Abstract
Purpose
This study aimed to examine the association between the systemic immune-inflammation index (SII) (ie, neutrophil count × platelet count/lymphocyte count), the age-adjusted Charlson comorbidity index (ACCI), and overall survival (OS) in non-small cell lung cancer (NSCLC) patients undergoing first-line platinum-based chemotherapy (PBC), with a particular emphasis on the role of SII in supporting ACCI.
Patients and Methods
This retrospective study enrolled 353 cases treated between July 2013 and November 2020. Mann–Whitney U-test and Kruskal–Wallis test were employed to compare parameters between high and low SII groups. The cut-off values for SII and ACCI were determined using the X-tile software. Prognostic significance was evaluated through the utilization of Kaplan-Meier curves and Cox regression analysis.
Results
In a univariate Cox regression analysis, sex, age, TNM, lymph node, therapy, SII, and ACCI were associated with OS. After adjusting for confounders in the multivariate analysis, TNM, SII, and ACCI remained independent prognostic factors for OS. Furthermore, within the ACCI subgroups (ACCI<5 or ACCI≥5), a high SII was significantly associated with an increased risk of death. Patients with both a high ACCI and a high SII had the highest risk of death (p < 0.001), with a loss of approximately ten months of survival during the first three years after treatment.
Conclusion
SII was proven to be valuable in predicting OS and, when complemented by ACCI, can help tailor prognostic assessment and treatment strategies in assessing the survival of NSCLC patients with first-line PBC.
Keywords: non-small cell lung cancer, platinum-based chemotherapy, systemic immune-inflammation index, age-adjusted Charlson comorbidity index, prognostic, overall survival
Introduction
Non-small cell lung cancer (NSCLC), as the predominant subtype of lung cancer, is one of the most prevalent malignancies worldwide.1 New treatment modalities such as immunotherapy and targeted therapies have demonstrated impressive response rates and improved outcomes, especially for NSCLC patients with EGFR or ALK mutations.2–4 These modalities are expensive, and not all NSCLC patients have actionable mutations. First-line platinum-based chemotherapy (PBC) remains a commonly viable option owing to its greater affordability and accessibility for a broader patient population.5–8 It should be noted that the lack of effective clinical prognostic indicators still poses a challenge for PBC.9
Currently, commonly used prognostic indicators for NSCLC include clinical-pathological parameters and molecular biomarkers.10–12 Clinical-pathological parameters, such as TNM staging, lymph node metastasis, tumor size, and histological type, have been extensively used as prognostic factors for NSCLC patients.10–14 However, variability among physicians in evaluating these parameters may introduce subjectivity and uncertainty, potentially affecting the accuracy of prognosis assessment.11 Furthermore, relying on a single pathological marker may not provide a comprehensive and objective assessment of NSCLC prognosis. Therefore, it has been suggested that a combination of multiple markers may be necessary for a more accurate evaluation.12 Molecular biomarkers in NSCLC include tumor markers (eg, NSE, CEA, CYFRA21-1),15 gene expression levels (eg, EGFR, ALK, KRAS),16 and microRNA levels (eg, miR-21, miR-155, miR-34).17 Detection methods for molecular biomarkers are highly standardized and precise, providing more objective and reliable results. However, the cost and requirement for advanced laboratory techniques, as well as the limited availability of suitable samples, limit their clinical application.11 Therefore, it is necessary to develop a rapid, accurate, easily standardized, and cost-effective prognostic system for predicting the outcome of NSCLC patients treated with PBC.
Recently, considerable attention has been devoted to investigating the relationship between cancer and inflammation owing to the pivotal role played by inflammation in tumor initiation, progression, and invasion.18,19 It has been revealed that inflammatory signals have an impact on intracellular signaling pathways within tumor cells.19 To effectively evaluate a patient’s inflammatory status and prognosis, two markers are commonly employed: the systemic immune-inflammation index (SII) and the age-adjusted Charlson comorbidity index (ACCI).20–23 SII takes into account peripheral blood cell counts, reflecting changes in inflammation and immune status, while ACCI incorporates age and comorbidity burden.20–23
The combination of these indices enhances the accuracy of predicting treatment outcomes and prognosis. However, the role of combining SII and ACCI in predicting the treatment response and prognosis of NSCLC patients receiving PBC is still unclear. Therefore, this study aims to further investigate the application of pre-treatment SII in supporting ACCI for predicting the treatment response and prognosis of NSCLC patients receiving PBC, building upon previous research. The findings of this study can help create more accurate prognostic assessment tools for individualized treatment of NSCLC patients.
However, the role of combining SII and ACCI in predicting the treatment response and prognosis of NSCLC patients receiving PBC is still unclear. Therefore, this study aims to further investigate the application of pre-treatment SII in supporting ACCI for predicting the treatment response and prognosis of NSCLC patients receiving PBC, building upon previous research. The findings of this study can help create more accurate prognostic assessment tools for individualized treatment of NSCLC patients.
Materials and Methods
Patients
A total of 353 NSCLC patients, confirmed by histology or cytology, who received treatment at the Department of Respiratory Medicine, the First Affiliated Hospital of Nanchang University, from August 2013 to October 2020, were retrospectively enrolled. Our study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University. Informed consent was obtained from all patients prior to treatment. The data collection process was non-selective and continuous.
We enrolled the patients according to the following inclusion criteria: (i) patients confirmed with NSCLC based on histology or cytology; (ii) patients with primary lung cancer; (iii) patients aged 18 years or older; (iv) patients who have not received any anti-cancer treatment, including chemotherapy, radiotherapy, targeted therapy, or surgery, prior to diagnosis; (v) NSCLC patients who received PBC after diagnosis; and (vi) patients with complete case data.
