Abstract
We reviewed our 3-year experience in treating interruption of the aorta in adult patients. Clinical profiles, surgical management, and results of early and mid-term follow-up are presented.
From August 2001 through June 2003, 7 adult patients underwent an extra-anatomic bypass procedure to repair interruption of the aortic arch. Five patients underwent ventral aortic repair through a mid-sternotomy and an upper midline laparotomy, and 2 patients underwent repair through a left posterolateral thoracotomy. A bovine collagen-impregnated polyester fiber graft was used in 6 patients, and a Gore-Tex® graft was interposed in 1 patient. All repairs were performed without cardiopulmonary bypass.
Follow-up was complete in all patients. The mean follow-up was 17.28 ± 7.1 months (range, 9–31 months). No neurologic, renal, or gastrointestinal complications were noted in any patient. There was no in-hospital or late mortality or need for re-intervention. All patients were asymptomatic; however, 5 patients had mild residual hypertension. Graft patency in all the patients was confirmed by computed tomographic angiography.
Interruption of the aorta is rare in adults. Ventral aortic repair through a midline approach is our preferred technique for surgical repair of this entity, because it avoids the extensive network of collateral vessels on the chest wall, enables simultaneous treatment of associated lesions, and in all likelihood reduces morbidity and mortality.
Key words: Adult; aorta, thoracic/abnormalities; heart defects, congenital/complications; methods; coronary artery bypass, off-pump
Interruption of the aortic arch in the adult population is an extremely rare entity. It is often diagnosed and repaired during the neonatal period. Conventional repair of aortic interruption by end-to-end anastomosis, as done for coarctation of the aorta, is often a surgical challenge in view of the extensive collateral vessels that develop on the chest wall and inside the chest cavity. Anatomic bypass is therefore associated with a high risk of postoperative morbidity and mortality. Various extra-anatomic techniques to bypass the lesion and at the same time decrease the potential complications of traditional anatomic repair have been described.1–3
In August 2001, a 64-year-old man with type A interruption of the aortic arch was treated by interposing an 18-mm Dacron graft between the left subclavian artery and the descending thoracic aorta through a left posterolateral thoracotomy. This patient was re-explored 3 times for very heavy hemorrhaging from spontaneous rupture of collateral vessels. Our experience with this patient prompted us to adopt the midline approach for surgical correction of the lesion in subsequent patients. We review our recent surgical experience in the treatment of interruption of aortic arch in adults. Results of early and midterm clinical follow-up and radiologic evaluation of graft patency by computed tomographic (CT) angiography are reported.
Patients and Methods
From August 2001 through June 2003, 7 adult patients with isolated interruption of the aortic arch underwent surgical palliation by means of extra-anatomic bypass. There were 6 men and 1 woman, who ranged in age from 18 to 64 years (mean, 37.85 ± 15.21 years). Five patients had type A interruption (luminal discontinuity of the aorta distal to the left subclavian artery), and 2 patients had type B interruption (discontinuity of the aorta between the left common carotid and the left subclavian arteries). Associated lesions were bicuspid aortic valve (n = 1), berry aneurysm of the anterior communicating artery (n = 1), and significant mid left anterior descending coronary artery (LAD) occlusive disease (n = 1). The patients' characteristics are more fully described in Table I.
