Catheter ablation of atrial fibrillation is an evolving field. We've made a lot of progress, but we have not yet determined the best way to ablate this very challenging arrhythmia. Just about every other arrhythmia that we've tackled in the EP laboratory is very discrete, and you can use mapping to determine where the target site is; you ablate it, and that's that. But atrial fibrillation is such a complex arrhythmia that the best technique still is not clear.
Consider a hypothetical case of a 48-year-old man with a 3-year history of hypertension and chronic atrial fibrillation. His primary symptoms are fatigue and exercise intolerance. He was treated with 3 different drugs, and underwent cardioversion and felt better briefly, but each time he had an early recurrence. He's being treated with metoprolol and warfarin. He has an enlarged left atrium (48 mm) and an ejection fraction of 0.60. His current EKG shows atrial fibrillation. What are the possible options for this man? He could continue on his present regimen, but he's 48 years old; he wants to continue to be active, and his quality of life is impaired. Simply continuing rate control and warfarin is not a very attractive option for him.
Catheter ablation of the AV node isn't really going to do him any good, because his rate is already controlled. His real problem is a lack of AV synchrony, so ablating the AV node is not going to help him. Amiodarone followed by cardioversion might be effective, but you've got potential long-term toxicities to consider. In 2005, he would be considered an ideal candidate for catheter ablation.
In the late 1990s, a group in Bordeaux demonstrated that the muscle sleeves that surround the pulmonary veins can be very arrhythmogenic and very often supply the triggers that set off atrial fibrillation. This opened up a new therapeutic avenue: electrically isolating the pulmonary veins from the atrium. One key tool was the development of the circular ring catheter, which is positioned inside the ostia of the veins. The triggers inside the vein can no longer get out to the atrium to set off the fibrillation.
Subsequently, it became clear that there are limitations to catheter ablation. Over the long term, probably only about 60% of patients with paroxysmal atrial fibrillation were “cured.” And for chronic atrial fibrillation, pulmonary vein isolation had a success rate of only about 20% to 25%.
For the last 15 to 20 years, surgeons have been developing their own techniques to “cure” atrial fibrillation. Almost all of the surgical approaches included isolation of the pulmonary veins, but to get truly high surgical success rates (>90%), there had to be other areas of the atrium that were also surgically interrupted. About 3 or 4 years ago, we adopted a technique, first described in Italy, in which we encircled the pulmonary veins 1 or 2 centimeters away from their ostia, with additional ablation lines in the posterior left atrium and adjacent to the mitral valve (Fig. 1). To do this, you cannot simply use fluoroscopy and a standard catheter; there's no way to make continuous ablation lines with standard equipment and imaging. It requires sophisticated 3-dimensional mapping systems, such as electroanatomic mapping.
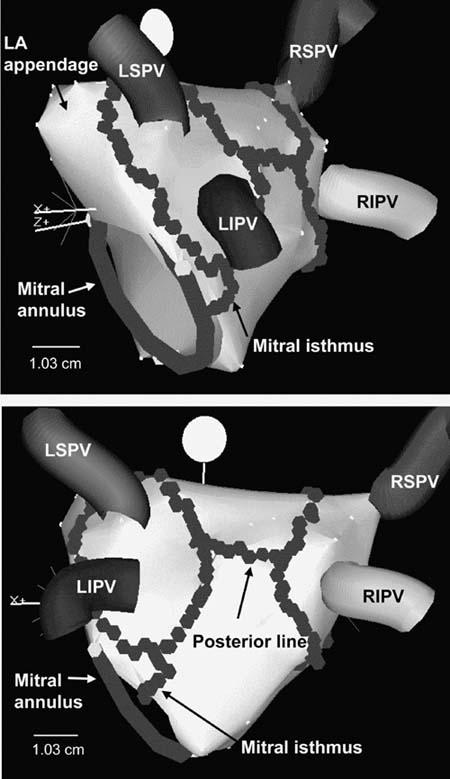
Fig. 1 Left atrial ablation to encircle the pulmonary veins
To date, we have performed this technique in close to 800 patients. We tended to avoid patients with severe structural heart disease, and many of the patients had either idiopathic atrial fibrillation or hypertension. At 3 years, our success rate was about 80% for paroxysmal atrial fibrillation and 70% for chronic atrial fibrillation. But even a 70% to 80% cure rate is not nearly as good as 99% for many of the other arrhythmias that we treat, like WPW or PSVT caused by AV node reentry.