Exclusion Criteria: (i) patients with small cell lung cancer; (ii) patients with severe organ dysfunction such as liver failure, renal failure, or heart failure; (iii) patients with a history of long-term immunosuppressive drug use; (iv) patients with primary or secondary immunodeficiency syndromes or concurrent hematological neoplasms; (v) patients under 18 years of age; and (vi) patients with secondary lung cancer or incomplete case data.
Data Collection
NSCLC patients were restaged according to the 8th edition of the TNM staging system for lung cancer, developed by the International Association for Lung Cancer Research (IASLC). General clinical data of the study subjects were collected, including gender, age, smoking history, education level, Eastern Cooperative Oncology Group (ECOG) performance status (PS) score, pathological classification, lung tumor resection, chemotherapy regimen, and presence of lymph node metastasis. The relevant hematological parameters prior to the first treatment after diagnosis were collected, including absolute neutrophil count, platelet count, and absolute lymphocyte count. The SII was calculated using the following formula:
SII = platelet count × neutrophil count / lymphocyte count (10^9/L) (1)
The Charlson comorbidity index (CCI) was calculated using the method reported by Charlson, which assigns a weighted score based on the presence of certain diseases: a score of one is assigned for conditions such as dementia, cerebrovascular disease, connective tissue disease, mild liver disease, diabetes, peripheral vascular disease, peptic ulcer disease, congestive heart failure, myocardial infarction, and chronic pulmonary disease; a score of two is assigned for conditions such as solid tumor or leukemia or lymphoma within five years, moderate or severe kidney disease, hemiplegia, and diabetes with organ damage; a score of three is assigned for patients with moderate or severe liver disease; and a score of six is assigned for patients with AIDS or metastatic solid tumors. The final ACCI value was obtained through an age-adjusted calculation of the CCI index. OS was analyzed as the evaluation index for efficacy.
Follow Up
OS was defined as the time from diagnosis of NSCLC to death or last follow-up, with a 3-year OS chosen as the endpoint for this study. All patients were followed up after the first treatment, and the included patients were followed up every 6 months. Survival data of patients were recorded through electronic medical records, telephone, and other means. A total of 353 patients were followed up until death from any cause or January 31, 2022, during the follow-up period, the positive outcome failed to manifest, and the loss to follow-up was presented as censored data.
Statistical Analysis
Statistical analysis was conducted using SPSS 27.0 software (SPSS Inc., Chicago, IL, USA). Normally distributed continuous variables were presented as mean ± standard deviation (SD). Non-normally distributed continuous variables were presented as median (interquartile range), while categorical variables were presented as frequency and percentage. Continuous and qualitative variables were compared using chi-square test, Fisher’s exact test, Mann–Whitney U-test, Kruskal–Wallis test, Student’s t-test, and one-way analysis of variance (ANOVA), respectively. Patients were grouped by optimal cut-off using the X-tile bioinformatics software (Yale University, New Haven, CT, USA). Statistically significant variables in the univariate Cox analysis were included in the multivariate Cox regression model to identify the independent factors of survival. The results of the survival analysis were expressed as hazard ratios and 95% confidence intervals (CI). OS was estimated from Kaplan-Meier curves with Rothman’s 95% confidence intervals (CI). The comparison among groups was performed by the Log rank test. A two-sided p< 0.05 was considered statistically significant.
Results
Patients Characteristics
A total of 353 patients were included in this study, with 163 patients (46.2%) demonstrating a high SII (≥889). There was no statistical significance in age, hypertension, diabetes, education, ECGO, tumor grade, and tumor size between the low SII group and the high SII group, this minimizes the impact of these factors on the disease prognosis to the greatest extent. Significant differences were observed between the groups with low and high SII in terms of gender, tobacco use, tumor types, therapy method, and organ metastasis. The high SII group had a statistically significant increase in the number of Stage III–IV patients. To ensure the robustness of the prognostic analysis, variables with significant imbalances were included as covariates in the multivariate analysis. This allowed us to assess the independent association between SII and clinical outcomes while controlling for confounding factors. The baseline characteristics of the patients stratified by SII (high vs low) are summarized in Table 1.
