Abstract
Barriers to replication of viruses in potential host cells may occur at several levels. Lack of suitable and functional receptors on the host cell surface, thereby precluding entry of the virus, is a frequent reason for noninfectivity, as long as no alternative way of entry (e.g., pinocytosis, antibody-dependent adsorption) can be exploited by the virus. Other barriers can intervene at later stages of the virus life cycle, with restrictions on transcription of the viral genome, incorrect translation and posttranslational processing of viral proteins, inefficient viral assembly, and release or efficient early induction of apoptosis in the infected cell. The data we present here demonstrate that replication of caprine arthritis-encephalitis virus (CAEV) is restricted in a variety of human cell lines and primary tissue cultures. This barrier was efficiently overcome by transfection of a novel infectious complete-proviral CAEV construct into the same cells. The successful infection of human cells with a vesicular stomatitis virus (VSV) G-pseudotyped Env-defective CAEV confirmed that viral entry is the major obstacle to CAEV infection of human cells. The fully efficient productive infection obtained with the VSV-G-protein-pseudotyped infectious CAEV strengthened the evidence that lack of viral entry is the only practical barrier to CAEV replication in human cells. The virus thus produced retained its original host cell specificity and acquired no propensity to propagate further in human cultures.
Lentiviruses, a genus of the family Retroviridae, infect mammalian hosts from several orders, including the primates (e.g., humans and several species of African monkeys), carnivores (feline), and artiodactyls (bovine, small ruminants, equine). They cause lifelong persistent infection, which may be essentially symptomless or results in a variety of inflammatory, degenerative, or immunosuppressive diseases in a variable proportion of infected hosts. Their epidemiological, economic, and public health impacts are considerable, and the insidious nature of the infection raises concerns about possible spread of lentiviral infections to novel host species. Lentiviruses have been considered to be highly species-specific pathogens. However, the phylogenetic relationship between them clearly indicates a common origin, so viral spread between distantly related taxa must presumably occur, even if only rarely (10, 15). In fact, several observations suggest possible passage from species to species and even jumping to other genera and families. It is clear now that human immunodeficiency virus type 1 (HIV-1) and HIV-2 in humans result from cross-species passages of lentiviruses naturally infecting nonhuman primates. The source of HIV-2 in humans (Hominidae family) appears to have been sooty mangabeys (Cercopithecidae family) harboring simian immunodeficiency virus smm (SIVsmm) (4, 14), whereas the source of HIV-1 has recently been proved to be chimpanzees (10, 15). Phylogenetic analyses also provided evidence that recombination events have occurred between divergent viruses in vivo, indicating coinfection with highly divergent viral strains can occur in HIV-infected humans and SIV-infected primates (10, 17, 31). Caprine arthritis-encephalitis virus (CAEV) and maedi-visna virus (MVV), the small-ruminant lentivirus prototypes, are pathogens closely related to HIV-1 and HIV-2. CAEV was isolated from goats and MVV was isolated from sheep (6, 26, 32). CAEV infection of goats and MVV infection of sheep are present worldwide and induce inflammatory disease mainly in the joints, mammary glands, lungs, and central nervous system (8, 25, 33). Unlike primate lentiviruses, CAEV and MVV do not induce immunodeficiency in infected sheep and goats and are not tropic for CD4+ T lymphocytes (11, 25). In a recent study, we demonstrated that CAEV replicates productively and efficiently in milk epithelial cells, suggesting their implication in virus transmission and pathogenesis (24). Several studies, including ours, demonstrated the presence of viruses genetically related to CAEV in sheep and the presence of viruses genetically related to MVV in goats from naturally infected flocks. These data suggested the absence of a barrier to CAEV and MVV cross-species infection in domestic small ruminants (18, 19, 39). The potential of these viruses to cross the species barrier and cause infection in other animals and humans has not yet been studied. We recently demonstrated that CAEV can cause productive persistent infection in mouflon (wild sheep), following experimental infection (13). Recent reports also suggested that some humans might harbor CAEV, perhaps as a result of consumption of untreated goat milk at an early age (A. Douvas, W. P. Cheevers, G. Ehresmann, D. T. Garcia, A. Levine, L. Xia, and J. Ju, Ninth Annu. Meet. Natl. Cooperat. Vacc. Dev., AIDS Weekly Plus, 2 June 1997).
The purpose of the present study was to investigate the potential of CAEV to infect human cells in vitro and the nature of any possible barrier that could prevent its replication in human cells. We show that, at the cellular level, the absence of suitable functional receptors is the only practical restriction.
MATERIALS AND METHODS
Cells and viruses.
