Abstract
Theiler's murine encephalomyelitis virus (TMEV) is a natural mouse pathogen which causes a lifelong persistent infection of the central nervous system (CNS) accompanied by T-cell-mediated myelin destruction leading to chronic, progressive hind limb paralysis. TMEV-induced demyelinating disease (TMEV-IDD) is considered to be a highly relevant animal model for the human autoimmune disease multiple sclerosis (MS), which is thought to be initiated as a secondary consequence of a virus infection. Although TMEV-IDD is initiated by virus-specific CD4+ T cells targeting CNS-persistent virus, CD4+ T-cell responses against self myelin protein epitopes activated via epitope spreading contribute to chronic disease pathogenesis. We thus examined the ability of antibodies directed against B7 costimulatory molecules to regulate this chronic virus-induced immunopathologic process. Contrary to previous studies showing that blockade of B7-CD28 costimulatory interactions inhibit the initiation of experimental autoimmune encephalomyelitis, treatment of SJL mice at the time of TMEV infection with murine CTLA-4 immunoglobulin or a combination of anti-B7-1 and anti-B7-2 antibodies significantly enhanced clinical disease severity. Costimulatory blockade inhibited early TMEV-specific T-cell and antibody responses critical in clearing peripheral virus infection. The inhibition of virus-specific immune responses led to significantly increased CNS viral titers resulting in increased damage to myelin-producing oligodendrocytes. Following clearance of the costimulatory antagonists, epitope spreading to myelin epitopes was accelerated as a result of the increased availability of myelin epitopes leading to a more severe chronic disease course. Our results raise concern about the potential use of B7-CD28 costimulatory blockade to treat human autoimmune diseases potentially associated with acute or persistent virus infections.
Theiler's murine encephalomyelitis virus (TMEV) is a picornavirus and a natural mouse pathogen which causes a lifelong persistent infection of the central nervous system (CNS) of susceptible mouse strains accompanied by T-cell-mediated myelin destruction leading to chronic, progressive hind limb paralysis (23, 24, 35). TMEV-induced demyelinating disease (TMEV-IDD) is considered to be a highly relevant animal model for the human autoimmune disease multiple sclerosis (MS). Both MS and TMEV-IDD are characterized by mononuclear cell infiltrates and areas of demyelination in the white matter of the CNS. In addition, MS also has a suspected virus etiology (17), as the incidence of MS varies according to a distinct geographical distribution, outbreaks of MS epidemics have been well documented, and higher relapse rates have been noted in patients after viral infection (11, 32).
Approximately 30 days after intracerebral inoculation of SJL animals with the BeAn 8386 strain of TMEV, mice demonstrate hind limb weakness and a waddling gait indicative of TMEV-IDD (23). Histological evidence shows a mononuclear cell infiltrate into the CNS consisting primarily of CD4+ T cells and macrophages (26). A wealth of evidence indicates that myelin destruction is T cell mediated (2, 3, 8, 39, 44). Demyelination is initiated by CD4+ T cells specific for virus epitopes. These T-cell responses arise within 7 to 10 days postinfection (2, 9, 46) and target CNS-persistent virus leading to macrophage-mediated bystander destruction of CNS myelin (16, 28, 29). Approximately 4 weeks after onset of clinical disease, i.e., 8 weeks postinfection, T-cell responses to myelin epitopes arise in an ordered temporal progression (30) consistent with a role for both virus- and myelin-specific responses in the chronic phase of disease. The appearance of myelin-specific responses and the lack of cross-reactivity between TMEV and myelin epitopes indicate that CNS autoimmunity arises by epitope spreading and is not due to molecular mimicry (i.e., shared virus and myelin epitopes) (27, 30, 42).
For complete activation, T cells require the delivery of at least two signals by antigen-presenting cells (APCs). Signal one is antigen specific and is delivered via the T-cell receptor by the peptide-major histocompatibility complex on the APC. The second “costimulatory” signal is largely provided via the CD28 molecule on T cells by its ligation with a member of the B7 family of molecules, B7-1 or B7-2, expressed on the APC (reviewed in references 12 and 20). CD28-mediated signaling results in the activation of both growth and survival factors for T cells (20). As the B7-CD28/CTLA-4 costimulatory system plays a critical role in determining the fate of immune responses (activation versus down-regulation), it serves as a promising therapeutic target for regulating autoimmune diseases and in other clinical situations where immune modulation is required (13, 43). Numerous studies from our laboratory and others clearly indicate that blockade of the B7-CD28 costimulatory pathway can prevent induction of several autoimmune diseases (7, 19, 33, 36), as well as serve as an effective therapy for established relapsing experimental autoimmune encephalomyelitis (EAE) (31).
Since both the initiation and progression of TMEV-IDD are T-cell-mediated events, we asked if treatment with antagonists of the B7-CD28 costimulatory pathway would be an effective means of interfering with disease initiation as has been previously reported for the various autoimmune disease models cited above. Interestingly, unlike other autoimmune disease models, treatment with CTLA-4 immunoglobulin (Ig) or a combination of antibodies against both B7-1 and B7-2 exacerbated the clinical disease course of TMEV-IDD. Blockade of B7-CD28 costimulation concomitant with virus infection resulted in significantly decreased TMEV-specific T-cell proliferative and antibody responses leading to an increased viral load in the CNS. During the chronic phase of disease, epitope spreading to self myelin epitopes was accelerated, presumably due to increased early direct virus-mediated damage to myelin-producing oligodendrocytes leading to accelerated release of myelin antigen, ultimately resulting in enhanced clinical disease. This study indicates that blockade of costimulatory interactions during the acute phase of a virus-initiated autoimmune disease such as TMEV-IDD, or perhaps during a virally induced MS relapse, may result in exacerbated clinical disease.
MATERIALS AND METHODS
Mice.
Five- to six-week-old female SJL mice were purchased from Jackson Laboratories (Bar Harbor, Maine). All mice were housed in Northwestern University animal care facility and were maintained on standard laboratory chow and water ad libitum.
Peptides.
Viral peptides VP270–86 (QEAFSHIRIPLPH) and VP324–37 (PIYGKTISTPSDY) and myelin peptides PLP139–151 (HSLGKWLGHPDKF) and PLP178–191 (NTWTTCQSIAFPSK) were synthesized on a synergy peptide synthesizer (Applied Biosystems, Foster City, Calif.) or purchased from Peptides International (Louisville, Ky.). Purity was confirmed by mass spectrometry.
Virus.
BeAn strain 8386 of TMEV was used in infection of mice and in proliferation and delayed-type hypersensitivity (DTH) assays. Virus was passaged in BHK-21 cells and partially purified from BHK lysate using polyethylene glycol precipitation and centrifugation through sucrose gradients (25).
Induction of TMEV-IDD and disease scores.
Mice were anesthetized with methoxyflurane and inoculated in the right cerebral hemisphere with 9 × 107 PFU of TMEV, BeAn 8386, in 30 μl of Dulbecco modified Eagle medium (DMEM). Mice were checked two or three times per week for clinical signs of disease until onset of disease when clinical symptoms were assessed two times per week. The clinical disease score is based on the development and progression of chronic gait abnormalities and spastic paralysis. Scores are assigned over a 6-point scale: 0, asymptomatic; 1, mild waddling gait; 2, severe waddling gait; 3, impaired righting reflexes; 4, impaired righting with accompanying dehydration; 5, total hind limb paralysis; 6, death.
Treatment of TMEV-infected mice with antagonists of costimulation.