Table 1.
Clinicopathological Characteristics of Enrolled NSCLC Patients Stratified by SII
| Variables | Categories | Overall | SII<889 | SII≥889 | P |
|---|---|---|---|---|---|
| Number (n, %) | 353 | 190 (53.8%) | 163 (46.2%) | ||
| Gender (n, %) | |||||
| Male | 227 (64.3%) | 108 (56.8%) | 119 (73.0%) | 0.002 | |
| Female | 126 (34.7%) | 82 (43.2%) | 44 (27.0%) | ||
| Age (years, mean±SD) | Year | 58±7.48 | 58.65±6.55 | 59.07±8.46 | 0.61 |
| Tobacco (n, %) | |||||
| Never | 178 (50.6%) | 107 (56.6%) | 71 (43.6%) | 0.04 | |
| Former | 17 (4.9%) | 7 (3.7%) | 10 (6.1%) | ||
| Current | 158 (44.5%) | 76 (39.7%) | 82 (50.3%) | ||
| Hypertension (n, %) | |||||
| Yes | 50 (14.2%) | 30 (15.8%) | 20 (12.3%) | 0.34 | |
| No | 303 (85.8%) | 160 (84.2%) | 143 (87.7%) | ||
| Diabetes (n, %) | |||||
| Yes | 11 (3.1%) | 5 (2.6%) | 6 (3.7%) | 0.57 | |
| No | 342 (96.9%) | 185 (97.4%) | 157 (96.3%) | ||
| Education (n, %) | |||||
| ≤12 years/completed high school | 329 (93.2%) | 179 (94.2%) | 150 (92.0%) | 0.42 | |
| >12 years | 24 (6.8%) | 11 (5.8%) | 13 (8.0%) | ||
| ECOG (n, %) | |||||
| 0 | 323 (91.5%) | 174 (91.6%) | 149 (91.4%) | 0.96 | |
| >0 | 30 (8.5%) | 16 (8.4%) | 14 (8.6%) | ||
| Tumor types (n, %) | |||||
| LUSC | 172 (48.7%) | 79 (41.6%) | 93 (57.1%) | 0.004 | |
| LUAD | 111 (58.4%) | 70 (42.9%) | |||
| Tumor grade (n, %) | |||||
| Highly differentiated | 53 (15.0%) | 22 (11.6%) | 31 (19.0%) | 0.72 | |
| Moderately differentiated | 64 (18.1%) | 31 (16.3%) | 33 (20.2%) | ||
| Poorly differentiated | 236 (66.9%) | 137 (72.1%) | 99 (60.8%) | ||
| Tumor size (median, IQR) | CM | 4.00 [3.00, 5.40] | 3.95 [3.00, 5.30] | 4.10 [2.95, 5.55] | 0.55 |
| Lymph node (n, %) | |||||
| N0 | 137 (38.8%) | 92 (48.4%) | 45 (27.6%) | <0.001 | |
| N1–N3 | 216 (61.2%) | 98 (51.6%) | 118 (72.4%) | ||
| Stage (n, %) | |||||
| I | 78 (22.1%) | 58 (30.5%) | 20 (12.3%) | <0.001 | |
| II | 103 (29.2%) | 76 (40.0%) | 27 (16.6%) | ||
| III | 76 (21.5%) | 24 (12.6%) | 52 (31.9%) | ||
| IV | 96 (27.2%) | 32 (16.8%) | 64 (39.3%) | ||
| Metastasis (n, %) | |||||
| One organ | 68 (19.2%) | 28 (87.5%) | 40 (62.5%) | 0.02 | |
| Two organs or more organs | 28 (7.9%) | 4 (12.5%) | 24 (27.5%) | ||
| Therapy (n, %) | |||||
| SC | 187 (52.9%) | 125 (65.8%) | 62 (38.0%) | <0.001 | |
| C | 166 (47.1%) | 65 (34.2%) | 101 (62.0%) | ||
| ACCI (median, IQR) | 4 [3, 7] | 4 [3, 6] | 6 [4, 8] | <0.001 | |
| ACCI (n, %) | |||||
| <5 | 177 (50.1%) | 115 (60.5%) | 62 (38.0%) | <0.001 | |
| ≥5 | 176 (49.9%) | 75 (39.5%) | 101 (62.0%) |
Abbreviations: SII, systemic immune-inflammation index; ECOG score, eastern cooperative oncology group score; ACCI, age-adjusted Charlson comorbidity index; IQR, interquartile range; LUSC, squamous cell lung cancer; LUAD, lung adenocarcinoma; SC, surgical resection with adjuvant chemotherapy; C, chemotherapy without surgery.
Survival Predictors
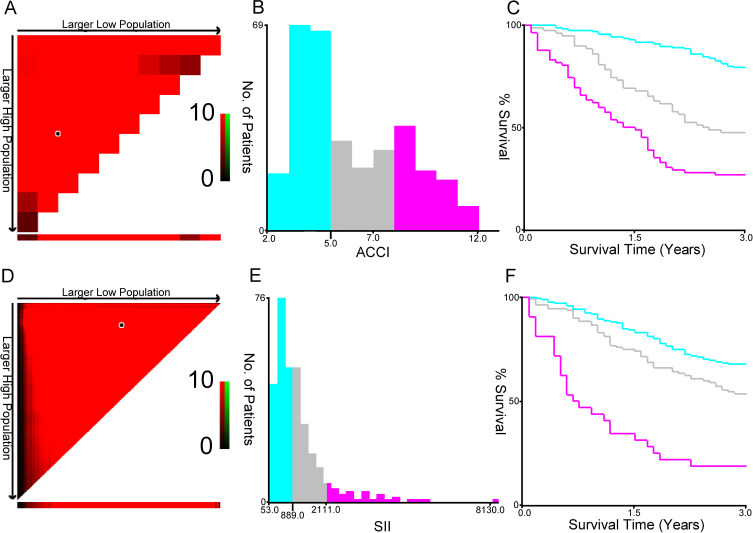
In order to investigate the potential prognostic significance of SII and ACCI in NSCLC patients, X-tile analysis was performed to identify the optimal threshold with the highest sensitivity and specificity in predicting mortality in 353 patients with available follow-up data. The resulting values were 889 for SII and 5 for ACCI. These thresholds were then used to assess the predictive value of SII and ACCI for survival prediction in NSCLC patients (see Figure 1).
Figure 1.
The optimal ACCI (A-C) and SII (D-E) cutoffs of 353 NSCLC patients using X-tile bioinformatics software. (A and D) The data were represented graphically in a right-triangular grid where each point represented the data from a given set of divisions. The plots showed the χ2 log-rank values produced, dividing them into three or two groups by the cut-off point. The optimal cut-points (5.0 and 889.0) were determined by locating the brightest pixel on the X-tile plot. The distribution of the number of patients was shown on the histogram (B and E), and corresponding populations were displayed on the Kaplan–Meier curve (C and F), respectively.
In a univariate survival analysis regarding baseline clinical and pathological factors, patients with stage III-IV disease (p < 0.001), high SII (≥ 889) (p < 0.001), and high ACCI (≥ 5) (p < 0.001) had a higher risk of death. Gender (p = 0.004), age (p = 0.008), lymph node involvement (p < 0.001), and therapy method (p < 0.001) also showed a tendency towards statistical significance (see Table 2). However, there was no significant association between survival and tobacco, education, ECOG, hypertension, diabetes, or tumor type. In the multivariate analysis, it was found that stage III-IV disease (p = 0.036), SII (p = 0.042), and ACCI (p = 0.007) were associated with 3-year OS. SII and ACCI were identified as independent predictive factors for a poor prognosis (see Table 2).