Goat synovial membrane (GSM) cells were derived from a carpal synovial membrane explant from a goat embryo as previously described (26) and were grown in Eagle's minimum essential medium (MEM; Gibco BRL, Cergy Pontoise, France), supplemented with 8% fetal bovine serum (FBS; Gibco-BRL). The large T-immortalized goat embryo fibroblast cell line TIGEF (9) was obtained by transfection of GSM cells with a replication origin-deleted simian virus 40 plasmid (pMK16-SV40-ori-). Goat macrophages were derived from peripheral mononuclear cells from blood samples of CAEV-negative yearling goats as described previously (3).
Human epithelial cell lines TE671 (derived from a human rhabdomyosarcoma [ATCC 184B5]), A431 (derived from a human epidermoid carcinoma [ATCC CRL-155]), HeLa (derived from human cervix carcinoma [ATCC CCL2]), and 293 (derived from transformed primary embryo kidney cell [ATCC: CRL-1573]) were grown in Dulbecco's modified Eagle's medium supplemented with 10% FBS. Human monocytic cell lines U937 (derived from a histiocytic lymphoma [ATCC CRL-1593]) and THP-1 (derived from the peripheral blood of a 1-year-old boy with monocytic leukemia [ATCC TIB-201]) were cultured in nonadherent conditions in RPMI 1640 medium supplemented with 10% FBS. Differentiation into adherent macrophages was induced by addition of 20 ng of phorbol 12-myristate 13-acetate (PMA) (Sigma, Isle D'Abeau, France)/ml of medium. Primary human macrophages were derived from blood samples of a healthy HIV-negative donor. Peripheral blood mononuclear cells were isolated by Ficoll-Hypaque gradient centrifugation and then were cultured in macrophage differentiation medium with 20% human serum in Teflon flasks as described previously (2) to induce the maturation of monocytes into macrophages. Differentiated macrophages were seeded into six-well plates for 4 to 24 h, nonadherent cells were removed, and macrophage monolayers were grown in macrophage differentiation medium. Primary human synovial cells were obtained from an arthritic HIV-negative patient and were expanded by culture in MEM supplemented with 10% FBS.
Plasmids.
The pTR-UF5 plasmid carrying the green fluorescent protein (GFP) gene under the transcriptional control of the human cytomegalovirus (CMV) early promoter/enhancer was obtained from Clontech, Montigny Le-Brotonneux, France). The pHCMV-G plasmid expressing the G glycoprotein of vesicular stomatitis virus (VSV) under control of the same CMV promoter/enhancer was kindly provided by J. Burns.
Construction of a plasmid containing the complete CAEV proviral genome.
We generated a full-length infectious provirus molecular clone of CAEV by using the recently described recombinant plasmids pK9Kb and pBSΔ (38). Double digestion of pK9Kb plasmid DNA with SalI (Promega, Charbonnieres-Les-Bains, France) and PflmI (New England Biolabs, Saint-Quentin Yvelines, France) endonucleases under the conditions recommended by the supplier released an 8.4-kb fragment corresponding to the complete CAEV-CO genome lacking the 3′ end of the env gene and the 3′ long terminal repeat (LTR). This fragment was separated by gel electrophoresis and harvested by the freeze-and-squeeze method. Then, DNA was harvested using the microcolumns (Millipore, Saint-Quentin Yvelines, France). This fragment was inserted into the pBSΔ plasmid double digested with XhoI (Promega) and PflmI endonucleases under the conditions recommended by the supplier. This reconstituted the complete proviral CAEV-CO genome in one plasmid, which was found to replicate well in transformed JM109 bacteria, with a lower recombination rate than that of DH5α, particularly at a low growth temperature (<30°C).
Production of CAEV-pBSCA virus stock.
GSM cells (5 × 105) were transfected with 5 μg of pBSCA plasmid DNA by using the Lipofectin method with Lipofectamine (Gibco BRL) as described previously (9). Medium was changed every 3 days, and the development of cytopathic effect (CPE) was monitored. When the monolayer reached 50% CPE, virus stock was harvested 8 to 16 h after replacement with fresh culture medium. Titer of the virus stock was determined by infection of GSM cells with serial dilutions and scoring CPE development as described previously (23, 29).
Infection of cell lines with CAEV-pBSCA virus.
Cells were seeded at 5 × 104 cells/well in a six-well plate, and 24 h later, they were inoculated with CAEV-pBSCA at multiplicities of infection of 0.1, 1.0, and 10. At day 2, inoculated cells were rinsed two or three times with fresh medium and then were cultured for three more days. Culture medium from each well was harvested daily, clarified by filtration through a 0.45-μm-pore-size membrane, and stored at −70°C for virus assay on permissive GSM cells. Inoculated cells were cocultured with 5 × 104 GSM indicator cells and observed for development of CPE. The presence of CAEV genome sequences was also investigated by direct PCR or reverse transcription-PCR (RT-PCR).
PCR and RT-PCR analysis.