Murine CTLA-4 Ig, a fusion protein between the mouse CTLA-4 molecule and the Fc portion of murine IgG2a, was obtained from Genetics Institute, Boston, Mass. Anti-B7-1 (16.10.A1) and anti-B7-2 monoclonal antibodies (MAbs; GL-1) were produced in an Acusyst Jr. bioreactor and purified as previously described (19). Antibodies were administered by intraperitoneal injection, beginning day 0 postinfection, every other day for a total of five treatments from day 0 to day 8 postinfection. Fifty micrograms of anti-B7-1 or anti-B7-2 antibody was given per injection for the single-treatment group, while 50 μg of each antibody in combination was administered to the double-treatment group for a total of 0.25 mg administered over the treatment regimen. Murine CTLA-4 Ig was given at 100 μg per injection, totaling 0.5 mg administered per animal. Control animals received 50 μg of hamster Ig (Cappell, Durham, N.C.) per antibody administration.
DTH analysis.
DTH responses were quantitated using a 24-h ear swelling assay. Prechallenge ear thickness was determined using a Mitutoyo model 7326 engineer's micrometer (Schlesinger's Tools, Brooklyn, N.Y.). Immediately thereafter, DTH responses were elicited by injecting 5 μg of VP270–86, 10 μg of PLP139–151, or 10 μg of PLP178–191 (in 10 μl of saline) into the dorsal surface of the ear using a 100-μl syringe fitted with a 30-gauge needle. The increase in ear thickness was determined 24 h after ear challenge. Results are expressed in units of 10−4 in. ± the standard error of the mean (SEM). Background swelling ranged between 3 × 10−4 and 10 × 10−4 in.
T-cell proliferation assays and IFN-γ assays.
Spleens of infected animals were removed and teased into a single-cell suspension over wire mesh in Hanks balanced salt solution (HBSS). Red blood cells were lysed by treatment with Tris-NH4Cl solution for 5 min at 37°C. Cells were washed with HBSS and resuspended in HL-1 media (BioWhittaker, Walkersville, Md.) supplemented with 1% l-glutamine and 1% penicillin-streptomycin (Gibco, Grand Island, N.Y.). Bulk splenocytes were plated in flat-bottom, 96-well plates (Costar, Corning, N.Y.) at 106 cells/well and stimulated with viral or myelin peptides in a range of concentrations (1 to 200 μM). Whole UV-inactivated virus was added at a concentration of 5 μg/well. Cell cultures were pulsed with 1 μCi of [3H]thymidine (TdR) (Amersham, Arlington Heights, Ill.) after 72 h and harvested 18 to 20 h thereafter (Packard, Meriden, Conn.). [3H]TdR incorporation was measured on a TopCount-NXT (Packard), and results are expressed as the means of triplicate cultures ± SEM (background counts subtracted). Supernatants collected at 24 and 48 h from replicate cultures were assayed for gamma interferon (IFN-γ) using Endogen (Woburn, Mass.) minikits by following the outlined protocol.
Assay of TMEV-specific antibody responses.
TMEV-specific antibody levels were determined by a specific enzyme-linked immunosorbent assay (ELISA). Ninety-six-well Maxisorp plates (Nunc, Naperville, Ill.) were coated overnight at room temperature with 1 μg of purified, UV-inactivated TMEV in phosphate-buffered saline (PBS). Plates were blocked the next day with 200 μl of PBS–2% bovine serum albumin–2% normal goat serum–0.05% Tween for 2 h. Plates were then washed four times with the same solution, and dilutions of serum samples were added in the blocking solution. Plates were incubated for 1 h and washed four times, and mouse anti-TMEV serum antibody was then detected by the addition of horseradish peroxidase-conjugated anti-mouse Ig isotypes (Caltag, San Francisco, Calif.) for 1 h at room temperature. Plates were washed and color was developed using tetramethylbenzidine substrate (Dako, Carpinteria, Calif.). The reactions was quenched by addition of 0.18 M H2SO4. Absorbance at 450 nm was measured using an ELISA reader (Molecular Devices, Menlo Park, Calif.) and analyzed using SOFTMax software. Purified BHK lysate was used as a negative control and plate blank. Results are expressed as sample optical density (OD) − blank OD.
Flow cytometry analysis.
All antibodies used were purchased from Pharmingen (San Diego, Calif.) except F4/80 fluorescein isothiocyanate (FITC), which was purchased from Caltag Laboratories. Splenocytes (2 × 106 per tube) were used for flow analysis. Cells to be stained were washed in isotonic saline–1% normal goat serum and blocked with 24G2 (anti-FcRIIIγ) supernatant and 1% normal mouse serum. Cells were then stained for three-color analysis with either anti-CD4 PerCP and anti-CD8 FITC or anti-F4/80 FITC and anti-B220 PerCP plus phosphatidylethanolamine (PE)-conjugated activation marker antibody for 30 min at 4°C in the dark. PE-conjugated isotype control antibodies were used to determine background staining levels. Cells were then washed twice in isotonic buffered saline-normal goat serum before analysis. Data were collected on a FACSCalibur flow cytometer (Becton-Dickinson, Mountain View, Calif.) and analyzed using Cellquest software.
Virus plaque assay.
Tissues from infected mice (two or three mice/time point) were pooled for analysis. Tissue was first weighed and then homogenized with a glass tissue homogenizer (Bellco, Vineland, N.J.) in sterile PBS. Tissue was diluted appropriately in DMEM–0.1% BSA and plated on a monolayer of BHK cells in 60- by 35-mm plastic tissue culture dishes (Nunc). Tissue homogenates were incubated for 45 min with shaking. Tissue homogenate was then removed, and the cells were overlayed with a warmed 1:1 mixture of DMEM and 0.1% BSA combined with noble agar solution. Plates were allowed to cool and placed at 33°C. Plaques were developed after 3 days using 0.025% neutral red (Sigma, St. Louis, Mo.) in PBS by overlaying the agar for approximately 1 h at 33°C, decanting, and allowing the plates to develop at 33°C for approximately 3 h.
Statistical analysis.
A statistical comparison of the percentages of animals showing clinical disease between any two groups of mice was performed by χ2 test using Fisher's exact probability. Comparisons of differences in mean peak disease severity and in immunological parameters between any two groups of mice were determined using Student's t test.
RESULTS
Blockade of B7-1 and B7-2 costimulation exacerbates the clinical and histologic course of TMEV-IDD.
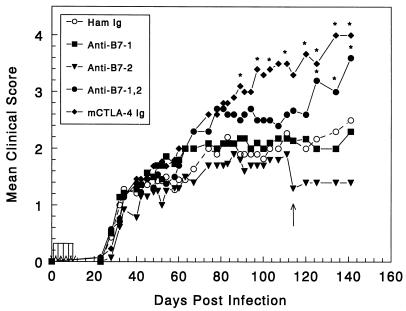
As blockade of B7-CD28-mediated costimulation has been shown to be effective in prevention and treatment of a variety of autoimmune diseases, we were interested to study the effects of costimulatory blockade for TMEV-IDD, a virus-induced, immune-mediated demyelinating disease with a chronic-phase autoimmune component (30). Six- to seven-week-old female SJL mice were infected with 9 × 107 PFU of TMEV. SJL mice were treated with costimulatory antagonists on days 0, 2, 4, 6, and 8 postinfection and were then monitored for clinical disease progression. As seen in Fig. 1, clinical signs of disease in all of the treatment groups began approximately 30 days postinfection, characteristic of a normal disease course. However, beginning around 60 to 70 days after TMEV infection, animals given mCTLA-4 Ig or combination injections of anti-B7-1 and anti-B7-2 showed a slightly increased mean clinical disease score. As disease progressed the CTLA-4 Ig-treated group (90 to 100 days postinfection), and slightly later the combined anti-B7 MAb-treated group (110 to 120 days postinfection), exhibited significantly increased mean clinical disease scores compared to the control mice treated with hamster Ig. Early administration of anti-B7-1 or anti-B7-2 MAbs alone had no significant effect on the progression of TMEV-IDD, perhaps indicating overlapping roles for these molecules in TMEV disease.