Table 2.
Univariate and Multivariate Analyzes of Cox Regression Model for Candidate Prognostic Factors for NSCLC Patients
| Univariate Cox Regression | Multivariate Cox Regression | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P | HR | 95% CI | P |
| Gender (vs female) | 1.817 | 1.213–2722 | 0.004 | 1.2 | 0.800–1.777 | 0.362 |
| Age (vs <60 years) | 1.626 | 1.137–2.324 | 0.008 | 1.097 | 0.758–1.588 | 0.624 |
| Tobacco (vs no or ever) | 1.366 | 0.955–1.954 | 0.088 | |||
| Education (vs >12 years) | 0.65 | 0.266–1.591 | 0.346 | |||
| ECOG (vs <1) | 1.373 | 0.756–2.493 | 0.297 | |||
| Hypertension (vs no) | 0.953 | 0.555–1.637 | 0.863 | |||
| Diabetes (vs yes) | 0.811 | 0.258–2.549 | 0.719 | |||
| LUAD vs LUSC | 1.085 | 0.760–1.550 | 0.653 | |||
| Stage III-IV vs stage I-II | 4.011 | 2.711–5.934 | <0.001 | 1.732 | 0.943–3.180 | 0.036 |
| Lymph node (vs N0) | 2.936 | 1.913–4.507 | <0.001 | 1.259 | 0.702–2.258 | 0.44 |
| Metastasis (vs One organ) | 0.736 | 0.446–1.213 | 0.229 | |||
| Therapy (vs SC) | 3.712 | 2.537–5.433 | <0.001 | 1.595 | 0.978–2.603 | 0.061 |
| ACCI (vs <5) | 4.597 | 3.049–6.932 | <0.001 | 2.147 | 1.236–3.728 | 0.007 |
| SII (vs <889) | 2.593 | 1.458–3.804 | <0.001 | 1.673 | 0.972–2.233 | 0.046 |
Abbreviations: ECOG score, eastern cooperative oncology group score; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; ACCI, age-adjusted Charlson index; SII, systemic Immune-Inflammation. HR, hazard ratio; CI, confidence interval.
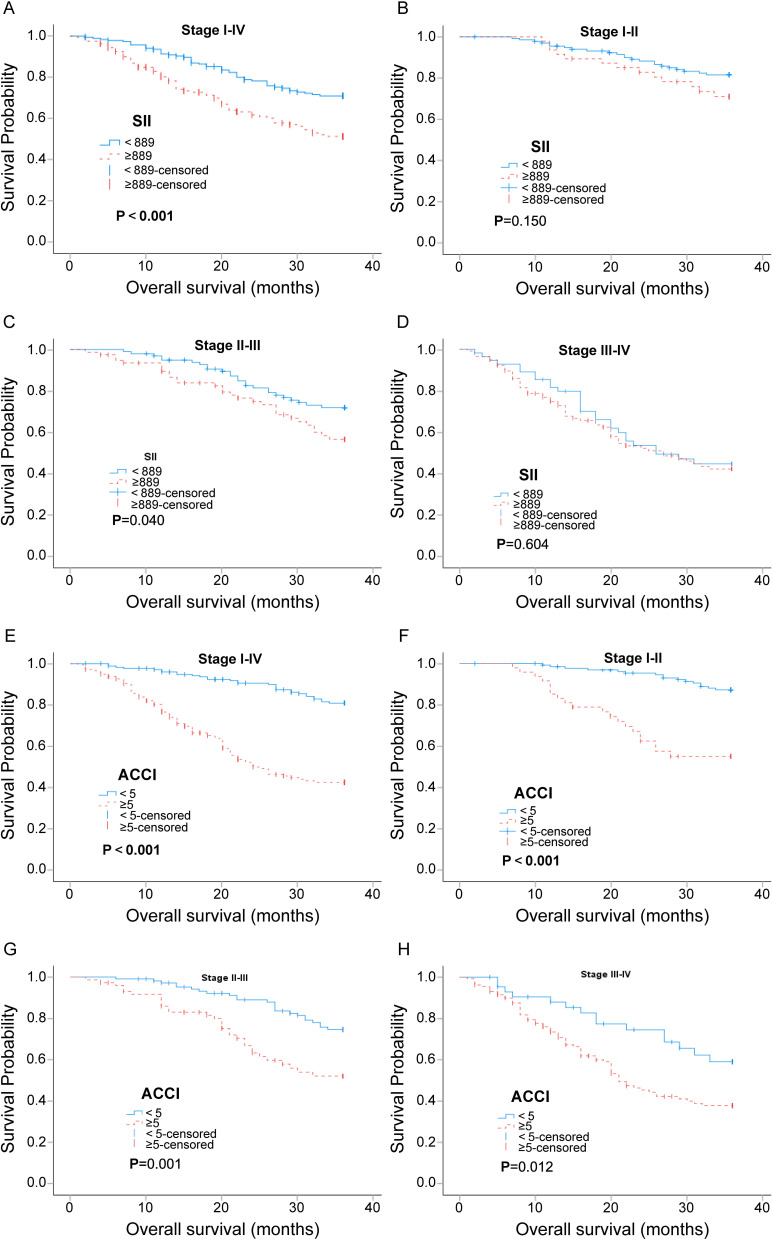
To evaluate the prognostic value of SII and ACCI in different tumor stages, patients were stratified according to the TNM stage. The results are shown in Figure 2. Except for the fact that there was no statistically difference in survival between the SII groups (high and low) in stages I–II and III–IV, the survival differences brought by different SII and ACCI groups were statistically significant in the corresponding other groups, and high SII or high ACCI predicted a poor prognosis. It indicated that SII and ACCI were strong predictors of tumor prognosis (see Figure 2).