One difficulty has been left atrial flutter, which occurred in close to 20% of our patients; this also happens not infrequently after surgical treatments for atrial fib. Small gaps in the ablation lines can set up a substrate that supports atrial flutter. As the gaps fill in with fibrosis over time, the flutter may go away, but 5% of our patients have needed a second procedure to treat flutter. A rare but major concern is atrial esophageal fistula; it has occurred in 2 of our 800 patients, and one of these instances was fatal. The esophagus is very sensitive to thermal injury. In patients who develop these fistulas, there's no pericardial effusion and no evidence of atrial perforation. Importantly, though, the posterior wall of the left atrium can be as thin as 1.6 or 1.7 mm; a typical lesion created by radiofrequency energy is 4 to 5 mm deep. A lot of patients have fat between the left atrial wall and the esophagus, but some patients don't, and they are probably the ones who are the most at risk. The radiofrequency energy appears to result in thermal injury to the outside of the esophagus and sets up a necrotic process that spreads toward the atrium. Patients may present with endocarditis, because the GI flora enter the blood stream. Very often there may be a vegetation at the site of the fistula.
There's probably no entirely safe way to use radiofrequency energy on the posterior wall of the atrium, and you can't predict the position of the esophagus relative to the left atrium because it changes with time. One way we've dealt with this risk in the last several months has been to have patients swallow 5 cc of barium when they come to our laboratory. It coats the walls of the esophagus so that, in most cases, we can see where it is over the course of the next 90 minutes or so. We don't ablate any areas that are close to the esophagus. Also, the risk of the fistula is not specific to our newer encircling procedure—it can occur with ostial ablation as well.
Various mechanisms participate in generating atrial fibrillation (Fig. 2). The pulmonary veins supply the premature depolarizations that trigger paroxysmal atrial fib. There are reentry and rotor waves in the left atrial substrate that may use the pulmonary veins as anchor points. There's a muscle sleeve around the coronary sinus that can also function in a manner analogous to that of the sleeve around a pulmonary vein. And, last, autonomic innervation may also play a role in atrial fibrillation. Our encircling approach (Fig. 3) addresses several of these mechanisms. The large circles afford at least some degree of pulmonary vein isolation. The ablation lines may be going through some of the rotors that are active, and in the mitral isthmus area, we may be transecting the location of the vein of Marshall. We have found, though, that these long lines are very rarely complete lines of block. It's very, very difficult to get complete block using only radiofrequency energy. Then again, we have come to realize that perhaps we don't need to have complete block. But with this approach, there's almost no way to avoid the posterior wall where, at least in some patients, you're going to encounter the area of the esophagus. So, in our recent practice, we have stopped trying to make complete lines of block or even lines at all. There are several advantages to this approach. First, we don't see nearly as much atrial flutter, because we avoid the small gaps that predispose to flutter. We're not committed to going across the posterior wall where the esophagus might be. We can also individualize the therapy and decrease the duration and the amount of radiofrequency.