TABLE I. Summary of Information on Patients and Procedures
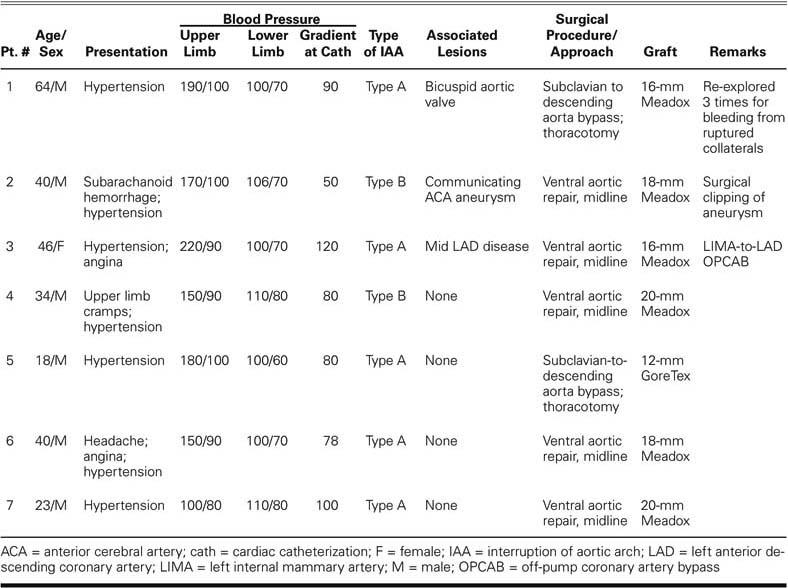
All patients had upper-limb arterial hypertension that required antihypertensive medication. In addition, feeble or absent femoral arterial pulsations, palpable collateral vessels, and interscapular pulsations were noted. One patient presented with subarachnoid hemorrhage secondary to a ruptured anterior communicating artery aneurysm and underwent surgical clipping before repair of the aortic interruption.
All patients underwent preoperative evaluation by routine chest radiography, 2-dimensional echocardiography, and cardiac catheterization and angiography via the transbrachial and transfemoral routes. Magnetic resonance angiography was performed in 1 patient (Fig. 1). The gradient across the interrupted segment varied between 50 and 120 mm Hg, as measured at catheterization (Table I).
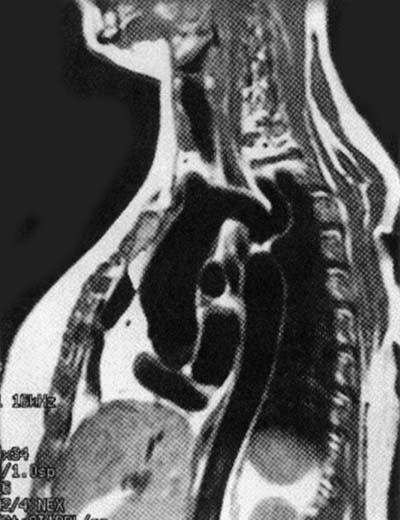
Fig. 1 Magnetic resonance angiogram shows type A interruption.
Surgical Technique
Single-stage extra-anatomic bypass was performed in all patients except for 2 who already had undergone surgical correction through a left posterolateral thoracotomy; in these 2 patients, we placed a subclavian-to-descending thoracic aorta bypass graft. Ventral aortic repair through a midline sternotomy, which was extended into an upper midline laparotomy, was performed in 5 patients. The supraceliac abdominal aorta was dissected and looped. The distal anastomosis to this portion of the aorta was performed first. The graft was then tunneled through a fenestration in the right hemidiaphragm, carried anterior to the inferior vena cava along the lateral border of the right atrium, and anastomosed proximally on the right lateral aspect of the ascending aorta. Both the proximal and distal anastomoses were done with a running 4-0 polypropylene suture in an end-to-side fashion, with a side-biting clamp placed on the aorta. All the repairs were performed without cardiopulmonary bypass. One patient had concomitant off-pump coronary artery bypass grafting with a left internal mammary artery-to-LAD graft.
The grafts used for extra-anatomic bypass in 6 patients were bovine-collagen-impregnated polyester-fiber grafts ranging in size from 12 to 20 mm (Meadox™ woven Hemashield®, Meadox-Boston Scientific Corporation; Oakland, NJ); in 1 patient, a 14-mm Gore-Tex® graft (W.L. Gore & Associates, Inc.; Flagstaff, Ariz) was used for the procedure.
Postoperative Course
Elective ventilation, antihypertensive medication titrated against the blood pressure, and monitoring of the hemodynamic parameters in the intensive care unit during the first 48 hours after surgery was the routine protocol followed in all the patients. Gradual weaning from mechanical ventilatory support and oral antihypertensive therapy were initiated once the patients had adequate respiratory effort and stable hemodynamic values.