Figure 2.
Kaplan-Meier curves with 95% confidence intervals for overall stage, stage I–II, stage II–III, and stage III–IV patients stratified with SII groups (≥ 889 and < 889) (A-D) and ACCI groups (ACCI<5 or ACCI≥5) (E-H). P<0.05 (bilateral) indicated statistical significance.
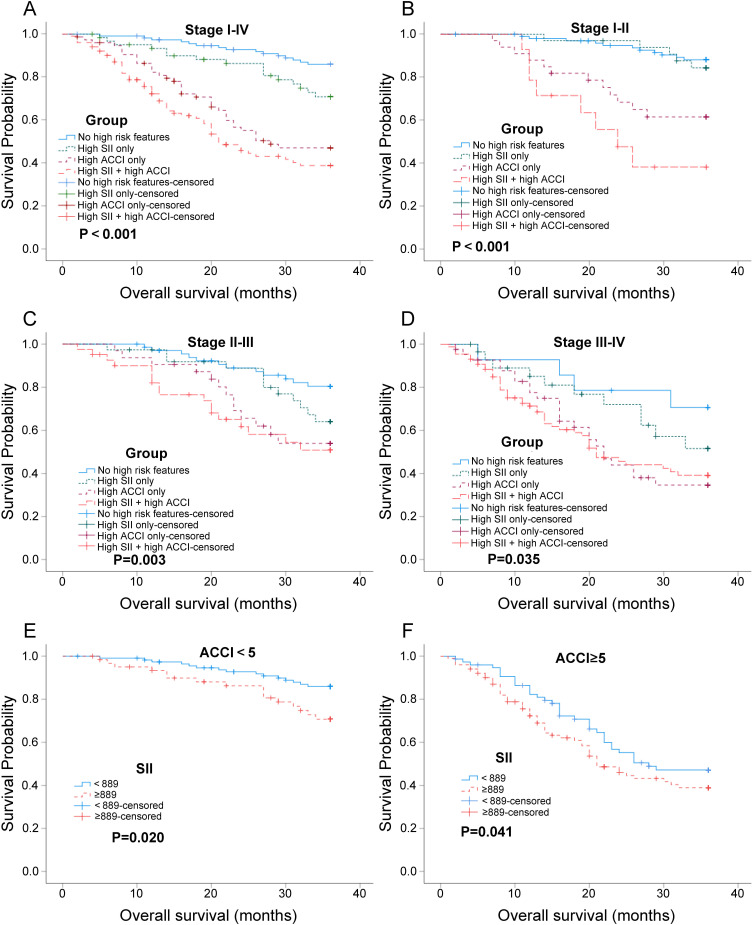
Based on the X-tile analysis, we conducted a subgroup analysis to evaluate the clinical utility of SII in assisting ACCI in predicting the prognosis of NSCLC. The patients were divided into four groups: no high-risk features group, high SII only group, high ACCI only group, and high SII + high ACCI group. We observed that the clinical outcomes of high SII + high ACCI group were inferior to the other three groups. This indicates that the combination of high SII and high ACCI is associated with a poorer prognosis (Figure 3A-D). Additionally, we performed a subgroup analysis using ACCI risk groups (Figure 3E-F). It was found that patients with high SII in the ACCI groups (high and low) had a higher risk of mortality. This finding suggests that in NSCLC patients with ACCI groups (high and low), high SII levels are associated with an increased risk of mortality. Therefore, SII can provide additional prognostic information beyond ACCI in identifying high-risk patients within the ACCI groups, and the prognostic significance of SII may not be overshadowed by the aggressive nature of ACCI itself. Overall, these results highlight the potential utility of SII as a prognostic marker in NSCLC, particularly in combination with ACCI risk stratification.
Figure 3.
Kaplan-Meier survival analyses depicting survival of NSCLC patients depending on SII and ACCI. (A-D) Kaplan-Meier survival analysis of patients in four groups (no high-risk features group, high SII only group, high ACCI only group, and high SII + high ACCI group) at different tumor stages (stages I–IV, stages I–II, stage II–III, and stage III–IV); (E and F) Kaplan-Meier survival analysis of patients in SII groups (≥ 889 and < 889) depending on the ACCI groups (ACCI<5 or ACCI≥5). P<0.05 (bilateral) indicated statistical significance.
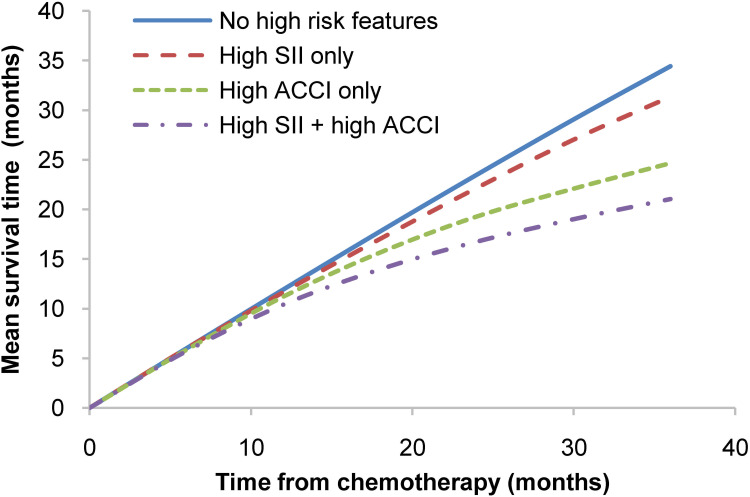
Quantitative survival analysis demonstrated a significant distinction in survival among predefined subgroups. Patients with both high SII and high ACCI had a reduction of approximately 10 months in survival after 36 months compared to those with no high-risk features (see Figure 4). In a multiple comparison analysis, there were no significant differences in survival between patients with high SII only, high ACCI only, or no risk features when comparing each of these groups individually. However, a significant difference in survival was observed between patients with both high SII and high ACCI and patients in each of the other three groups, including high SII only (p < 0.001), high ACCI only (p = 0.041), and no risk features (p < 0.001).