FIG. 1.
Early treatment with CTLA-4 Ig or a combination of anti-B7-1 and anti-B7-2 antibodies exacerbates clinical disease in Theiler's virus-infected SJL mice. Five- to six-week-old female SJL mice were infected intracerebrally with 9 × 107 PFU of TMEV. Beginning on the day of infection mice (n = 14 mice per group) were treated every other day for five total treatments with hamster control Ig or costimulatory antagonists according to the protocol in Materials and Methods. The mice were monitored for disease onset and progression of clinical symptoms for 142 days postinfection. ∗, clinical score significantly greater than that of hamster Ig-treated controls, P < 0.05. Disease course is representative of three or four separate experiments. Arrows indicate where animals were sacrificed for assay purposes.
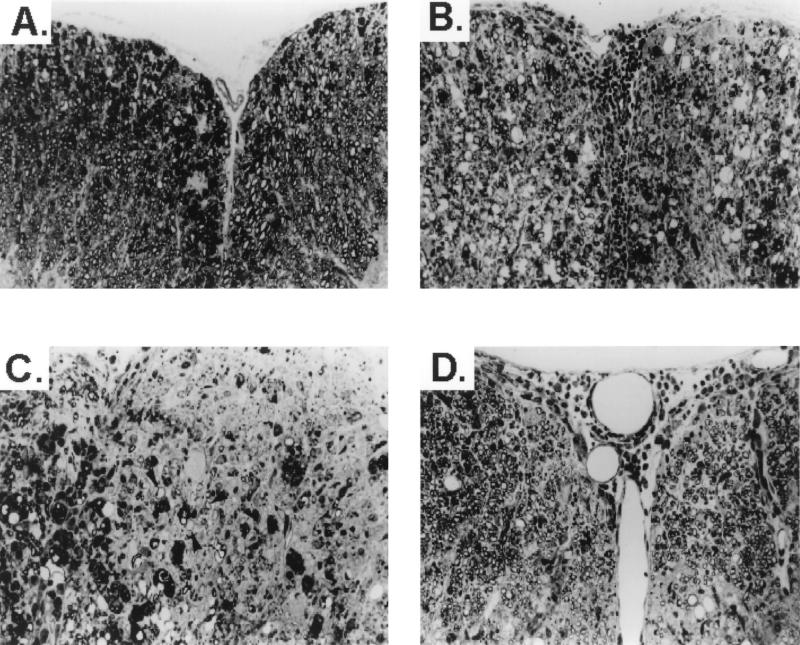
Representative mice from the treatment groups were analyzed for histologic evidence of inflammation and demyelination at 60 days postinfection. CNS sections from mice treated with CTLA-4 Ig exhibited more severe inflammation and demyelination (Fig. 2B and C) than TMEV-infected control mice treated with hamster Ig (Fig. 2A). Interestingly, there was extensive remyelination in the CTLA-4 Ig-treated mice examined at 116 days postinfection and the remyelination was mediated equivalently by oligodendrocytes and Schwann cells (Fig. 2D).
FIG. 2.
CTLA-4 Ig treatment leads to enhanced CNS myelin destruction and increased remyelination in TMEV-infected mice. (A) Section of spinal cord of hamster IgG-treated, TMEV-infected animal 60 days postinfection showing moderate myelin degeneration in both anterior columns. (B) Section from the spinal cord of a CTLA-4 Ig-treated mouse 63 days postinfection showing severe inflammation and demyelination in both anterior columns. (C) Section from a different area of the spinal cord of the CTLA-4 Ig-treated animal shown in panel B showing an area of active demyelination with numerous macrophages on the left and an area of chronic demyelination on the right. Arrowheads, axons surrounded by thin myelin, characteristic of remyelination. (D) Section from CTLA-4 Ig-treated animal 116 days postinfection shows mild residual inflammation, with both anterior columns showing extensive remyelination. Remyelinating cells are equally distributed between oligodendrocytes and Schwann cells. All sections are 1-μm-thick Epon-embedded sections stained with toluidine blue. Magnification, ×220.
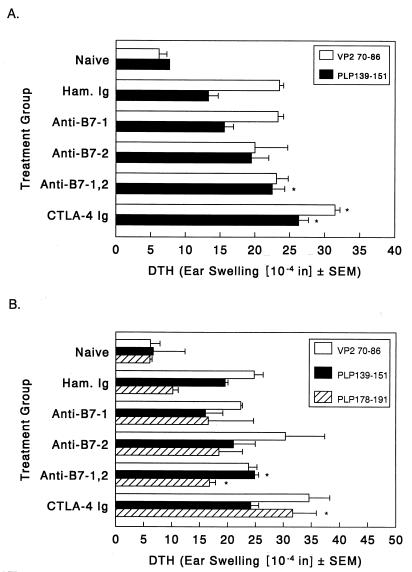
Blockade of B7-1 and B7-2 costimulation enhances virus-specific DTH and epitope spreading to myelin epitopes during chronic disease.
Normally, TMEV-infected SJL mice develop T-cell responses to the dominant epitope on proteolipid protein (PLP139–151) via epitope spreading approximately 55 to 60 days postinfection, i.e., 3 to 4 weeks after the onset of clinical-disease signs (30). PLP139–151-specific DTH responses were significantly enhanced above control values in the group treated with anti-B7-1 plus anti-B7-2 and the group treated with CTLA-4 Ig 50 days postinfection (Fig. 3A). There was also an enhanced response to the immunodominant viral peptide, VP270–86, in the CTLA-4 Ig-treated mice. Interestingly, the enhanced autoimmune responses at this early time point are not yet reflected by an increased disease score. However, myelin-specific DTH responses to PLP epitope PLP139–151 as well as to secondary PLP peptide PLP178–191 were still enhanced at 105 days postinfection (Fig. 3B), when clinical disease is exacerbated. The response to PLP178–191 is particularly significant since responses to this epitope are not detected in the peripheral immune system until approximately 150 days postinfection (unpublished results). Thus, increased clinical-disease severity in the groups treated with anti-B7-1 plus anti-B7-2 and CTLA-4 Ig is accompanied by increased and accelerated epitope spreading in these animals.
FIG. 3.
In vivo DTH responses to virus and myelin epitopes in costimulatory antagonist-treated mice. Three representative animals from each treatment regimen were analyzed for DTH response at 48 (A) and 105 days (B) postinfection. Mice were ear challenged with either 5 μg of VP270–86, 10 μg of PLP139–151, or 10 μg of PLP178–191, and ear swelling was measured 24 h later. Results are expressed as the change in ear swelling. ∗, DTH responses significantly above that of hamster Ig-treated controls, P < 0.05.