PCR analysis was performed on cell lysates by using Gag and actin primers as previously described (34). To increase the sensitivity of DNA amplification, two rounds of PCR were performed. After the second round of amplification, a fragment of 512 bp was expected. We used the human β-actin gene as an internal control for the integrity of the DNA lysates. After the second round of amplification using the actin primers, a fragment of 390 bp was expected as previously described (34).
Total cellular RNA was isolated from cell monolayers by using the acid guanidium thiocyanate method (5). RNA was reverse transcribed using Moloney murine leukemia virus reverse transcriptase and random hexamer primers as reported previously (23). PCR analysis was performed using the Gag primers GEX5 and GEX3 previously described (34).
Transfection of pBSCA into TE671 and THP-1 cell lines.
TIGEF and TE671 cells were transfected using the calcium phosphate method (12). The cells were seeded at 5 × 105 cells/25-cm2 flask and then, 24 h later, were transfected with 5 μg of pBSCA plasmid DNA. The cell monolayers were washed with phosphate-buffered saline 16 to 18 h later to remove the precipitate, and the medium was replaced. Culture medium of transfected cells was harvested at different times after transfection, clarified by filtration through a 0.45-μm-pore-size membrane, and stored at −70°C for infectious virus assay.
THP-1 (5 × 106) cells were seeded into 25-cm2 flasks and then 24 h later were transfected by the DEAE-dextran method (36). pBSCA DNA (10 μg) was adsorbed onto DEAE-dextran (500 mg/ml), added to the cells, and incubated for 1 h at 37°C. The cells were then incubated for 2.5 min in Tris-buffered saline, rinsed with medium lacking FBS, and then incubated in regular growth medium. Aliquots of transfected cells were cultured in medium containing 20 ng of PMA/ml 24 h posttransfection. Culture in the presence of PMA induced differentiation of about 30% of the THP-1 cells in culture. Medium was harvested from transfected cells every 24 h, clarified, and stored at −70°C for infectious virus assay.
The pTR-UF5 plasmid was used to control the efficiency of transfection. Expression of GFP was evaluated by FACScan analysis 72 h after transfection.
Radioimmunoprecipitation of virus-specific proteins.
Radioimmunoprecipitation of viral proteins was performed as previously described (3). Briefly, cells were seeded into six-well plates at 48 h posttransfection. The culture monolayers were preincubated for 2 h in MEM lacking methionine and cysteine, and then the proteins were radiolabeled for 16 to 18 h with 100 μCi of [35S]methionine-cysteine (Promix; Amersham, Orsay, France) in 1 ml of the same medium. Virus-specific proteins released into the supernatant or accumulated inside the cells were immunoprecipitated using the hyperimmune serum (G9615) from a goat which had received several injections of a mixture of three different CAEV and MVV-K1514 isolates. Clarified cell culture medium and cell lysates were incubated overnight at 4°C in the presence of 10 μl of G9615 serum and Sepharose protein A. Immunoprecipitated proteins were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and specific virus proteins were visualized by standard autoradiography.
RESULTS
Construction of a full-length infectious CAEV molecular clone.
The proviral genome of CAEV-CO was initially cloned in two fragments (28), and despite several attempts in many laboratories, there was no success in obtaining an infectious molecular clone that contains the complete proviral sequence of CAEV. To ensure a homogeneous virus source for infection and transfection of the different cell lines used in this study, we first constructed an infectious full-length proviral molecular clone of the CO strain of CAEV. The pK9Kb plasmid, which lacks about 400 bp of the 3′ end of the CAEV genome (38), was digested with SalI and PflmI to release an 8.4-kb CAEV genome lacking the 3′ end of the env gene and the 3′ LTR. This fragment was inserted between the XhoI and PflmI sites of the pBSΔ second plasmid, which contains the missing 3′ end of CAEV (Fig. 1). The resulting complete-genome construct (pBSCA) was stable in JM109 bacteria cultured at a temperature of <30°C to limit recombination, and no deletion in the viral genome was detected after repeated rounds of amplification. After transfection of pBSCA into GSM cells (23) or goat TIGEF cells (9), infectious cytopathic virus (CAEV-pBSCA) was released into the culture medium with titers in the range of 106 50% tissue culture infective doses (TCID50s)/ml at days 6 and 7.
FIG. 1.
Construction of pBSCA and pBSCAδE5 molecular clones. pK9Kb plasmid DNA was double digested with SalI and PflmI to release an 8.4-kb fragment corresponding to the complete CAEV-CO genome lacking the 3′ end of the env gene and 3′ LTR. This fragment was gel purified and then inserted into the pBSΔ plasmid DNA doubly digested with XhoI and PflmI. The isolated molecular clone corresponds to a reconstituted complete infectious proviral CAEV-CO genome (pBSCA). pBSCAδE5 was derived from the pBSCA plasmid by deletion of an 0.4-kb BamHI fragment in the envelope-coding sequence.
