, there is an increased risk of blood loss during liver resection, and transfusion requirements also increase accordingly [5,15]. These results can be explained by the pathophysiology of SOS. Through in vivo studies performed in rats, it has been found that drugs administered for chemotherapy or radiotherapy, as well as other factors, can activate and damage sinusoidal endothelial cells. The destruction of this endothelial cell lining creates gaps in the sinusoidal barrier, allowing RBCs, leukocytes, and other cellular debris to penetrate the space of Disse. The slowed sinusoidal lining cells obstruct the sinusoidal flow, leading to post-sinusoidal hypertension [16,17]. As a result, patients with SOS are at increased risk of bleeding during hepatectomy. Although there is some debate regarding the contribution of SOS to increased postoperative complications and perioperative blood loss, many studies have reported that SOS can increase blood loss during hepatectomy, and our study also observed this phenomenon.
As mentioned earlier, as seen in most papers, regimen specificity has been observed in liver toxicity. In our study, however, this was not confirmed. It is speculated that this discrepancy may be due to the high prevalence of patients in our study who were exposed to multiple regimens repetitively and in combination.
It’s difficult to predict the development of SOS before surgery. Previous studies have suggested that factors such as female sex, the number of chemotherapy cycles, the interval between the end of chemotherapy and liver resection, and preoperative abnormal liver function tests may be associated with the incidence of SOS. However, the exact cause of SOS has not yet been revealed. Therefore, a definitive predictive model has yet to be established. Pathological confirmation remains the most reliable way to diagnose SOS. In this study, we investigated the risk factors for SOS, but we found no significant differences in the aforementioned factors between the SOS (+) and SOS (−) groups. However, some factors in the SOS (+) group were slightly higher than in the SOS (−) group. The chemotherapy-free interval was shorter by about 2 weeks in the SOS (+) group (P = 0.069). The increase in spleen volume, as indicated by the splenic index, was greater in the SOS (+) group (P = 0.173). These findings may be limited by the small number of objects, and additional studies on a larger scale will be necessary to confirm these results.
Zhao et al. [5] published that SOS was associated with an increase in the incidence of postoperative complications. Similar results were obtained in this study as well. There was no significant difference observed when comparing the occurrence of SOS and all complications. However, it appears that the occurrence of liver surgery-specific complications, such as ascites, pleural effusion, and bile leakage, were associated with SOS. PHLF was more common in patients with SOS, but the difference was statistically insignificant, possibly due to the small sample size. Therefore, a larger study with a greater number of patients may yield results with significant differences.
The occurrence of SOS seems to be linked to early recurrence, particularly intrahepatic recurrence, but it has been controversial as to whether it can negatively impact OS. However, there have been reports suggesting that SOS may reduce the chemotherapy response, thereby having a negative impact on OS [8,18]. In our study, patients with SOS also had a higher incidence of intrahepatic recurrences and demonstrated lower survival rates.
There are a few limitations in our study. First, this study was a retrospective analysis, which means that there was variability in the chemotherapy and perioperative procedures for all patients. Therefore, the results we obtained may not fully reflect the degree of CALI, due to the diversity in the timing of hepatectomy after the end of chemotherapy, perioperative CT scans, and laboratory tests performed. Secondly, this study was conducted in a single center; therefore, further large-scale studies are necessary to confirm our findings. Thirdly, patients who did not undergo liver resection after chemotherapy could not be analyzed because there was no pathologic data. However, the pathologic data of all patients who underwent surgery at our hospital was reviewed by a single pathologist, and consistent classification was possible, which helped to decrease selection bias.
The occurrence of SOS resulting from neoadjuvant chemotherapy has a negative impact on peri-operative and postoperative outcomes. SOS results in significant bleeding during hepatectomy, requiring frequent blood transfusion. Moreover, it leads to rapid intrahepatic recurrence and poor OS. It should be noted that the possibility of SOS may be higher if the interval from chemotherapy to surgery is short or the increase in SI is large. This requires further research through large-scale and randomized controlled trial studies in the future.
Footnotes
Fund/Grand Support: None.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: HJC.
- Formal Analysis: YW.
- Investigation: YW, SHL.
- Methodology: HJC, THH, YKY.
- Project Administration: HJC, THH, YKY.
- Writing – Original Draft: YW, SHL.
- Writing – Review & Editing: HJC, YC, SEP, THH, YKY.