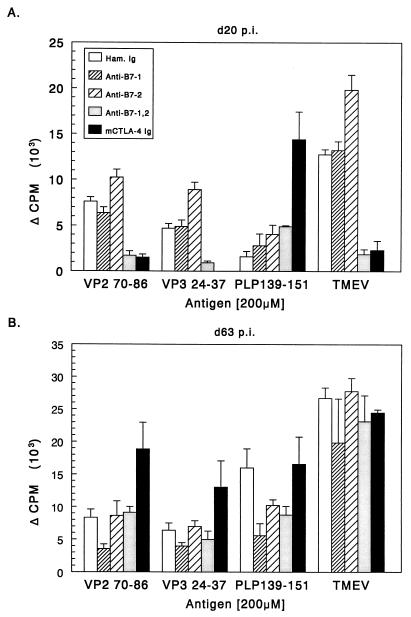
Blockade of B7-1 and B7-2 costimulation inhibits early T-cell proliferative and cytokine responses to TMEV but enhances myelin-specific responses.
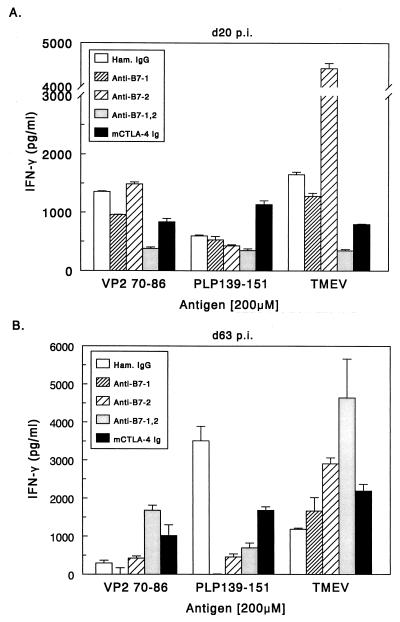
On day 20 postinfection, approximately the time when peripheral viremia is cleared and infected animals show potent responses to viral antigen (3), we assayed T-cell proliferative responses against both TMEV and myelin epitopes. At 20 days postinfection, splenic T cells from control mice proliferated (Fig. 4A) and produced IFN-γ (Fig. 5A) in response to UV-inactivated TMEV and to the dominant viral epitopes VP270–86 and VP324–37, but not in response to the immunodominant myelin epitope PLP139–151. Animals treated with anti-B7-1 alone and anti-B7-2 alone can also respond to whole virus and to the VP270–86 epitope. Stimulation index (SI) calculations show that all single-antibody treatment groups had good proliferative responses to whole TMEV and VP2 peptide (SI range, 3.8 to 11.1). Mice treated with either anti-B7-1 plus anti-B7-2 or CTLA-4 Ig in which both B7 molecules are targeted, failed to proliferate or produce significant levels of IFN-γ upon stimulation with whole virus or the viral epitopes. Interestingly, animals treated with CTLA-4 Ig during the first few days of viral infection exhibited significant proliferative and IFN-γ responses to the myelin peptide PLP139–151 (SI = 4.6) at this early time point, perhaps indicating accelerated epitope spreading in these animals. Therefore, it appears that blocking both B7-1 and B7-2 inhibits the ability of the animals to activate virus-specific cells but may lead to autoimmune responses by promoting direct release of self antigens from infected oligodendrocytes (see below). At 63 days postinfection (Fig. 4B and 5B) the groups treated with CTLA-4 Ig and anti-B7-1 plus anti-B7-2 exhibited significant proliferative and IFN-γ responses to virus, viral peptides, and PLP139–151. Th2 cytokines were undetectable in any treatment groups at either time point. This suggests that treatment with costimulatory antagonists at the time of viral infection may affect the early responses to viral antigens, but that animals recover the ability to mount Th1 responses against virus peptides as disease progresses and the virus persists in the CNS of the animal. The early decrease in virus-specific antigen responses in the periphery may delay viral clearance in the periphery and increase viral persistence in the CNS. An increase in viral load in the CNS could lead to increased myelin damage, thereby accelerating epitope spreading to endogenous myelin epitopes.
FIG. 4.
Splenic T-cell proliferative responses to viral and myelin epitopes in costimulatory antagonist-treated mice. Splenocytes from three representative animals were taken at 20 (A) and 63 days (B) postinfection. Splenocytes (106/well) were challenged with 5 μg of UV-inactivated TMEV or 200 μM concentrations of the indicated peptides for 72 h and pulsed with [3H]TdR for an additional 18 to 20 h. All groups made equivalent responses to concanavalin A (1 ng/ml) stimulation. Results are expressed as changes in counts per minute (backgrounds subtracted).
FIG. 5.
IFN-γ responses to viral and myelin epitopes in costimulatory antagonist-treated mice. Forty-eight-hour culture supernatants taken from the proliferation assays shown in Fig. 3 were used in IFN-γ ELISAs. ELISA analysis was performed as outlined in Materials and Methods. Results are means.
Blockade of B7-1 and B7-2 costimulation impairs antivirus antibody production.
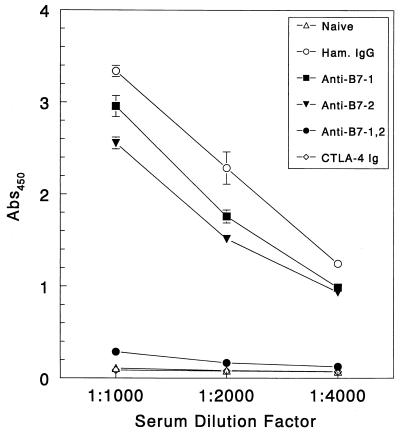
Anti-TMEV antibody responses are required for infected animals to properly keep viral replication in check and eventually clear the virus from the periphery (41). Suppression of B-cell responses in susceptible animals using anti-IgM antibodies accelerates and increases demyelination in treated animals (38). The importance of the antiviral antibody response early in disease led us to investigate whether treating with costimulatory antagonists during the early stages of viral infection would inhibit anti-TMEV antibody responses. We used pooled sera from three animals per treatment regimen in an analysis of total anti-TMEV IgG responses at 13 days postinfection to show that vigorous anti-TMEV IgG responses were produced by the control hamster Ig-treated mice, as well as the anti-B7-1- and the anti-B7-2-treated mice (Fig. 6). However, anti-TMEV IgG production was severely impaired in the groups treated with anti-B7-1 plus anti-B7-2 and CTLA-4 Ig. This lack of antibody response can be attributed to blockade of Th-cell activation required to produce IgG antibodies and control viral replication. Analysis of serum anti-TMEV IgG levels 54 days postinfection shows that the groups treated with CTLA-4 Ig and anti-B7-1 plus anti-B7-2 made anti-TMEV-specific antibody, although at lower levels than the other treatment groups (data not shown). Recovery of antibody responsiveness later in disease parallels a similar recovery of both proliferative responses and IFN-γ secretion seen in mice treated with the costimulatory molecule antagonists (Fig. 4 and 5), indicating that the effects of CD28 costimulation blockade are not long lasting (over 50 days). However, blockade early in disease dramatically affects early antiviral responses and eventually leads to increased disease severity.
FIG. 6.
TMEV-specific antibody responses are abolished in virus-infected mice treated with CTLA-4 Ig or anti-B7-1 plus anti-B7-2 MAbs. Serum was collected 13 days postinfection from three representative mice per group and pooled for analysis. Anti-TMEV antibody levels were determined on triplicate samples as detailed in Materials and Methods. Results are expressed as the net absorbance at 450 nm (OD for representative blank wells subtracted). Values are representative of three or four separate experiments.