Lack of sensitivity of human cells to CAEV-pBSCA infection.
Human cell lines of epithelial (HeLa, A431, TE671) or monocyte/macrophage (THP-1, U937) origin, primary monocytes/macrophages from a healthy volunteer, and synovial cells from a rheumatic patient were inoculated with CAEV-pBSCA at a multiplicity of infection of 0.1, 1, and 10. Induction of CPEs in pure cultures and in cocultures with the indicator GSM cells was examined by microscopic observation. Search for viral proteins in the lysate and culture medium of inoculated cells was performed by radioimmunoprecipitation assay. PCR and RT-PCR techniques with CAEV-specific primers were used for detection of viral genomes. The results of all these examinations were consistently negative for all primary and continuous human cells we tested. In contrast, the TIGEF cell line used as a positive control produced easily detectable virus, viral proteins, and viral genomes. Lysates of 107 human cells readily supported amplification of the 393-bp actin gene fragment used as an internal control, but the 512-bp gag-specific fragment of the CAEV-pBSCA virus genome was never observed (data not shown).
Transfection of human cells with CAEV-pBSCA DNA.
The human epithelial cell lines TE671 and 293 were transfected with 5 μg of CAEV-pBSCA plasmid DNA for 5 × 105 cells by using the calcium phosphate method (12), and the THP-1 human monocyte/macrophage line was transfected with 10 μg of CAEV-pBSCA plasmid DNA for 107 cells by using the DEAE-dextran method (36). Transfection controls using the pTR-UF5 plasmid expressing the GFP gene under the control of the CMV promoter were carried out in the same conditions. Sixty-two percent of TE671 cells, 78% of 293 cells, and 61% of TIGEF cells expressed the GFP protein as detected by cytofluorometry 72 h postinfection. Transfection of THP-1 cell lines resulted in only 2% of the GFP-expressing cells.
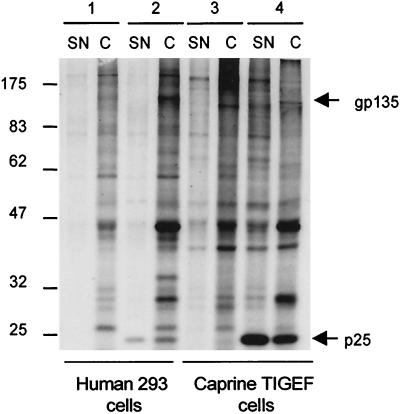
The CAEV-pBSCA-transfected cells developed no visible CPE, but following coculture with the indicator GSM cells, typical giant multinucleated cells developed (not shown). Viral proteins were detected in the lysate and culture medium of transfected but not nontransfected cells after metabolic radiolabeling with [35S]methionine-cysteine and immunoprecipitation with a polyvalent hyperimmune antiserum (G9615) from a goat multiply infected with three different isolates of CAEV and with the K-1514 strain of maedi-visna virus. The protein profiles shown in Fig. 2 indicate that the major Gag proteins and Env glycoproteins were correctly translated and processed. The supernatant medium contained the p25 Gag protein and the gp135 Env glycoprotein, suggesting that viral particles were released from the transfected cells. Culture medium collected from TE671 or THP-1 human cells, or from TIGEF positive control cells at 72 h after transfection with CAEV-pBSCA plasmid DNA, was shown to contain titers ranging from 103 to 104 TCID50s of infectious cytopathic CAEV per ml when tested on GSM cells. The THP-1 cells required the PMA treatment to induce their differentiation to macrophages with consequent viral expression, as already observed for HIV-1 productive infection in these cells.
FIG. 2.
Radioimmunoprecipitation of viral proteins from pBSCA-transfected human and goat cells by using a polyvalent hyperimmune serum. pBSCA plasmid DNA containing the complete proviral CAEV-CO genome was introduced by transfection into the human 293 epithelial (lanes 1 and 2) and the goat TIGEF fibroblastic (lanes 3 and 4) cell lines. The major CAEV p25 Gag protein and gp135 Env glycoproteins were detected in the pBSCA-transfected cells (lanes 2 and 4) but not in the nontransfected cells (lanes 1 and 3). These proteins were present both in the cell lysate (C) and in the supernatant (SN) of the pBSCA-transfected cells. The high-molecular-weight protein marker bandings are shown on the left.
