Abstract
Extensive aortic disease, such as atherosclerosis with aneurysms or dissections that involve the ascending aorta, can complicate the choice of a cannulation site for cardiopulmonary bypass.
To date, the standard peripheral arterial cannulation site has been the common femoral artery; however, this approach carries the risk of atheroembolism due to retrograde aortic perfusion, or it is undesirable because of severe iliofemoral disease.
Arterial perfusion through the axillary artery provides sufficient antegrade aortic flow, is more likely to perfuse the true lumen in the event of dissection, and is associated with fewer atheroembolic complications.
From September 2000 through March 2004, 27 patients underwent right axillary artery cannulation for acute ascending aortic dissection (n = 16), ascending aortic aneurysm (n = 9), or coronary artery bypass grafting (n = 2). Direct artery cannulation was performed in the first 4 patients, and the last 23 patients were cannulated through a longitudinal arteriotomy via an 8-mm woven Dacron graft. Seventeen patients underwent hypothermic circulatory arrest and antegrade cerebral perfusion.
Two patients died intraoperatively: one due to low cardiac output and one due to diffuse bleeding. One patient suffered mild right-arm paresthesia postoperatively, but recovered completely. Axillary artery cannulation was successful in all patients; it provided sufficient arterial flow, and there were no intraoperative problems with perfusion.
In the presence of extensive aortic or iliofemoral disease, arterial perfusion through the axillary artery is a safe and effective means of providing sufficient arterial inflow during cardiopulmonary bypass. In this regard, it is an excellent alternative to standard femoral artery cannulation.
Key words: Aneurysm, dissecting/complications; aortic aneurysm/complications; arteriosclerosis/complications; axillary artery; cardiopulmonary bypass/methods; catheterization, peripheral/methods; extracorporeal circulation; femoral artery; reperfusion/methods; vascular surgical procedures
The femoral artery is the most common cannulation site for cardiopulmonary bypass (CPB) in cases of extensive aortic disease or when CPB is required before sternotomy or thoracotomy. However, this procedure is associated with a significant risk of embolization to the brain or the heart (by retrograde perfusion) of atherosclerotic plaque or of thrombi from the iliofemoral arterial wall and abdominal aortic wall. Furthermore, the prospect of an aortic dissection that extends into the femoral artery makes this approach undesirable.1,2
Arterial cannulation through the axillary artery may present a valid alternative in these circumstances, and in cases of dissection, at least in regard to the vessels that perfuse the cerebrum, it perfuses the true lumen more reliably.3 It is easy and safe to perform, and it provides sufficient arterial inflow during CPB.
We report our preliminary experience with axillary artery cannulation through a small infraclavicular incision for repair of acute aortic dissections and ascending aortic aneurysms, and for coronary artery bypass grafting (CABG).
Patients and Methods
From September 2000 through March 2004, we operated on 27 patients for acute ascending aortic dissection (n = 16), ascending aortic aneurysm (n = 9), and CABG (n = 2), with the use of the axillary artery for arterial inflow. These patients ranged in age from 38 to 85 years (median, 64 years); 20 were men and 7 were women (Table I).
TABLE I. Clinical Profile of Patients Undergoing Axillary Artery Cannulation for Cardiopulmonary Bypass

The following were our indications for axillary artery cannulation and peripheral CPB: aortic aneurysmal disease, dissection, and severe atherosclerosis that precluded ascending aortic cannulation and clamping. In 3 patients, axillary artery cannulation followed an initial attempt at femoral artery cannulation. Aortic disease was identified and evaluated before operation by means of echocardiography, computed tomography, angiography, or magnetic resonance imaging. No specific diagnostic procedure was used for viewing the axillary arteries before surgery. Seventeen cases, comprising the 16 ascending aortic dissections and one of the CABG cases, were handled as emergency procedures.
Surgical Technique
The patient was placed in the standard supine position. For better exposure of the right axillary artery, the arm was positioned near the body with the hand down to the side and the elbow slightly flexed, almost as if the hand were placed in an imaginary pants pocket. The patient's upper body was elevated 20° and tilted toward the left, so that the skin of the overlying infraclavicular fossa was nearly on a horizontal plane. Arterial pressure was monitored via 3 arterial lines placed routinely in both radial arteries and in the right femoral artery. An infraclavicular skin incision 8 to 10 cm in length was made, running from a point just lateral to the sternal head of the clavicle to the upper deltopectoral groove. The pectoralis major fascia was incised and its fibers split, exposing the axillary vein. The pectoralis minor was retracted laterally.
The axillary artery was easily exposed under the vein and gently mobilized for 2 cm, without touching the medial and lateral cords of the brachial plexus. An umbilical tape was looped around the artery, and gentle traction was applied. After heparinization, the artery was cannulated either directly (4 patients) or indirectly (23 patients) through a longitudinal arteriotomy via an 8-mm woven Dacron graft (Fig. 1). This graft had been anastomosed with 5–0 polypropylene suture after a side-bite clamp had been applied across the artery. It was then connected to a 20-F straight arterial cannula, and the pressure was compared to the contralateral radial systemic pressure, to confirm that there was no gradient.