Blockade of B7-1 and B7-2 costimulation alters peripheral levels of costimulatory molecule expression.
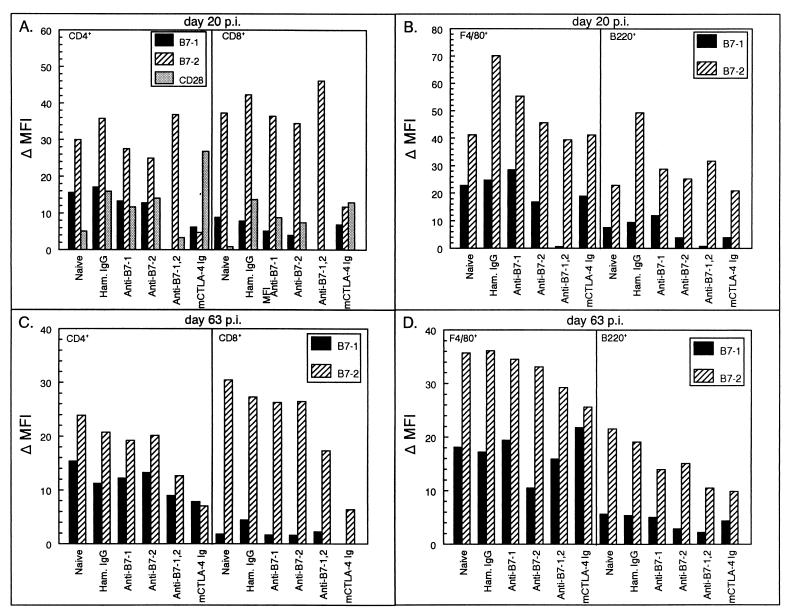
Because the initial peripheral response to virus is critical in viral clearance, inhibition of this response early in disease in mice treated with costimulatory antagonists could indicate a lack of activation of virus-specific cells. Flow cytometry analysis was performed on splenocytes from treated mice taken 12 days after the last antibody treatment (20 days postinfection). Early in disease, CTLA-4 Ig treatment decreased levels of B7-2 on the surfaces of both CD4+ and CD8+ T cells (Fig. 7A) as well as the number of B7-2+ CD4+ and CD8+ cells (8 and 21.5%, respectively, versus >50% in all other groups; data not shown). B7-1 surface expression was decreased by both the CTLA-4 Ig and anti-B7-1 plus anti-B7-2 treatments for both T-cell subsets, with CD4+ populations less than 5% B7-1+ compared to ∼40% in other treatment groups and <3% CD8+ compared to 10% B7-1+ in other groups. The combination treatment also decreased B7-1 expression on the surfaces of splenic F4/80+ macrophages (4% B7-1+) and B220+ B cells (2% B7-1+) (Fig. 7B). In groups treated with either anti-B7-1 or anti-B7-2 alone, surface expression of B7-1 and B7-2 and the percentage of positive cells were comparable to levels in hamster Ig-treated controls. Thus, it is unlikely that the decreased levels of B7-1 or B7-2 observed in the groups treated with CTLA-4 Ig and anti-B7-1 plus anti-B7-2 are due to interference by the treatment antibodies with the detection antibodies used in the flow analysis, but rather is likely due to an alteration in surface levels of costimulatory molecules due to antagonist treatment. Treatment with the costimulatory antagonists also altered the level of the T-cell costimulatory molecule CD28, decreasing CD28 levels in the combination therapy-treated mice and increasing CD28 levels in CTLA-4 Ig-treated mice. Analysis of other T-cell activation markers showed no difference between the treatment groups.
FIG. 7.
Phenotypic analysis of costimulatory molecule expression on splenic lymphocytes of TMEV-infected SJL mice treated with costimulatory molecule antagonists. Splenocytes from three representative animals per treatment group were harvested at 20 (A and B) and 63 days (C and D) postinfection and analyzed for cell surface expression of B7-1 and B7-2. For the analysis, cell gates were placed on CD4+, CD8+, F4/80+, or B220+ populations by histogram and these specific cell populations were analyzed for their levels of costimulatory molecule expression. Results are changes in mean fluorescent intensity (MFI) (MFI of cells stained with anti-B7 MAb − MFI of cells stained with isotype control antibody).
Interestingly, B7-1 and B7-2 expression levels were still altered later in the disease course (63 days postinfection), when clinical disease in the murine CTLA-4 Ig and anti-B7-1 plus anti-B7-2 treatment groups began to accelerate above control levels (Fig. 7C and D). B7-2 levels on both T-cell and APC populations in the CTLA-4 Ig-treated mice remained low even months after cessation of treatment, with numbers of APCs expressing B7-2 decreasing dramatically over time. F4/80+ B7-2+ cells decreased from 64 to 33% while B220+ B7-2+ cells dropped from 55 to 22% (data not shown). B7-2 surface expression was also still decreased in mice treated with the combination of anti-B7-1 and anti-B7-2 antibodies despite a comparable number of cells expressing B7-2. In contrast, B7-1 surface expression was only minimally affected at this later time point. However, while numbers of T cells expressing B7-1 in the groups treated with CTLA-4 Ig and anti-B7-1 plus anti-B7-2 increased over time (∼20% CD4+ and <10% CD8+) the numbers remain lower than those for other treatment groups. This long-lasting alteration in peripheral costimulatory molecule expression may contribute to the increased disease severity and accelerated epitope spreading in the animals treated with CTLA-4 Ig and anti-B7-1 plus anti-B7-2. Sustained down-regulated levels of costimulatory molecule expression, as well as the numbers of cells expressing the individual costimulatory molecules, may thus influence immune activation events long after costimulatory antagonists are cleared.
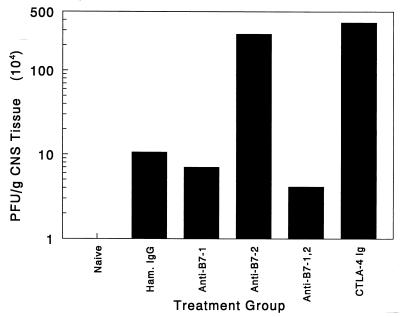
Blockade of B7-1 and B7-2 costimulation significantly enhances early CNS viral titers.
Early decreases in virus-specific proliferation, IFN-γ secretion, and IgG antibody production in the animals treated with CTLA-4 Ig and anti-B7-1 plus anti-B7-2 indicate that initial antiviral immunity was temporarily inhibited in these groups. This TMEV-unresponsive state could lead to an increased rate of viral replication delaying clearance of the peripheral viremia. The subsequent increase in viral load in the periphery and impaired antiviral immune responses may subsequently lead to an increased level of viral persistence in the CNS. To test this hypothesis, TMEV titers from spinal cord tissue of antagonist-treated and control animals were analyzed. Spinal cord tissue isolated on day 21 postinfection shows that treatment with CTLA-4 Ig fusion protein increases viral load by 40-fold over the hamster control Ig-treated group. Interestingly, mice treated with both anti-B7-1 and anti-B7-2 had CNS virus levels comparable to that of the control mice, while mice treated with anti-B7-2 alone displayed an increased CNS virus load at this time point. This suggests that B7-2-mediated costimulatory events may play a critical role in regulating the virus levels in the CNS. This is supported by the flow cytometry analysis (Fig. 7) wherein cell surface levels of B7-2 are decreased in animals treated with CTLA-4 Ig and anti-B7-1 plus anti-B7-2. Perhaps decreased cell surface levels of B7-2 on peripheral or CNS APCs, as is seen in these treatment groups, delays early host cytotoxic T lymphocyte responses, which have been shown to play a critical role in limiting CNS viral replication (6, 18). While the anti-B7-2-treated animals showed a high viral load in the CNS early in disease, the group also exhibited efficient proliferation and effector functions (Fig. 4 and 5) in response to stimulation by viral antigen, which is not displayed in the combination therapy and which may explain the increased viral titers seen in this group. Thus, in the anti-B7-2 treatment group, high viral load is accompanied by a fully competent antiviral response. However, complete blockade of CD28 costimulation in the groups treated with CTLA-4 Ig and anti-B7-1 plus anti-B7-2 also interferes with the host's ability to activate virus-specific proliferative responses, IFN-γ, and antibody production leading to increased viral persistence and accelerated myelin damage in the CNS.