Figure 4.
Relationships between mean survival time and follow-up time among the four groups (no high-risk features group, high SII only group, high ACCI only group, and high SII + high ACCI group) of patients.
Discussion
The present study investigated SII and ACCI in predicting survival outcomes in NSCLC patients with PBC. We have validated the prognostic values of SII for OS and proposed its sequential integration with ACCI, thus representing that SII can complement ACCI in predicting survival in this patient population. To the best of our knowledge, few studies have analyzed both SII and ACCI as prognostic factors in this clinical setting.
CCI has been validated as a stratification of comorbidities influencing overall survival in multiple solid tumors.23,24 While limited research exists regarding the role of ACCI in predicting the prognosis of NSCLC with PBC, emerging evidence suggests that ACCI has the potential to predict treatment efficacy and survival rates in this patient population. Specifically, ACCI is an effective prognostic indicator for overall mortality in patients with inoperable early-stage NSCLC treated with stereotactic ablative radiotherapy25,26 and in patients with advanced lung cancer receiving palliative treatment.27,28 Furthermore, ACCI outperforms the Elixhauser comorbidity index in prognostic prediction.29 In our study, patients with ACCI ≥ 5 demonstrated significantly worse overall survival, and high ACCI emerged as an independent predictor of death in the multivariate analysis. Notably, a patient’s ACCI score, regardless of their SII level, negatively impacts their overall health condition. This suggests that high ACCI can translate into relatively apparent oncological benefits, assessing overall health condition, is more important than solely evaluating blood markers to predict prognosis.
Inflammatory biomarkers have shown promising prognostic value in various oncology fields, including NSCLC.30,31 The SII, based on the parameters of neutrophils, platelets, and lymphocytes,32 is a novel inflammation-related biomarker. Neutrophils promote tumor invasion and metastasis through the secretion of vascular endothelial growth factors,33 while platelets can be activated by tumor cells to provide protection against lymphocyte attack and contribute to tumor development.34,35 Hence, SII reflects the balance between inflammatory activators and regulatory factors associated with cancer development. Considering the composition of the SII, this biomarker holds the potential for optimal predictive ability in terms of survival outcomes.32 Nevertheless, further research and clinical validation are necessary to fully establish the clinical utility of the SII and its application in personalized cancer treatment strategies.
In this study, we revealed that elevated SII was independently correlated with the poor OS of NSCLC patients with PBC. In addition, we investigated the prognostic value of SII in sub-group analysis and found that there was no significant difference in survival between different SII groups in stage I–II and stage III-IV, indicating the shortfalls of this index in predicting survival. When adjusting for comorbidities using ACCI, it was found that patients with high SII in the ACCI groups (high and low) showed a statistically significant association with OS outcomes. This suggested that ACCI combined with SII was better than ACCI alone in predicting prognosis. A high ACCI supplemented with SII ≥ 893 provided genuine discrimination in overall mortality in patients. It indicated the superiority of SII supporting ACCI in predicting the prognosis of NSCLC patients treated with PBC.
To our knowledge, this study is one of the few comprehensive investigations into the prognostic value of SII in conjunction with TNM stage, ACCI, and PBC in NSCLC patients. Several previous studies have shown that SII is a significant prognostic factor in NSCLC patients.36–38 For instance, in a meta-analysis conducted by Wang et al38, which included nine studies with a total of 2441 patients, pretreatment SII was found to be a useful prognostic indicator with a higher prognostic value compared to the neutrophil-to-lymphocyte ratio (NLR) or the platelet-to-lymphocyte ratio (PLR). Fu et al39 also reported that an SII ≥ 479 was independently predictive of recurrence-free survival (RFS) (P=0.034) and OS (P<0.001) in NSCLC patients, even after adjusting for age, sex, and TNM stage. Similar outcomes were observed by Rossana et al36, albeit with a higher SII threshold of ≥ 1270. We confirmed the prognostic value of SII as a supporting marker for ACCI in NSCLC patients undergoing PBC. Considering the relative affordability and routine nature of SII and the easy availability of ACCI, the combination of SII and ACCI was presented as a non-invasive and easily accessible candidate for a prognostic factor in NSCLC.
Additionally, other biological models, such as methylation modification on mRNA40 and circulating tumor DNA (ctDNA),41 have been limited in their widespread application due to challenges associated with detection difficulty and high cost. Consequently, this study has provided an economical, direct, and effective approach to aid clinicians in effectively predicting the prognosis of NSCLC patients undergoing PBC treatment. This not only complements the existing utilization of tumor staging for prognostication in NSCLC but also pioneers novel avenues for future research on prognostic prediction in NSCLC patients. Furthermore, it can significantly assist physicians in making clinical treatment decisions. It should be noted that the stringent inclusion criteria employed in this study may restrict its widespread clinical applicability. For instance, when patients present potential infections or underlying diseases, it could potentially impact the prognostic predictive ability of this model.
There are several limitations in relation to this study. Firstly, it is an observational analysis that relies on retrospective data collection and has a limited follow-up period. Secondly, although a comprehensive evaluation of comorbidities is a routine part of the pre-surgical workup, the assessment of ACCI was based on self-reported comorbidities without a standardized survey procedure. Finally, this study only establishes a preliminary prognostic assessment, and further confirmatory studies are required. The short follow-up period restricts our ability to conduct robust analyses and detect significant differences between groups. To address these limitations, future studies will include an extended follow-up period and a larger sample size. Whereas, these incipient results from the retrospective study can be used as a theoretical basis for the next multicenter study on a large-scale population.