To study comparatively the profile of CAEV production in human and caprine transfected cells, viruses in the culture medium were harvested daily and titrated in permissive GSM cells. The titers obtained were used to derive kinetic curves that are shown in Fig. 3. Whereas TIGEF cells showed a continual titer increase, reaching 106 TCID50s/ml by day 10, the supernatants from both transfected human cell lines reached maximum titers of 103 to 104 TCID50s/ml at day 4, then progressively decreased to 10 TCID50s/ml at day 10 posttransfection. This might indicate that the virus produced remained unable to infect human cells, thereby limiting the spread of infection, while the increase in TIGEF cells resulted from subsequent infections of these permissive cells. We confirmed that supernatants from the pBSCA-transfected human cells collected at day 4 (at the highest titer point) were unable to infect all tested human cell lines, as demonstrated by the absence of specific viral sequences in the treated cell's nucleic acids by PCR. However, the supernatant was still infectious and replication competent in goat cells.
FIG. 3.
Comparative kinetics of production of CAEV-pBSCA virus from human (TE671 and THP-1) and goat (TIGEF) cells. pBSCA plasmid DNA containing CAEV-CO genome was introduced by transfection into human (TE671 and THP-1) and goat (TIGEF) cell lines. Culture medium of transfected cells was harvested on days 2, 4, 6, 8, and 10 posttransfection, clarified by filtration through a 0.45-μm-pore-size membrane, and titrated for infectious virus in GSM cells as described in Materials and Methods.
Infection of human cells with VSV-G-protein-pseudotyped CAEV.
First, an Env-defective mutant of pBSCA was generated by deleting a 400-bp fragment in the SU part of the env gene also causing a shift of the reading frame for the residual part of the gene (pBSCAδE5; Fig. 1). Following transfection into GSM cells, this construct produced Gag proteins but no infectious progeny. It could be rescued by cotransfection with plasmid DNA expressing the complete env and rev sequences. Recombination events between these two plasmids generated replication-competent viruses (data not shown).
Cotransfection of pBSCAδE5 and pHCMV-G, a plasmid coding for the G glycoprotein of VSV (1), into TIGEF cells led to production of pseudotyped particles which were collected on day 4 and used to inoculate TE671 and THP-1 human cell lines. Cellular proteins were metabolically labeled with [35S]methionine-cysteine at day 5 postinfection and CAEV-specific proteins were immunoprecipitated with the hyperimmune G9615 serum (Fig. 4A). Uninfected THP-1 cells or those infected with transfection product from the nonpseudotyped pBSCAδE5 produced viral proteins neither in their cell lysate nor in their supernatant medium (samples 1 and 2). The cells infected with the pseudotyped particles produced abundant viral Gag proteins (sample 3). As only about 120 amino acids were deleted in the envelope glycoproteins, the band observed near the position expected for gp135 probably corresponds to the truncated nonfunctional Env glycoprotein.
FIG. 4.
Radioimmunoprecipitation of CAEV-specific proteins from human cell lines. (A) Immunoprecipitation of CAEV proteins from THP-1 human cell line either uninfected (lanes 1), infected with the supernatant from pBSCAδE5-transfected cells (lanes 2), or infected with supernatant from pBSCAδE5 and pHCMV-G cotransfected cells (lanes 3). (B) Immunoprecipitation of CAEV proteins from the TE671 human cell line either uninfected (lanes 1), infected with supernatant from pBSCA-transfected cells (lanes 2), or infected with supernatant from pBSCA and pHCMV-G cotransfected cells (lanes 3). CAEV viral proteins were detected both in the cell lysate (C) and in the supernatant (SN) of the human cell lines infected with VSV-G pseudotyped particles (A3 and B3). The high-molecular-weight protein marker bandings are shown on the left.
The results of these experiments indicated that an envelope-defective CAEV can be complemented with VSV-G protein envelope and thereby enabled to enter human cells and to direct the expression of viral proteins. However, they could not indicate whether these infected cells are capable of producing infectious particles since they lack a functional envelope. We therefore repeated the experiment using the replication-competent pBSCA and pHCMV-G to cotransfect the TIGEF cells. The produced pseudotyped virus was used to inoculate the human 293 and TE671 cells. Cellular proteins were metabolically labeled with [35S]methionine-cysteine, and CAEV-specific proteins were immunoprecipitated from cell lysates and supernatants from cells infected with the pseudotyped virus, but not from those infected with CAEV-pBSCA alone (Fig. 4B). Both Gag proteins and Env glycoproteins were detected, and the virus-bearing supernatant was infectious for susceptible goat cells with titers of up to 106 TCID50s/ml, although this virus was still incapable of infecting human cells (not shown).