DISCUSSION
TMEV-IDD is a CD4+ T-cell-mediated demyelinating disease of mice which serves as a model of human MS, a disease suspected to have a viral etiology (1, 4, 11, 17, 32). Demyelination is initiated by virus-specific CD4+ T cells targeting CNS-persistent virus thereby leading to macrophage-mediated bystander destruction of CNS myelin (2, 9, 16, 28, 29, 46). However, the chronic phase of disease is associated with the development of autoimmune responses to multiple myelin epitopes which arise via epitope spreading in an ordered temporal progression consistent with a role for both virus- and myelin-specific responses in the chronic phase of disease (27, 30).
T cells require both T-cell receptor and CD28-mediated costimulatory signals to undergo full activation (12, 20). We and others have shown that blockade of B7-CD28 costimulatory interactions is an effective means to regulate the induction and progression of EAE and other autoimmune diseases (7, 10, 19, 31, 33, 36). Since myelin destruction in TMEV-IDD is a T-cell-mediated pathologic process, we were interested in determining if blockade of B7-CD28 costimulatory interactions would also be efficacious in regulating the initiation and/or progression of a disease characterized by a persistent CNS virus infection. Interestingly, we report that treatments which abrogate autoimmune disease in EAE, NOD diabetes, and lupus models of autoimmunity exacerbated disease severity in TMEV-IDD when administered at the time of virus infection. Treatment of TMEV-infected SJL mice beginning on the day of virus infection with anti-B7-1 or anti-B7-2 alone had no effect on disease progression, indicating that either B7-1 or B7-2 can provide costimulation for TMEV-specific CD4+ T-cell responses. However, total blockade of B7-mediated signals by either treatment with a combination of anti-B7-1 and anti-B7-2 antibodies or with the fusion protein murine CTLA-4 Ig significantly increased clinical and histologic disease severity in TMEV-infected mice (Fig. 1 and 2). Interestingly, the increase in clinical disease severity was delayed, with differences only becoming apparent 50 to 60 days after the final antibody treatment. This slow development of exacerbated clinical disease in the groups treated with CTLA-4 Ig and anti-B7-1 plus anti-B7-2 MAb was associated with inhibition of early antivirus immune responses leading to a significantly increased CNS virus load.
Our results show that blocking both B7-1 and B7-2 early in the disease course significantly inhibits early TMEV-specific immune responses, including both Th1 reactivity (Fig. 4 and 5) and antibody responses (Fig. 6), resulting in a greatly increased CNS viral load (Fig. 8).. The diminution of virus-specific immunity by interfering with costimulation is not surprising. It is well known that interfering with costimulation causes immunosuppression in vivo. Complete blockade of CD28 costimulation often diminishes both antigen-specific antibody responses and proliferative responses and can lead to specific unresponsiveness in antigen-specific T cells (21, 22). Interestingly, the early immunosuppression and increase in CNS virus replication ultimately, after clearance of the B7 antagonists, lead to enhanced antiviral responses later in disease, facilitating accelerated and increased T-cell responses via epitope spreading to the immunodominant myelin PLP139–151 epitope. It is these accelerated antimyelin responses which are likely responsible for the enhanced clinical and histologic disease. One potential explanation consistent with our results which would account for both the enhanced antivirus and myelin-specific responses is that the lack of the early antiviral response leads to the production of increased levels of virus antigen and to direct virus damage of myelin-producing oligodendrocytes, thus enhanced release of myelin epitopes. Following clearance of the costimulatory antagonists the enhanced levels of virus and myelin antigens would lead to elevated virus-specific immune responses and to earlier and more potent autoimmune Th1 responses to the immunodominant myelin epitope as indicated by enhanced proliferative responses and IFN-γ secretion (Fig. 4 and 5). This is also supported by the enhanced macrophage infiltration and demyelination (Fig. 2) observed in CTLA-4 Ig-treated mice at 60 days postinfection; these are accompanied by increased Schwann cell remyelination, an indication of severe myelin damage and attempted repair (37, 40).
FIG. 8.
Viral titers in the CNS of costimulatory antagonist-treated mice are significantly enhanced in CTLA-4 Ig-treated mice. Spinal cords from three representative animals per treatment group were isolated 21 days postinfection, pooled, and homogenized in PBS. Viral plaque assays were carried out as outlined in Materials and Methods to determine the amount of live virus persisting in TMEV-infected mice. Results are representative of three or four separate experiments.
It is interesting to compare the present results with our previous results which showed that induction of TMEV-specific immunological tolerance induced by the intravenous administration of TMEV-pulsed, ethylene carbodiimide-fixed APCs within the first several weeks following TMEV infection resulted in long-term protection from clinical and histologic demyelinating disease (16). Tolerization with TMEV-coupled syngeneic splenocytes decreased TMEV-specific DTH responses at 60 days postinfection and also led to a significant diminution of serum anti-TMEV IgG2a antibody levels (15, 16). Since this form of tolerance is thought to work by blocking delivery of costimulatory signals in an antigen-specific manner, it is somewhat surprising that this therapy prevented development of TMEV-IDD. However, this tolerization regimen is known to target primarily Th1 responses, resulting in long-term unresponsiveness, while Th2 reactivity is unaffected and tolerant mice actually make enhanced levels of Th2-directed IgG1 antiviral antibodies (15, 34). Thus, unlike what was found for the short-term blockade of antivirus responses using either CTLA-4 Ig or anti-B7-1 plus anti-B7-2, the “tolerant” mice are most likely able to control viral replication via their enhanced ability to produce Th2-directed IgG1 antibodies, thus avoiding significant direct virus-induced pathology of oligodendrocytes, while at the same time proinflammatory cytokine production by the pathologic TMEV-specific Th1 cells is suppressed.