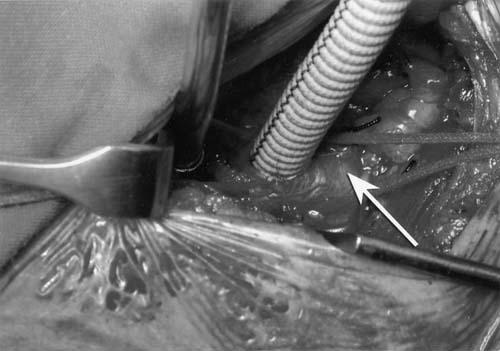
Fig. 1 A Dacron graft (8 mm) is anastomosed to the right axillary artery.
Venous outflow was usually obtained from the right atrium after sternotomy, or through the right femoral vein in the event of aortic rupture into the pericardium. Cardiopulmonary bypass was then established, and arterial flow of 2.2 to 2.8 L/min/m2 was easily obtained at normothermia. During extracorporeal circulation, the systemic pressure was monitored through the contralateral radial line, because pressure tended to be higher on the side of the axillary cannulation. After full flow was achieved without observable problems, cooling was started. The left ventricle was vented through the right superior pulmonary vein. The mid-ascending aorta was clamped, cold blood cardioplegic solution was administered (both retrograde via the coronary sinus and antegrade via the coronary ostia), and proximal aortic reconstruction was initiated. If circulatory arrest was necessary, the patient was cooled to 22°C, the table was placed in the Trendelenburg position, cardiopulmonary bypass was stopped, and the cross-clamp was removed. The arch was inspected from inside. In the event of dissection, the type and extent of arch replacement were determined in accordance with the nature of the lesion.
While arterial flow was resumed, the arch vessels were carefully occluded sequentially from right to left with soft vascular clamps, or, in some instances, the left subclavian artery was occluded by the manually inflated balloon of a conventional 12-F retrograde cardioplegia cannula. Preparation and isolation of the left subclavian artery was therefore unnecessary. Careful visual inspection and digital examination helped ensure that vascular clamps were not placed on atherosclerotic areas. During the sequential occlusion and while we concomitantly increased arterial flow up to 10 mL/kg body weight, we carefully observed backflow via the arch vessels, which had not yet been occluded. The brain perfusion pressure was monitored through the right radial artery during that time and was kept at 50 mmHg.
All arch reconstructions and distal anastomoses were performed with an open aortic anastomotic technique, while low-flow perfusion through the right axillary artery was continued. When the distal repair was complete, flow through the axillary artery was gradually increased as the soft clamps on the brachiocephalic vessels were removed. Air was removed from the vessels and grafts, which were then filled with blood, and the distal graft was cross-clamped. The normal flow rate was reached, and rewarming was begun in accordance with the time necessary for completion of the proximal repair.
At the end of the procedure, the axillary artery graft was clamped and cut a few millimeters above the anastomosis, then oversewn with 4–0 polypropylene suture. It is important to avoid narrowing the axillary artery or creating a stump that could become an aneurysm or a source of emboli.
Results
One patient (Patient 14, Table I) died intraoperatively due to low cardiac output associated with left ventricular dysfunction, which itself had been caused by severe coronary artery disease. A 2nd patient (Patient 25) died due to diffuse bleeding after the completion of a Bentall procedure, hemiarch replacement, and double-vessel CABG. Another patient (Patient 24) suffered mild right-arm paresthesia, possibly due to the axillary artery preparation, after undergoing elective surgery for an ascending aortic aneurysm; but he recovered completely.
Twenty-five patients survived the operation, and no patient had a cerebrovascular accident or other neurologic deficit. No axillary artery or vein thrombosis was recorded. The arterial inflow during CPB was sufficient in all cases. There were no in hospital deaths.
Discussion
Our recent experience with axillary artery cannulation shows that it can be safely used in cases in which peripheral CPB is required and femoral artery cannulation or retrograde aortic perfusion is not desired.
Atheroemboli generated during cardiac operations are a major source of morbidity and mortality.1 Antegrade perfusion is of paramount importance in cases of aortic atherosclerosis. Retrograde arterial perfusion through an atherosclerotic aorta has been shown to be associated with stroke and with visceral organ injury, a consequence of atheroemboli in both instances.4 Furthermore, significant ascending aortic atherosclerosis is often associated with severe abdominal atherosclerosis.5 Even in a setting of extensive aortic and innominate artery disease, the axillary artery is rarely affected and provides an excellent site for safe antegrade aortic perfusion, which may play a role in preventing embolic stroke.6 In surgery for acute type A dissection, cannulation of the femoral artery and retrograde aortic perfusion can result in pressurization of the false lumen and subsequent brain and visceral malperfusion, with devastating results.