DISCUSSION
The ability of small-ruminant lentiviruses to cause cross-species infection in human cells is subject to some controversy. There are reports on morphological and cytopathic changes in human astrocytes that have been inoculated with maedi-visna virus (20, 21); however, no observation of nucleic acid or viral protein expression in these cells was reported. Other recent observations (L. S. Tiley, personal communication) have reported the ability of maedi-visna virus to penetrate and replicate in some human cell lines. CAEV, on the other hand, has not been shown to replicate in human cells, and our present study shows that CAEV-pBSCA does not naturally infect a range of human cell lines or cells derived from primary human tissues. In addition, similar results were obtained with a French field isolate (CAEV-3112), suggesting that the restriction is not a property of CAEV-pBSCA (data not shown). The difference between the two viruses might reside in their use of different presently unknown receptors and/or co-receptors. We show in the present study that the restriction of CAEV replication in human cells depends only on its inability to penetrate the cell. Human cells do not form syncytia when cocultivated with CAEV-infected goat cells (data not shown). Interestingly, however, both transfection with infectious proviral DNA and infection with CAEV pseudotyped with VSV-G protein envelope resulted in efficient viral production and, in the relevant circumstances, production of fully infectious virus. The resulting virus retained the phenotypic character and biological properties of the parental CAEV, in that it was still incapable of infecting human cells.
The lack of replication of CAEV in human cells contrasts with the situation when similar cells are infected or transfected with feline immunodeficiency virus (FIV). This virus can readily enter human cells, and the FIV provirus is integrated into their genome (16, 27), but no virus is released into the supernatant medium of these cells. In this particular case, the major restriction in the process is the lack of active transcription of the provirus from the FIV LTR in human cells (22, 35). When the provirus is modified to be under transcriptional control of CMV promoter, abundant virus is produced and released in the culture medium of human cells (27). However, a further restriction concerning the proper functioning of FIV Rev in human cells has been described (37). The CAEV genome appears to be correctly expressed in human cells, and the titers (on caprine cells) of virus produced are comparable to those obtained from permissive goat cells. Furthermore, despite a low transfection efficacy of pBSCA into the THP-1 monocyte/macrophage human cell line (around 2%), these transfected cells produced much more infectious virus than the TE671 rhabdomyosarcoma cells in which 62% of the cells were transfected. This demonstrates that the preference for CAEV replication in the monocyte/macrophage cell lineage is still maintained in the human cellular context.
Production of CAEV particles pseudotyped with a polytropic functional envelope, which can target human cells, restores the ability of the virus to complete a full life cycle. Human cells infected with the pseudotyped virus maintained virus production for at least 25 days postinfection, suggesting stable integration of the provirus into the host chromosome. These data clearly suggest that CAEV might be considered as potentially able to cross the species barrier to human cells by a single acquisition of novel receptor specificity or by the cooperation of a helper virus. We also cannot exclude the possibility of infection of human cells following cell-to-cell contact with infected goat cells as we recently described for small-ruminant cells resistant to infection by ovine field isolates (34).
These findings also provide important information for the development of nonprimate lentiviral vectors for gene transfer. Indeed, to prevent these vectors from neutralization by human serum, CAEV-based vectors should be produced in human cell lines as described with murine leukemia virus-based vectors (7). The data here presented bring clear evidence that human cells can be used to derive packaging cell lines for production of CAEV-based vectors.
ACKNOWLEDGMENTS
This work was supported by grants from INRA and DGER. We thank Pasteur-Merieux-Connaught and the Fondation Merieux for grant support of the salary of L. Mselli-Lakhal.
We thank Timothy Greenland for helpful discussion and proofreading of the manuscript. We thank J. Burns for kindly providing the pHCMV-G plasmid.
REFERENCES