Conclusion
This study presents high SII as a potential marker for predicting survival outcomes in patients receiving first-line PBC for NSCLC. We have validated the SII as a complementary indicator to the widely used ACCI. Our findings indicate that patients with high SII and high ACCI experienced a reduction in survival time of approximately 10 months within a 36-month period after treatment. Furthermore, we have confirmed the utility of the SII in stratifying the risk of death in patients with low ACCI. We believe that incorporating the SII into existing prognostic tools could provide valuable clinical insights, particularly for patients undergoing first-line PBC for NSCLC.
Acknowledgments
The authors thank all patients who participated in this study.
Funding Statement
This work was supported by The Natural Science Foundation of Jiangxi Province, China (Grant No. 20192BAB205088), The National Natural Science Fund of China (Grant No. 82260085), the Scientific and Technological Research Projects of Jiangxi Province Department of Education, China (Grant No. GJJ210231) and the Science and Technology Planning of Jiangxi Province Department of Health Commission, China (Grant No. 202310455) and the Chinese Medicine Science and Technology Project of Jiangxi Province Department of Health Commission, China (Grant No. 2022B901) were supported the data collection and statistical analysis of the study.
Abbreviations
SII, systemic immune-inflammation index; ACCI, age-adjusted Charlson comorbidity index; OS, overall survival; NSCLC, non-small cell lung cancer; PBC, platinum-based chemotherapy; IASLC, International Association for Lung Cancer Research; ECOG score, eastern cooperative oncology group score; PS, performance status; CCI, Charlson Comorbidity Index; SD, standard deviation; ANOVA, one-way analysis of variance; CI, confidence intervals; IQR, interquartile range; LUSC, squamous cell lung cancer; LUSD, lung adenocarcinoma; SC, surgical resection with adjuvant chemotherapy; C, chemotherapy without surgery.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study was conducted in accordance with the guidelines of the Declaration of Helsinki. Informed consent was obtained from all patients participating in the study, approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University (Jiangxi Province, China). This study only involved inquiries on medical system records, and did not use the patient’s blood and surgically resected tissue specimens.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- 3.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–2394. doi: 10.1056/NEJMoa1214886 [DOI] [PubMed] [Google Scholar]
- 4.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 5.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884 [DOI] [PubMed] [Google Scholar]
- 6.Ohe Y, Ohashi Y, Kubota K, et al. Randomized Phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: four-arm cooperative study in Japan. Ann Oncol. 2007;18(2):317–323. doi: 10.1093/annonc/mdl377 [DOI] [PubMed] [Google Scholar]
- 7.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543–3551. doi: 10.1200/JCO.2007.15.0375 [DOI] [PubMed] [Google Scholar]
- 8.Zhu J, Sharma DB, Gray SW, et al. Carboplatin and paclitaxel with vs without bevacizumab in older patients with advanced non-small cell lung cancer. JAMA. 2012;307(15):1593–1601. doi: 10.1001/jama.2012.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang SH, Chu CY, Hsu YC, et al. How platinum-induced nephrotoxicity occurs? Machine learning prediction in non-small cell lung cancer patients. Comput Meth Programs Biomed. 2022;221:106839. doi: 10.1016/j.cmpb.2022.106839 [DOI] [PubMed] [Google Scholar]
- 10.Mitsudomi T, Tan D, Yang JCH, et al. Expert consensus recommendations on biomarker testing in metastatic and nonmetastatic NSCLC in Asia. J Thorac Oncol. 2023;18(4):436–446. doi: 10.1016/j.jtho.2022.10.021 [DOI] [PubMed] [Google Scholar]
- 11.Kerr K, Bibeau F, Thunnissen E, et al. The evolving landscape of biomarker testing for non-small cell lung cancer in Europe. Lung Cancer. 2021;154:161–175. doi: 10.1016/j.lungcan.2021.02.026 [DOI] [PubMed] [Google Scholar]
- 12.Jamal-Hanjani M, Wilson GA, McGranahan N, et al. Tracking the evolution of non-small-cell lung cancer. N Engl J Med. 2017;376(22):2109–2121. doi: 10.1056/NEJMoa1616288 [DOI] [PubMed] [Google Scholar]
- 13.Le VH, Kha QH, Tuan Minh TN, et al. Development and validation of CT-based radiomics signature for overall survival prediction in multi-organ cancer. J Digit Imaging. 2023;36(3):911–922. doi: 10.1007/s10278-023-00778-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le VH, Tuan Minh TN, Kha QH, et al. A transfer learning approach on MRI-based radiomics signature for overall survival prediction of low-grade and high-grade gliomas. Med Biol Eng Comput. 2023;61(10):2699–2712. doi: 10.1007/s11517-023-02875-2 [DOI] [PubMed] [Google Scholar]
- 15.Dall’Olio FG, Abbati F, Facchinetti F, et al. CEA and CYFRA 21-1 as prognostic biomarker and as a tool for treatment monitoring in advanced NSCLC treated with immune checkpoint inhibitors. Ther Adv Med Oncol. 2020;12:1758835920952994. doi: 10.1177/1758835920952994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrelli F, Borgonovo K, Cabiddu M, et al. Relationship between skin rash and outcome in non-small-cell lung cancer patients treated with anti-EGFR tyrosine kinase inhibitors: a literature-based meta-analysis of 24 trials. Lung Cancer. 2012;78(1):8–15. doi: 10.1016/j.lungcan.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Gu J, Roth JA, et al. Pathway-based serum microRNA profiling and survival in patients with advanced stage non-small cell lung cancer. Cancer Res. 2013;73(15):4801–4809. doi: 10.1158/0008-5472.CAN-12-3273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saab S, Zalzale H, Rahal Z, et al. Insights into lung cancer immune-based biology, prevention, and treatment. Front Immunol. 