Major hemorrhage requiring delayed re-exploration was seen in a 64-year-old man who had undergone a left subclavian artery-to-descending thoracic aorta bypass. He had to be re-explored 72 hours after surgery and then needed 2 additional explorations for extensive hemorrhage. Each time, the source of bleeding was spontaneous rupture of one of the many collateral vessels in the vicinity of the aorta. Subsequently, this patient made an uneventful recovery.
None of the patients had any renal, neurologic, or gastrointestinal complications. The mean length of stay in the intensive care unit was 3 days (range, 2–10 days). The mean length of stay in the hospital was 12 days (range, 6–30 days).
Follow-Up
All patients were followed up in the outpatient clinic. Evaluation included clinical assessment, echocardiography, and CT angiography. Follow-up ranged from 9 to 31 months (mean, 17.28 ±7.1 months). All patients underwent echocardiography and CT angiography for imaging of the aorta and the graft.
All patients were in New York Heart Association functional class I and reported a symptom-free life. Two patients (28%) had normal blood pressure. The remaining 5 patients had residual mild hypertension that was well controlled with a single antihypertensive drug (β-blocker, ACE inhibitor, or calcium channel blocker).
From 6 months to 2 years after surgical repair, noninvasive evaluation for graft patency was carried out in all the patients by CT angiography. Good runoff in the distal circulation with patent grafts was demonstrated on follow-up imaging (Fig. 2).
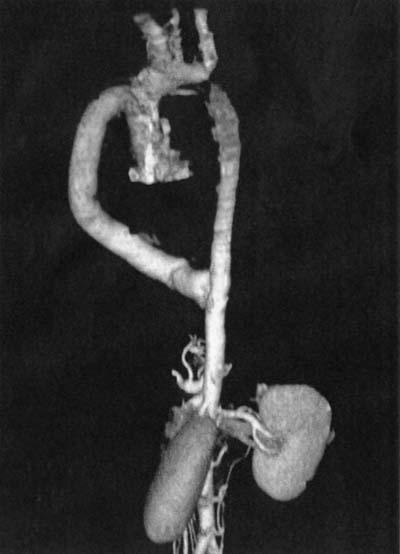
Fig. 2 Follow-up computed tomographic angiogram shows ventral aortic repair of type A interruption.
Discussion
Interruption of the aortic arch is defined as a loss of luminal continuity between the ascending and descending portions of the aorta. The lesion was first described by Steidele4 in 1778. Celoria and Patton5 proposed a classification system in 1959 that divides this clinical entity into 3 types, depending on the site of discontinuity, which may be distal to the left subclavian artery (Type A), between the left common carotid and the left subclavian arteries (Type B), or between the braciocephalic and the left common carotid arteries (Type C). Type B interruption is the most common (53%) followed by Type A (43%) and Type C (4%).
Interruption of the aortic arch is a rare congenital malformation that occurs with an incidence of 3 per million live births. It presents as severe congestive heart failure in the neonatal period, and 90% of affected neonates die at a median age of 4 days,6 if left untreated. The anomaly is so rare in adults that the world medical literature contains only 12 such reports of isolated interruption of the aortic arch.7 In the few cases reported in adults, the presentation varies from asymptomatic status to differential blood pressure recordings in the extremities and systemic arterial hypertension with its attendant complications. Survival into adulthood is dependent upon the development of substantial collateral circulation.6 These collateral vessels are subject to atrophy, atherosclerosis, and even spontaneous rupture, resulting in secondary complications.8
Of the 12 adult patients with interruption whose cases were reported in the literature,7–13 10 had undergone successful surgical repair, and most procedures were done in a single stage by means of an extra-anatomic approach.
Operations on the aortic arch are usually performed with cardiopulmonary bypass, deep hypothermia, and circulatory arrest.14 Extra-anatomic bypass is our preferred technique for the repair of these lesions. In addition, after our initial experience with excessive hemorrhage with the thoracotomy approach, we now use ventral aortic repair as the surgical method of choice. In all of our patients, we were able to successfully bypass the lesion without the need for cardiopulmonary bypass. We have not observed any neurologic, renal, or gastrointestinal complications, and no patient has died.