- 1.Burns J C, Friedmann T, Driever W, Burrascano M, Yee J K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chebloune Y, Karr B, Sheffer D, Leung K, Narayan O. Variations in lentiviral gene expression in monocyte-derived macrophages from naturally infected sheep. J Gen Virol. 1996;77:2037–2051. doi: 10.1099/0022-1317-77-9-2037. [DOI] [PubMed] [Google Scholar]
- 3.Chebloune Y, Sheffer D, Karr B M, Stephens E, Narayan O. Restrictive type of replication of ovine/caprine lentiviruses in ovine fibroblast cell cultures. Virology. 1996;222:21–30. doi: 10.1006/viro.1996.0394. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Luckay A, Sodora D L, Telfer P, Reed P, Gettie A, Kanu J M, Sadek R F, Yee J, Ho D D, Zhang L, Marx P A. Human immunodeficiency virus type 2 (HIV-2) seroprevalence and characterization of a distinct HIV-2 genetic subtype from the natural range of simian immunodeficiency virus-infected sooty mangabeys. J Virol. 1997;71:3953–3960. doi: 10.1128/jvi.71.5.3953-3960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chomczynsky P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 6.Cork L C, Narayan O. The pathogenesis of viral leukoencephalomyelitis-arthritis of goats. I. Persistent viral infection with progressive pathologic changes. Lab Investig. 1980;42:6596–6602. [PubMed] [Google Scholar]
- 7.Cosset F L, Takeuchi Y, Battini J L, Weiss R A, Collins M K. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J Virol. 1995;69:7430–7436. doi: 10.1128/jvi.69.12.7430-7436.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crawford T B, Adams D S, Cheevers W P, Cork L C. Chronic arthritis in goats caused by a retrovirus. Science. 1980;207:997–999. doi: 10.1126/science.6153243. [DOI] [PubMed] [Google Scholar]
- 9.Da Silva Teixeira M F, Lambert V, Mselli-Lakhal L, Chettab A, Chebloune Y, Mornex J F. Immortalization of caprine fibroblasts permissive for replication of small ruminant lentiviruses. Am J Vet Res. 1997;58:6579–6584. [PubMed] [Google Scholar]
- 10.Gao F, Bailes E, Robertson D L, Chen Y, Rodenburg C M, Michael S F, Cummins L B, Arthur L O, Peeters M, Shaw G M, Sharp P M, Hahn B H. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature. 1999;397:436–441. doi: 10.1038/17130. [DOI] [PubMed] [Google Scholar]
- 11.Gorrell M D, Brandon M R, Sheffer D, Adams R J, Narayan O. Ovine lentivirus is macrophagetropic and does not replicate productively in T lymphocytes. J Virol. 1992;66:2679–2688. doi: 10.1128/jvi.66.5.2679-2688.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham F L, Eb A J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 13.Guiguen F, Mselli-Lakhal L, Fornazero C, Du J, Favier C, Durand M, Grezel D, Chebloune Y. Experimental infection of mouflon-domestic sheep hybrids with caprine arthritis-encephalitis virus. Am J Vet Res. 2000;61:456–461. doi: 10.2460/ajvr.2000.61.456. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch V M, Olmsted R A, Murphey-Corb M, Purcell R H, Johnson P R. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature. 1989;339:389–392. doi: 10.1038/339389a0. [DOI] [PubMed] [Google Scholar]
- 15.Huet T, Cheynier R, Meyerhans A, Roelants G, Wain-Hobson S. Genetic organization of a chimpanzee lentivirus related to HIV-1. Nature. 1990;345:356–359. doi: 10.1038/345356a0. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda Y, Tomonaga K, Kawaguchi Y, Kohmoto M, Inoshima Y, Tohya Y, Miyazawa T, Chieeko K, Mikami T. Feline immunodeficiency virus can infect a human cell line (MOLT4) but establishes a state of latency in the cells. J Gen Virol. 1996;77:1623–1630. doi: 10.1099/0022-1317-77-8-1623. [DOI] [PubMed] [Google Scholar]
- 17.Jin M J, Rogers J, Phillips-Conroy J E, Allan J S, Desrosiers R C, Shaw G M, Sharp P M, Hahn B H. Infection of a yellow baboon with simian immunodeficiency virus from African green monkeys: evidence for cross-species transmission in the wild. J Virol. 1994;68:8454–8460. doi: 10.1128/jvi.68.12.8454-8460.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karr B M, Chebloune Y, Leung K, Narayan O. Genetic characterization of two phenotypically distinct North American ovine lentiviruses and their possible origin from caprine arthritis-encephalitis virus. Virology. 1996;225:1–10. doi: 10.1006/viro.1996.0569. [DOI] [PubMed] [Google Scholar]
- 19.Leroux C, Vuillermoz S, Mornex J F, Greenland T. Genomic heterogeneity in the pol region of ovine lentiviruses obtained from bronchoalveolar cells of infected sheep from France. J Gen Virol. 1995;76:1533–1537. doi: 10.1099/0022-1317-76-6-1533. [DOI] [PubMed] [Google Scholar]
- 20.Macintyre E H, Wintersgill C J, Watter A E. Visna virus infection of sheep and human cells in vitro—an ultrastructural study. J Cell Sci. 1973;13:173–191. doi: 10.1242/jcs.13.1.173. [DOI] [PubMed] [Google Scholar]