2020;11:159. doi: 10.3389/fimmu.2020.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang H, Hegde S, Knolhoff BL, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med. 2016;22(8):851–860. doi: 10.1038/nm.4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Cao D, Huang Y, et al. The prognostic and clinicopathological significance of systemic immune-inflammation index in bladder cancer. Front Immunol. 2022;13:865643. doi: 10.3389/fimmu.2022.865643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hung SP, Chen PR, Ho TY, et al. Prognostic significance of the preoperative systemic immune-inflammation index in patients with oral cavity squamous cell carcinoma treated with curative surgery and adjuvant therapy. Cancer Med. 2021;10(2):649–658. doi: 10.1002/cam4.3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takada Y, Kawashima H, Ohno E, et al. The impact of the age-adjusted Charlson comorbidity index as a prognostic factor for endoscopic papillectomy in ampullary tumors. J Gastroenterol. 2022;57(3):199–207. doi: 10.1007/s00535-022-01853-z [DOI] [PubMed] [Google Scholar]
- 23.Barz M, Bette S, Janssen I, et al. Age-adjusted Charlson comorbidity index in recurrent glioblastoma: a new prognostic factor? BMC Neurol. 2022;22(1):32. doi: 10.1186/s12883-021-02532-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 25.Lee IH, Chen GY, Chien CR, et al. A retrospective study of clinicopathologic and molecular features of inoperable early-stage non-small cell lung cancer treated with stereotactic ablative radiotherapy. J Formos Med Assoc. 2021;120(12):2176–2185. doi: 10.1016/j.jfma.2020.12.028 [DOI] [PubMed] [Google Scholar]
- 26.Holmes OE, MacRae R, Cook G, et al. Age-not Charlson co-morbidity index-predicts for mortality after stereotactic ablative radiotherapy for medically inoperable stage I non-small cell lung cancer. Clin Transl Radiat Oncol. 2017;5:37–41. doi: 10.1016/j.ctro.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stamenovic D, Messerschmidt A, Schneider T. Surgery for lung tumors in the elderly: a retrospective cohort study on the influence of advanced age (over 80 years) on the development of complications by using a multivariate risk model. Int J Surg. 2018;52:141–148. doi: 10.1016/j.ijsu.2018.02.008 [DOI] [PubMed] [Google Scholar]
- 28.Calvo-Espinos C, De Gaona-Lana E, Gonzalez-Anguren C, et al. Assessment of the impact of comorbidity on the survival of cancer patients treated by palliative care teams. Palliat Support Care. 2015;13(4):1049–1055. doi: 10.1017/S1478951514000832 [DOI] [PubMed] [Google Scholar]
- 29.Yang CC, Fong Y, Lin LC, et al. The age-adjusted Charlson comorbidity index is a better predictor of survival in operated lung cancer patients than the Charlson and Elixhauser comorbidity indices. Eur J Cardiothorac Surg. 2018;53(1):235–240. doi: 10.1093/ejcts/ezx215 [DOI] [PubMed] [Google Scholar]
- 30.Tong YS, Tan J, Zhou XL, et al. Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small cell lung cancer. J Transl Med. 2017;15(1):221. doi: 10.1186/s12967-017-1326-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi: 10.1158/1078-0432.CCR-14-0442 [DOI] [PubMed] [Google Scholar]
- 32.Shen YJ, Qian LQ, Ding ZP, et al. Prognostic value of inflammatory biomarkers in patients with stage I lung adenocarcinoma treated with surgical dissection. Front Oncol. 2021;11:711206. doi: 10.3389/fonc.2021.711206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang Y, Peng B, Liu J, et al. Systemic immune-inflammation index and bone mineral density in postmenopausal women: a cross-sectional study of the national health and nutrition examination survey (NHANES) 2007-2018. Front Immunol. 2022;13:975400. doi: 10.3389/fimmu.2022.975400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol. 2018;11(1):125. doi: 10.1186/s13045-018-0669-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palacios-Acedo AL, Mège D, Crescence L, et al. Platelets, thrombo-inflammation, and cancer: collaborating with the enemy. Front Immunol. 2019;10:1805. doi: 10.3389/fimmu.2019.01805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berardi R, Santoni M, Rinaldi S, et al. Pre-treatment systemic immune-inflammation represents a prognostic factor in patients with advanced non-small cell lung cancer. Ann Transl Med. 2019;7(20):572. doi: 10.21037/atm.2019.09.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo W, Cai S, Zhang F, et al. Systemic immune-inflammation index (SII) is useful to predict survival outcomes in patients with surgically resected non-small cell lung cancer. Thorac Cancer. 2019;10(4):761–768. doi: 10.1111/1759-7714.12995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Chen B, Wang L, et al. Systemic immune-inflammation index is a promising noninvasive marker to predict survival of lung cancer: a meta-analysis. Medicine. 2019;98(3):e13788. doi: 10.1097/MD.0000000000013788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu F, Deng C, Wen Z, et al. Systemic immune-inflammation index is a stage-dependent prognostic factor in patients with operable non-small cell lung cancer. Transl Lung Cancer Res. 2021;10(7):3144–3154. doi: 10.21037/tlcr-21-267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang K, Yang Y, Yu L, et al. Methylation modification is a poor prognostic factor in non-small cell lung cancer and regulates the tumor microenvironment: mRNA molecular structure and function. Int J Biol Macromol. 2024;2:137214. doi: 10.1016/j.ijbiomac.2024.137214 [DOI] [PubMed] [Google Scholar]
- 41.Hong TH, Hwang S, Dasgupta A, et al. Clinical utility of tumor-naïve presurgical circulating tumor DNA detection in early-stage NSCLC. J Thorac Oncol. 2024;9:S1556–0864(24)00666–X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.