Early and midterm follow-up results show a return to normal functional class, effectively controlled arterial hypertension, excellent graft patency on radiologic evaluation, and no graft-related complications.
We conclude that ventral aortic repair without cardiopulmonary bypass through a midline approach is a safe and effective means for the treatment of this rare pathologic condition and is also the best method to treat select cases of adult aortic coarctation, as reported by de Oliveira and colleagues.15
Footnotes
Address for reprints: Dr. Anil Bhan, MCh, Additional Professor, Department of Cardiothoracic and Vascular Surgery, All India Institute of Medical Sciences, Ansari Nagar, New Delhi - 110 029, India. E-mail: anil_bhan@hotmail.com
References
- 1.Siderys H, Graffis R, Halbrook H, Kasbeckar V. A technique for management of inaccessible coarctation of the aorta. J Thorac Cardiovasc Surg 1974;67:568–70. [PubMed]
- 2.Wukasch DC, Cooley DA, Sandiford FM, Nappi G, Reul GJ Jr. Ascending aorta-abdominal aorta bypass: indications, technique, and report of 12 patients. Ann Thorac Surg 1977;23:442–8. [DOI] [PubMed]
- 3.Robicsek F, Hess PJ, Vajtai P. Ascending-distal abdominal aorta bypass for treatment of hypoplastic aortic arch and atypical coarctation in the adult. Ann Thorac Surg 1984; 37:261–3. [DOI] [PubMed]
- 4.Steidele RJ. Samml Chir U Med Beob (Vienna) 1778;2: 114.
- 5.Celoria GC, Patton RB. Congenital absence of the aortic arch. Am Heart J 1959;58:407–13. [DOI] [PubMed]
- 6.Collins-Nakai RL, Dick M, Parisi-Buckley L, Fyler DC, Castaneda AR. Interrupted aortic arch in infancy. J Pediatr 1976;88:959–62. [DOI] [PubMed]
- 7.Messner G, Reul GJ, Flamm SD, Gregoric ID, Opfermann UT. Interrupted aortic arch in an adult single-stage extra-anatomic repair. Tex Heart Inst J 2002;29:118–21. [PMC free article] [PubMed]
- 8.Prasad SV, Gupta SK, Reddy KN, Murthy JS, Gupta SR, Somnath HS. Isolated interrupted aortic arch in adult. Indian Heart J 1988;40:108–12. [PubMed]
- 9.Kauff MK, Bloch J, Baltaxe HA. Complete interruption of the aortic arch in adults. Radiology 1973;106:53–7. [DOI] [PubMed]
- 10.Milo S, Massini C, Goor DA. Isolated atresia of the aortic arch in a 65-year-old-man. Surgical treatment and review of published reports. Br Heart J 1982;47:294–7. [DOI] [PMC free article] [PubMed]
- 11.Todoric M, Martinovic N, Jablanov J, Albreht M, Aleksandrov R, Prcovic M. Interrupted aortic arch—case report of a patient successfully operated on in adulthood [in Croatian]. Acta Chir Iugosl 1985;32:201–6. [PubMed]
- 12.Burton BJ, Kallis P, Bishop C, Swanton H, Pattison CW. Aortic root replacement and extraanatomic bypass for interrupted aortic arch in an adult. Ann Thorac Surg 1995;60: 1400–2. [DOI] [PubMed]
- 13.Ogino H, Miki S, Matsubayashi K, Ueda Y, Nomoto T. Two-stage repair for aortic regurgitation with interrupted aortic arch. Ann Thorac Surg 1998;65:1151–3. [DOI] [PubMed]
- 14.Katsumata T, Westaby S. Operation for mid-arch coarctation. Ann Thorac Surg 1999;67:1386–90. [DOI] [PubMed]
- 15.Almeida de Oliveira S, Lisboa LA, Dallan LA, Abreu F CA, Rochitte CE, de Souza JM. Extraanatomic aortic bypass for repair of aortic arch coarctation via sternotomy: midterm clinical and magnetic resonance imaging results. Ann Thorac Surg 2003;76:1962–6. [DOI] [PubMed]