References
- 1.Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14:89–103. doi: 10.5114/pg.2018.81072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McWhirter D, Kitteringham N, Jones RP, Malik H, Park K, Palmer D. Chemotherapy induced hepatotoxicity in metastatic colorectal cancer: a review of mechanisms and outcomes. Crit Rev Oncol Hematol. 2013;88:404–415. doi: 10.1016/j.critrevonc.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Colorectal cancer facts & figures 2020–2022. American Cancer Society; 2020. [Google Scholar]
- 4.Valderrama-Treviño AI, Barrera-Mera B, Ceballos-Villalva JC, Montalvo-Javé EE. Hepatic metastasis from colorectal cancer. Euroasian J Hepatogastroenterol. 2017;7:166–175. doi: 10.5005/jp-journals-10018-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao J, van Mierlo KM, Gómez-Ramírez J, Kim H, Pilgrim CH, Pessaux P, et al. Systematic review of the influence of chemotherapy-associated liver injury on outcome after partial hepatectomy for colorectal liver metastases. Br J Surg. 2017;104:990–1002. doi: 10.1002/bjs.10572. [DOI] [PubMed] [Google Scholar]
- 6.Gosavi R, Chia C, Michael M, Heriot AG, Warrier SK, Kong JC. Neoadjuvant chemotherapy in locally advanced colon cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2021;36:2063–2070. doi: 10.1007/s00384-021-03945-3. [DOI] [PubMed] [Google Scholar]
- 7.Stevenson HL, Prats MM, Sasatomi E. Chemotherapy-induced sinusoidal injury (CSI) score: a novel histologic assessment of chemotherapy-related hepatic sinusoidal injury in patients with colorectal liver metastasis. BMC Cancer. 2017;17:35. doi: 10.1186/s12885-016-2998-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamandl D, Klinger M, Eipeldauer S, Herberger B, Kaczirek K, Gruenberger B, et al. Sinusoidal obstruction syndrome impairs long-term outcome of colorectal liver metastases treated with resection after neoadjuvant chemotherapy. Ann Surg Oncol. 2011;18:421–430. doi: 10.1245/s10434-010-1317-4. [DOI] [PubMed] [Google Scholar]
- 9.Imai K, Emi Y, Iyama KI, Beppu T, Ogata Y, Kakeji Y, et al. Splenic volume may be a useful indicator of the protective effect of bevacizumab against oxaliplatin-induced hepatic sinusoidal obstruction syndrome. Eur J Surg Oncol. 2014;40:559–566. doi: 10.1016/j.ejso.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Soubrane O, Brouquet A, Zalinski S, Terris B, Brézault C, Mallet V, et al. Predicting high grade lesions of sinusoidal obstruction syndrome related to oxaliplatin-based chemotherapy for colorectal liver metastases: correlation with post-hepatectomy outcome. Ann Surg. 2010;251:454–460. doi: 10.1097/SLA.0b013e3181c79403. [DOI] [PubMed] [Google Scholar]
- 11.Miyata T, Takamura H, Kin R, Nishiki H, Hashimoto A, Fujii Y, et al. Spleen volume as a predictive biomarker for thrombocytopenia and liver dysfunction after oxaliplatin-based chemotherapy. Anticancer Res. 2020;40:3361–3370. doi: 10.21873/anticanres.14319. [DOI] [PubMed] [Google Scholar]
- 12.Angitapalli R, Litwin AM, Kumar PR, Nasser E, Lombardo J, Mashtare T, et al. Adjuvant FOLFOX chemotherapy and splenomegaly in patients with stages II-III colorectal cancer. Oncology. 2009;76:363–368. doi: 10.1159/000210025. [DOI] [PubMed] [Google Scholar]
- 13.Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA NASH Clinical Research Network (CRN) Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53:810–820. doi: 10.1002/hep.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 15.Aloia T, Sebagh M, Plasse M, Karam V, Lévi F, Giacchetti S, et al. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol. 2006;24:4983–4990. doi: 10.1200/JCO.2006.05.8156. [DOI] [PubMed] [Google Scholar]
- 16.Carreras E, Diaz-Ricart M. The role of the endothelium in the short-term complications of hematopoietic SCT. Bone Marrow Transplant. 2011;46:1495–1502. doi: 10.1038/bmt.2011.65. [DOI] [PubMed] [Google Scholar]
- 17.Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, et al. Sinusoidal obstruction syndrome/veno-occlusive disease: current situation and perspectives: a position statement from the European Society for Blood and Marrow Transplantation (EBMT) Bone Marrow Transplant. 2015;50:781–789. doi: 10.1038/bmt.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viganò L, Capussotti L, De Rosa G, De Saussure WO, Mentha G, Rubbia-Brandt L. Liver resection for colorectal metastases after chemotherapy: impact of chemotherapy-related liver injuries, pathological tumor response, and micrometastases on long-term survival. Ann Surg. 2013;258:731–742. doi: 10.1097/SLA.0b013e3182a6183e. [DOI] [PubMed] [Google Scholar]