Interestingly, although the percentage and numbers of splenic lymphoid subsets were not significantly different from those of controls in any of the treatment groups, administration of CTLA-4 Ig and anti-B7-1 plus anti-B7-2 led to profound and, for B7-2, sustained down-regulation of costimulatory molecule expression on peripheral lymphoid and APC populations (Fig. 7). This early down-regulation of B7 expression on APC populations would help to account for the early suppressed antivirus T-cell and antibody responses likely resulting from a combination of TMEV-specific cells becoming anergic due to direct blockade of CD28-mediated costimulatory signals and antagonist-mediated down-regulation of their cell surface expression. Effects of the different treatment regimens on cell surface molecule expression may explain some differences in disease profiles resulting from treatment with anti-B7-1 plus anti-B7-2 and CTLA-4 Ig. Figure 7 shows that both treatments alter costimulatory molecule expression but that each treatment has a distinct effect on B7-1 and B7-2 expression. CTLA-4 Ig appears to have a dramatic and sustained effect on B7-2 levels, while the combination therapy more profoundly changes levels of B7-1 on the APC populations early in disease development (Fig. 7B). This altered surface molecule expression may indicate that the treatment molecules interact with their respective ligands using different methods. It has been proposed that the kinetics of CTLA-4 Ig fusion protein binding is significantly different from the kinetics of binding of the separate antibodies to B7-1 or B7-2. This difference in kinetics and whether the molecules do or do not cross-link the surface molecule may contribute to the differing levels of cell surface molecule expression and may ultimately contribute to the difference in viral titers seen in Fig. 8. However, it appears that complete blockade of costimulatory molecules in either manner significantly affects TMEV-IDD progression by interfering with the initiation of an efficient antiviral immune response. Additionally, the spreading of T-cell reactivity to endogenous myelin epitopes in relapsing EAE in the SJL mouse is highly dependent on B7-1-mediated costimulation (14, 31); thus it is possible that the “relative” increase in the capacity of B7-1 to deliver costimulatory signals after clearance of the B7 antagonists promoted the enhanced levels of Th1 responses to the PLP139–151 epitope and to the later recovery of responses to the immunodominant viral epitopes. Up-regulation of B7-1 costimulatory molecules has also been reported in lesions from MS patients (5, 45), implicating these molecules in immune-mediated CNS myelin damage.
Overall, the current data raise an important caution regarding the potential use of antagonists of the B7-CD28 costimulatory pathway in (auto)immune-mediated diseases associated with persistent virus infections. The results clearly show that blockade of this critical costimulatory pathway during a period of active viral replication leads to suppression of virus-specific immune responses and subsequent exacerbation of clinical demyelination. In fact our preliminary data show that treatment of TMEV-infected SJL mice with CTLA-4 Ig or anti-B7-1 plus anti-B7-2 beginning on either day 25 or day 45 postinfection (after initial virus clearance but prior to initiation of PLP139–151 responses) results in significantly decreased clinical disease accompanied by decreased myelin-specific T-cell responses. The ability of antagonists of CD28 costimulation to down-regulate antimyelin responses in TMEV-infected mice during chronic disease is encouraging for use as therapy in ongoing human autoimmune disease. Treatment with costimulatory antagonists during autoimmune disease would target T cells specific for self proteins, regardless of their antigen specificity. However, if autoimmune diseases such as MS are initiated and/or exacerbated by viral infection, treatment with antagonists of costimulatory molecules will have to be carefully applied, as blocking of costimulation may interfere with not only antiself responses but also antiviral responses necessary in sustained regulation of persistent viral infection.
ACKNOWLEDGMENTS
This study was supported in part by USPHS NIH grants NS23349 and NS34819.
REFERENCES
- 1.Allen I, Brankin B. Pathogenesis of multiple sclerosis–the immune diathesis and the role of viruses. J Neuropathol Exp Neurol. 1993;52:95–105. doi: 10.1097/00005072-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Clatch R J, Lipton H L, Miller S D. Characterization of Theiler's murine encephalomyelitis virus (TMEV)-specific delayed-type hypersensitivity responses in TMEV-induced demyelinating disease: correlation with clinical signs. J Immunol. 1986;136:920–927. [PubMed] [Google Scholar]
- 3.Clatch R J, Melvold R W, Miller S D, Lipton H L. Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease in mice is influenced by the H-2D region: correlation with TMEV-specific delayed-type hypersensitivity. J Immunol. 1985;135:1408–1414. [PubMed] [Google Scholar]
- 4.Cook S D, Rohowsky-Kochan C, Bansil S, Dowling P C. Evidence for multiple sclerosis as an infectious disease. Acta Neurol Scand. 1995;161:34–42. doi: 10.1111/j.1600-0404.1995.tb05854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Simone R, Giampaolo A, Giometto B, Gallo P, Levi G, Peschle C, Aloisi F. The costimulatory molecule B7 is expressed on human microglia in culture and in multiple sclerosis acute lesions. J Neuropathol Exp Neurol. 1995;54:175–187. doi: 10.1097/00005072-199503000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Dethlefs S, Brahic M, Larsson-Sciard E L. An early, abundant cytotoxic T-lymphocyte response against Theiler's virus is critical for preventing viral persistence. J Virol. 1997;71:8875–8878. doi: 10.1128/jvi.71.11.8875-8878.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finck B K, Linsley P S, Wofsy D. Treatment of murine lupus with CTLA4lg. Science. 1994;265:1225–1227. doi: 10.1126/science.7520604. [DOI] [PubMed] [Google Scholar]
- 8.Friedmann A, Frankel G, Lorch Y, Steinman L. Monoclonal anti-I-A antibody reverses chronic paralysis and demyelination in Theiler's virus-infected mice: critical importance of timing of treatment. J Virol. 1987;61:898–903. doi: 10.1128/jvi.61.3.898-903.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerety S J, Karpus W J, Cubbon A R, Goswami R G, Rundell M K, Peterson J D, Miller S D. Class II-restricted T cell responses in Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease. V. Mapping of a dominant immunopathologic VP2 T cell epitope in susceptible SJL/J mice. J Immunol. 1994;152:908–918. [PubMed] [Google Scholar]
- 10.Girvin A M, Dal Canto M C, Rhee L, Saloman B, Sharpe A H, Bluestone J A, Miller S D. A critical role for B7/CD28 costimulation in EAE: a comparative study using costimulatory molecule-deficient mice and monoclonal antibody blockade. J Immunol. 2000;164:136–143. doi: 10.4049/jimmunol.164.1.136. [DOI] [PubMed] [Google Scholar]
- 11.Johnson R T. The virology of demyelinating diseases. Ann Neurol. 1994;36(Suppl.):S54–S60. doi: 10.1002/ana.410360715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.June C H, Bluestone J A, Nadler L M, Thompson C B. The B7 and CD28 receptor families. Immunol Today. 1994;15:321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 13.Karandikar N J, Vanderlugt C L, Bluestone J A, Miller S D. Targeting the B7/CD28:CTLA-4 costimulatory system in CNS autoimmune disease. J Neuroimmunol. 1998;89:10–18. doi: 10.1016/s0165-5728(98)00058-7. [DOI] [PubMed] [Google Scholar]
- 14.Karandikar N J, Vanderlugt C L, Eagar T, Tan L, Bluestone J A, Miller S D. Tissue-specific up-regulation of B7-1 expression and function during the course of murine relapsing experimental autoimmune encephalomyelitis. J Immunol. 1998;161:192–199. [PubMed] [Google Scholar]