- 21.Macintyre E H, Wintersgill C J, Watter A E. Prolonged culture of visna virus in human astrocytes. Beitr Pathol. 1974;152:163–178. doi: 10.1016/s0005-8165(74)80117-4. [DOI] [PubMed] [Google Scholar]
- 22.Miyazawa T, Kawaguchi Y, Kohmoto M, Sakuragi J, Adaci A, Fukasawa M, Mikami T. Production of feline immunodeficiency virus in feline and non-feline non-lymphoid cell lines by transfection of an infectious molecular clone. J Gen Virol. 1992;73:1543–1546. doi: 10.1099/0022-1317-73-6-1543. [DOI] [PubMed] [Google Scholar]
- 23.Mselli-Lakhal L, Favier C, Da Silva Teixeira M F, Chettab A, Legras C, Ronfort C, Verdier G, Mornex J F, Chebloune Y. Defective RNA packaging is responsible for low transduction efficiency of CAEV-based vectors. Arch Virol. 1998;143:681–695. doi: 10.1007/s007050050323. [DOI] [PubMed] [Google Scholar]
- 24.Mselli-Lakhal L, Guiguen F, Fornazero C, Du J, Favier C, Durand J, Grezel D, Balleydier S, Mornex J F, Chebloune Y. Goat milk epithelial cells are highly permissive to CAEV infection in vitro. Virology. 1999;259:67–73. doi: 10.1006/viro.1999.9752. [DOI] [PubMed] [Google Scholar]
- 25.Narayan O. Lentiviruses are etiological agents of chronic diseases in animals and acquired immunodeficiency syndrome in humans. Can J Vet Res. 1990;54:42–48. [PMC free article] [PubMed] [Google Scholar]
- 26.Narayan O, Clements J E, Strandberg J D, Cork L C, Griffin D E. Biological characterization of the virus causing leukoencephalitis and arthritis in goats. J Gen Virol. 1980;50:69–79. doi: 10.1099/0022-1317-50-1-69. [DOI] [PubMed] [Google Scholar]
- 27.Poeschla E M, Wong-Staal F, Looney D J. Efficient transduction of nondividing human cells by feline immunodeficiency virus lentiviral vectors. Nat Med. 1998;4:354–357. doi: 10.1038/nm0398-354. [DOI] [PubMed] [Google Scholar]
- 28.Pyper J M, Clements J E, Gonda M A, Narayan O. Sequence homology between cloned caprine arthritis encephalitis virus and visna virus, two neurotropic lentiviruses. J Virol. 1986;58:665–670. doi: 10.1128/jvi.58.2.665-670.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reed L, Muench H. A simple method for estimating fifty per cent points. Am J Hyg. 1938;27:413–497. [Google Scholar]
- 30.Saltarelli M J, Querat G, Konings D A, Vigne R, Clements J E. Nucleotide sequence and transcriptional analysis of molecular clones of CAEV which generate infectious virus. Virology. 1990;179:347–364. doi: 10.1016/0042-6822(90)90303-9. [DOI] [PubMed] [Google Scholar]
- 31.Sharp P M, Robertson D L, Hahn B H. Cross-species transmission and recombination of 'AIDS' viruses. Philos Trans R Soc Lond B Biol Sci. 1995;349:41–47. doi: 10.1098/rstb.1995.0089. [DOI] [PubMed] [Google Scholar]
- 32.Sigurdsson B. Observations on three slow infections of sheep: maedi, paratuberculosis, rida, a slow encephalitis of sheep, with general remarks on infections which develop slowly, and some of their special characteristics. Br Vet J. 1954;110:255–270. [Google Scholar]
- 33.Sigurdsson B, Palson P A, Grimsson H. Visna, a demyelinating transmissible disease of sheep. J Neuropathol. 1957;16:389–403. doi: 10.1097/00005072-195707000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Singh D K, Chebloune Y, Mselli-Lakhal L, Karr B M, Narayan O. Ovine lentivirus-infected macrophages mediate productive infection in cell types that are not susceptible to infection with cell-free virus. J Gen Virol. 1999;80:1437–1444. doi: 10.1099/0022-1317-80-6-1437. [DOI] [PubMed] [Google Scholar]
- 35.Sparger E E, Shacklett B L, Renshaw-Gegg L, Barry P A, Pedersen N C, Elder J H, Luciw P A. Regulation of gene expression directed by the long terminal repeat of the feline immunodeficiency virus. Virology. 1992;187:165–177. doi: 10.1016/0042-6822(92)90305-9. [DOI] [PubMed] [Google Scholar]
- 36.Sussman D J, Milman D. Short-term, high-efficiency expression of transfected DNA. Mol Cell Biol. 1984;4:1641–1643. doi: 10.1128/mcb.4.8.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomonaga K, Miyazawa T, Kawaguc Y, Kohmoto M, Inoshima Y, Mikami T. Comparison of the rev transactivation of the feline immunodeficiency virus in feline and non-feline cell lines. J Vet Med Sci. 1994;56:199–201. doi: 10.1292/jvms.56.199. [DOI] [PubMed] [Google Scholar]
- 38.Turelli P, Petursson G, Guiguen F, Mornex J F, Vigne R, Querat G. Replication properties of dUTPase-deficient mutants of caprine and ovine lentiviruses. J Virol. 1996;70:1213–1217. doi: 10.1128/jvi.70.2.1213-1217.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valas S, Benoit C, Guionaud C, Perrin G, Mamoun R Z. North American and French caprine arthritis-encephalitis viruses emerge from ovine maedi-visna viruses. Virology. 1997;237:307–318. doi: 10.1006/viro.1997.8800. [DOI] [PubMed] [Google Scholar]