Cannulating the femoral artery requires the extra step of recannulating the aortic graft after the distal anastomosis has been performed, to assure the perfusion of the true lumen in cases of dissection. It is unusual for the right axillary artery to be involved with an intimal flap from an acute dissection.7 On the other hand, associated dissection of the femoral artery is more frequent, and sufficient arterial inflow is much less likely to be achieved than with cannulation of the axillary artery.
Unlike the femoral vessels, the axillary artery benefits from rich collateral flow from the thyrocervical trunk to the suprascapular and transverse cervical arteries, which helps to avert upper extremity ischemia during total distal occlusion.8
The management of porcelain aorta during CABG requires peripheral CPB. Avoiding manipulation, cannulation, and clamping of the ascending aorta is important. Arterial cannulation of the axillary artery, in combination with hypothermic fibrillatory arrest for performing the distal anastomosis and for constructing the proximal anastomosis to the innominate artery, is an established alternative for the management of porcelain aorta during CABG.9 Another advantage of performing right axillary perfusion is that it enables circulatory arrest to be changed to selective cerebral perfusion by means of balloon occlusion of the arch vessels, should the surgeon expect brain ischemic time to be prolonged.10
In our series, we used the right axillary artery, and cannulation was feasible in all cases. Direct arterial cannulation was performed in the first 4 cases; later, we preferred a synthetic graft-to-artery anastomosis with cannulation of the graft, especially in patients with a small body surface area and therefore a small vessel. We had no instances of wound infection. Despite preoperative disease states that placed our patients at high risk of stroke and visceral end-organ injury, we saw no clinically demonstrable permanent postoperative deficits.
A contraindication for the use of the axillary artery for cannulation is known severe atherosclerotic disease of the axillary or subclavian artery. However, these vessels are rarely involved in the atherosclerotic process.3
In summary, we have found that axillary artery cannulation provides a safe and feasible alternative to the historically used femoral artery, by enabling antegrade aortic flow through the true lumen, ensuring adequate arterial inflow, and minimizing the risk of retrograde atheroemboli. Use of the axillary artery may decrease the risk of stroke and visceral organ malperfusion that is associated with peripheral CPB in this high-risk group of patients.
Footnotes
Address for reprints: George Lazopoulos, MD, PhD, Ellinikou Stratou 24 Str., 15237 Athens, Greece. E-mail: lazopoulosg@ath.forthnet.gr
References
- 1.Sabik JF, Lytle BW, McCarthy PM, Cosgrove DM. Axillary artery: an alternative site of arterial cannulation for patients with extensive aortic and peripheral vascular disease. J Thorac Cardiovasc Surg 1995;109:885–91. [DOI] [PubMed]
- 2.Neri E, Massetti M, Capannini G, Carone E, Tucci E, Diciolla F, et al. Axillary artery cannulation in type A aortic dissection operations. J Thorac Cardiovasc Surg 1999;118: 324–9. [DOI] [PubMed]
- 3.Pasic M, Schubel J, Bauer M, Yankah C, Kuppe H, Weng YG, Hetzer R. Cannulation of the right axillary artery for surgery of acute type A aortic dissection. Eur J Cardiothorac Surg 2003;24:231–6. [DOI] [PubMed]
- 4.Price DL, Harris J. Cholesterol emboli in cerebral arteries as a complication of retrograde aortic perfusion during cardiac surgery. Neurology 1970;20:1209–14. [DOI] [PubMed]
- 5.Blauth CI, Cosgrove DM, Webb BW, Ratliff NB, Boylan M, Piedmonte MR, et al. Atheroembolism from the ascending aorta. An emerging problem in cardiac surgery. J Thorac Cardiovasc Surg 1992;103:1104–12. [PubMed]
- 6.Kouchoukos NT. Adjuncts to reduce the incidence of embolic brain injury during operations on the aortic arch. Ann Thorac Surg 1994;57:243–5. [DOI] [PubMed]
- 7.Whitlark JD, Goldman SM, Sutter FP. Axillary artery cannulation in acute ascending aortic dissections. Ann Thorac Surg 2000;69:1127–9. [DOI] [PubMed]
- 8.Gates JD, Bichell DP, Rizzo RJ, Couper GS, Donaldson MC. Thigh ischemia complicating femoral vessel cannulation for cardiopulmonary bypass. Ann Thorac Surg 1996; 61:730–3. [DOI] [PubMed]
- 9.Leyh RG, Bartels C, Notzold A, Sievers HH. Management of porcelain aorta during coronary artery bypass grafting. Ann Thorac Surg 1999;67:986–8. [DOI] [PubMed]
- 10.Sabik JF, Nemeh H, Lytle BW, Blackstone EH, Gillinov AM, Rajeswaran J, Cosgrove DM. Cannulation of the axillary artery with a side graft reduces morbidity. Ann Thorac Surg 2004;77:1315–20. [DOI] [PubMed]