- 15.Karpus W J, Peterson J D, Miller S D. Anergy in vivo: down-regulation of antigen-specific CD4+ Th1 but not Th2 cytokine responses. Int Immunol. 1994;6:721–730. doi: 10.1093/intimm/6.5.721. [DOI] [PubMed] [Google Scholar]
- 16.Karpus W J, Pope J G, Peterson J D, Dal Canto M C, Miller S D. Inhibition of Theiler's virus-mediated demyelination by peripheral immune tolerance induction. J Immunol. 1995;155:947–957. [PubMed] [Google Scholar]
- 17.Kurtzke J F. Epidemiologic evidence for multiple sclerosis as an infection. Clin Microbiol Rev. 1993;6:382–427. doi: 10.1128/cmr.6.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsson-Sciard E L, Dethlefs S, Brahic M. In vivo administration of interleukin-2 protects susceptible mice from Theiler's virus persistence. J Virol. 1997;71:797–799. doi: 10.1128/jvi.71.1.797-799.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lenschow D J, Ho S C, Sattar H, Rhee L, Gray G, Nabavi N, Herold K C, Bluestone J A. Differential effects of anti-B7-1 and anti-B7-2 mAb treatment on the development of diabetes in the NOD mouse. J Exp Med. 1995;181:1145–1155. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenschow D J, Walunas T L, Bluestone J A. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 21.Lenschow D J, Zeng Y, Thistlethwaite J R, Montag A, Brady W, Gibson M G, Linsley P S, Bluestone J A. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992;257:789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 22.Linsley P S, Wallace P M, Johnson J, Gibson M G, Greene J L, Ledbetter J A, Singh C, Tepper M A. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 23.Lipton H L. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975;11:1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipton H L, Dal Canto M C. Chronic neurologic disease in Theiler's virus infection of SJL/J mice. J Neurol Sci. 1976;30:201–207. doi: 10.1016/0022-510x(76)90267-7. [DOI] [PubMed] [Google Scholar]
- 25.Lipton H L, Friedmann A. Purification of Theiler's murine encephalomyelitis virus and analysis of the structural virion polypeptides: correlation of the polypeptide profile with virulence. J Virol. 1980;33:1165–1172. doi: 10.1128/jvi.33.3.1165-1172.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipton H L, Kratochvil J, Sethi P, Dal Canto M C. Theiler's virus antigen detected in mouse spinal cord 2 1/2 years after infection. Neurology. 1984;34:1117–1119. doi: 10.1212/wnl.34.8.1117. [DOI] [PubMed] [Google Scholar]
- 27.Miller S D, Clatch R J, Pevear D C, Trotter J L, Lipton H L. Class II-restricted T cell responses in Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease. I. Cross-specificity among TMEV substrains and related picornaviruses, but not myelin proteins. J Immunol. 1987;138:3776–3784. [PubMed] [Google Scholar]
- 28.Miller S D, Gerety S J. Immunologic aspects of Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease. Semin Virol. 1990;1:263–272. [Google Scholar]
- 29.Miller S D, Gerety S J, Kennedy M K, Peterson J D, Trotter J L, Tuohy V K, Waltenbaugh C, Dal Canto M C, Lipton H L. Class II-restricted T cell responses in Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease. III. Failure of neuroantigen-specific immune tolerance to affect the clinical course of demyelination. J Neuroimmunol. 1990;26:9–23. doi: 10.1016/0165-5728(90)90115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller S D, Vanderlugt C L, Begolka W S, Pao W, Yauch R L, Neville K L, Katz-Levy Y, Carrizosa A, Kim B S. Persistent infection with Theiler's virus leads to CNS autoimmunity via epitope spreading. Nat Med. 1997;3:1133–1136. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- 31.Miller S D, Vanderlugt C L, Lenschow D J, Pope J G, Karandikar N J, Dal Canto M C, Bluestone J A. Blockade of CD28/B7-1 interaction prevents epitope spreading and clinical relapses of murine EAE. Immunity. 1995;3:739–745. doi: 10.1016/1074-7613(95)90063-2. [DOI] [PubMed] [Google Scholar]
- 32.Nathanson N, Miller A. Epidemiology of multiple sclerosis: critique of evidence for a viral etiology. Am J Epidemiol. 1978;107:451–461. doi: 10.1093/oxfordjournals.aje.a112564. [DOI] [PubMed] [Google Scholar]
- 33.Perrin P J, Scott D, Quigley L, Albert P S, Feder O, Gray G S, Abe R, June C H, Racke M K. Role of B7:CD28/CTLA-4 in the induction of chronic relapsing experimental allergic encephalomyelitis. J Immunol. 1995;154:1481–1490. [PubMed] [Google Scholar]
- 34.Peterson J D, Karpus W J, Clatch R J, Miller S D. Split tolerance of Th1 and Th2 cells in tolerance to Theiler's murine encephalomyelitis virus. Eur J Immunol. 1993;23:46–55. doi: 10.1002/eji.1830230109. [DOI] [PubMed] [Google Scholar]
- 35.Pevear D C, Calenoff M, Rozhon E, Lipton H L. Analysis of the complete nucleotide sequence of the picornavirus Theiler's murine encephalomyelitis virus indicates that it is closely related to cardioviruses. J Virol. 1987;61:1507–1516. doi: 10.1128/jvi.61.5.1507-1516.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Racke M K, Scott D E, Quigley L, Gray G S, Abe R, June C H, Perrin P J. Distinct roles for B7-1 (CD80) and B7-2 (CD86) in the initiation of experimental allergic encephalomyelitis. J Clin Investig. 1995;96:195–203. doi: 10.1172/JCI118274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez M. Mechanisms of virus-induced demyelination and remyelination. Ann N Y Acad Sci. 1988;540:240–251. doi: 10.1111/j.1749-6632.1988.tb27066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez M, Kenny J J, Thiemann R L, Woloschak G E. Theiler's virus-induced demyelination in mice immunosuppressed with anti-IgM and in mice expressing the xid gene. Microb Pathog. 1990;8:23–35. doi: 10.1016/0882-4010(90)90005-b. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez M, Lafuse W P, Leibowitz J, David C S. Partial suppression of Theiler's virus-induced demyelination in vivo by administration of monoclonal antibodies to immune-response gene products (Ia antigens) Neurology. 1986;36:964–970. doi: 10.1212/wnl.36.7.964. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez M, Miller D J. Immune promotion of central nervous system remyelination. Prog Brain Res. 1994;103:343–355. doi: 10.1016/s0079-6123(08)61148-6. [DOI] [PubMed] [Google Scholar]
- 41.Rossi C P, Cash E, Aubert C, Coutinho A. Role of the humoral immune response in resistance to Theiler's virus infection. J Virol. 1991;65:3895–3899. doi: 10.1128/jvi.65.7.3895-3899.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubio N, Cuesta A. Lack of cross-reaction between myelin basic proteins and putative demyelinating virus envelope proteins. Mol Immunol. 1989;26:663–668. doi: 10.1016/0161-5890(89)90049-7. [DOI] [PubMed] [Google Scholar]
- 43.Vanderlugt C L, Karandikar N J, Bluestone J A, Miller S D. The functional significance of epitope spreading and its regulation by costimulatory interactions. Immunol Rev. 1998;164:63–72. doi: 10.1111/j.1600-065x.1998.tb01208.x. [DOI] [PubMed] [Google Scholar]
- 44.Welsh C J, Tonks P, Nash A A, Blakemore W F. The effect of L3T4 T cell depletion on the pathogenesis of Theiler's murine encephalomyelitis virus infection in CBA mice. J Gen Virol. 1987;68:1659–1667. doi: 10.1099/0022-1317-68-6-1659. [DOI] [PubMed] [Google Scholar]
- 45.Windhagen A, Newcombe J, Dangond F, Strand C, Woodroofe M N, Cuzner M L, Hafler D A. Expression of costimulatory molecules B7-1 (CD80), B7-2 (CD80), and interleukin 12 cytokine in multiple sclerosis lesions. J Exp Med. 1995;182:1985–1996. doi: 10.1084/jem.182.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yauch R L, Kerekes K, Saujani K, Kim B S. Identification of a major T cell epitope within VP3(24-37) of Theiler's virus in demyelination-susceptible SJL/J mice. J Virol. 1995;69:7315–7318. doi: 10.1128/jvi.69.11.7315-7318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]