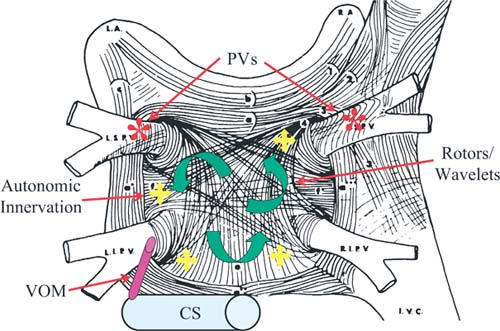
Fig. 2 Mechanisms that contribute to atrial fibrillation
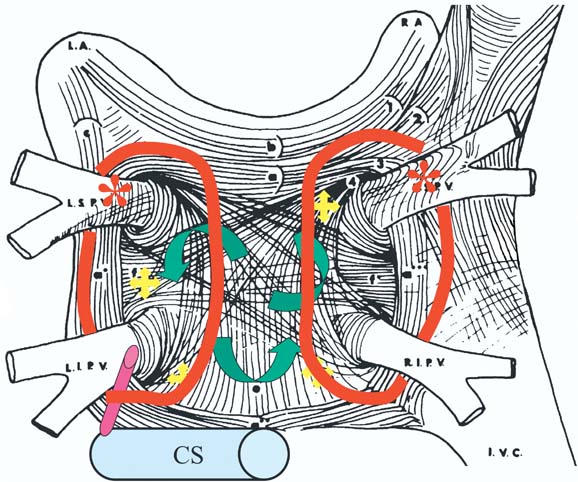
Fig. 3 Left atrial circumferential ablation
We started using this sort of modified approach systematically in July of 2004, and we have information on 133 patients that were done in a period of 5 months up to December of 2004. The average duration of the RF applications was 30 minutes, including both paroxysmal and chronic fibrillation; the total procedure time is fairly short (99 minutes). In patients with paroxysmal atrial fibrillation, none have required a redo procedure as of yet; 9% have flutters, 73% are in sinus rhythm, and 18% have had recurrences of atrial fib. Many of these are early recurrences that we hope will resolve. What happens in the first couple of months usually has no long-term prognostic sig-nificance. With chronic atrial fib, 68% are in sinus rhythm, 25% have had an early recurrence of atrial fib, and 7% have had atrial flutter.
Finally, a few comments about the AFFIRM trial. This was a large-scale trial of 4,000 patients with atrial fibrillation treated with either a rhythm control strategy or a rate control strategy. The study showed that there was no particular advantage to rhythm control, and there were actually some disadvantages. Mortality tended to be lower in the rate control arm, and there were fewer hospitalizations in the rate control arm. However, AFFIRM did not truly compare sinus rhythm with rate control of atrial fib. It compared 2 strategies: rate control with fairly benign drugs and continued anticoagulation, versus rhythm control with antiarrhythmic drugs that have a lot of adverse effects and side-effects and more variable anticoagulation. All this was in a relatively asymptomatic population with a mean age of 70 years. Therefore, AFFIRM really has nothing to do with the younger, symptomatic patients that we consider for catheter ablation.
Recently, there was a descriptive comparison of 1,100 patients who were treated either by ablation or by medical therapy, with 3 years of follow-up. There was a 50% reduction in mortality and about a 50% reduction in heart failure in the patients who had a successful outcome from the catheter ablation procedure. Why should eliminating atrial fib improve mortality by 50%? Going back to AFFIRM, we find a very intriguing observation from the on-treatment analysis, which looked at patients who actually were in sinus rhythm versus patients who were in atrial fibrillation. There was 50% less mortality in the patients who were in sinus rhythm; but in patients treated with antiarrhythmic drugs, mortality was 50% higher. Therefore, all of the benefit of being out of atrial fib appeared to be negated by the adverse affects of antiarrhythmic drugs.
If we could develop a technique that eliminated atrial fib without exposing patients to the adverse affects of antiarrhythmic drugs, it is possible that there could be long-term benefits on survival.
I'd like to close with our current selection criteria for patients undergoing ablation:
Symptomatic atrial fibrillation.
Age cutoff of about 70 years (the risk goes up after the age of 70; the success rate goes down).
Impaired quality of life. We generally don't use ablation as first-line therapy, although recently we've become a little more liberal with this.
Failure to respond to drug therapy.
Left atrial size <55 mm. If the atrium is much bigger than 55 mm, it's much less likely that we will be able to cure it with a catheter technique.
Footnotes
Presented at the Texas Heart Institute's Sixth Symposium on Cardiac Arrhythmias: New Pharmacologic and Interventional Strategies; held at the Houstonian Hotel, Club & Spa; 19 February 2005; Houston. E-mail: fmorady@umich.edu


