Abstract
Background
Leigh syndrome is a common mitochondrial disorder caused by gene mutations in the nucleus and mitochondria. When building mitochondrial complex I, the main subunit ND1 combines with the Q module to form a 273 kDa complex, which then adds Ndufa3, Ndufa8, and Ndufa13 to create an intermediate product of about 283 kDa called Q/Pp-a. Although Ndufa8 and Ndufa13 have been linked to mitochondrial diseases, the role of Ndufa3 in disease development is still not fully understood.
Methods
A family suspected of having Leigh syndrome was examined. Subjects (two brothers and a sister) underwent brain imaging, and their clinical symptoms were evaluated. Also, whole exome sequencing and minigene testing were performed by examining peripheral blood samples (2 ml) collected from the proband, his parents, and brothers.
Results
Three affected children showed early-onset symptoms, including abnormalities in muscle tone and delayed motor and language development. Symptoms were relatively mild. The second child of the second pregnancy experienced worsened muscle tone abnormalities after injury, slow wound healing, and sustained increased muscle tone up to a year after wound closure. His brain scans revealed lesions in the basal ganglia and brainstem, consistent with Leigh syndrome diagnosis. Genetic analysis identified compound heterozygous mutations in the Ndufa3 gene in all affected family members.
Conclusion
This is the first report of a family affected by Leigh syndrome associated with mutations in the Ndufa3 gene. Our analyses of clinical symptoms, radiological scans, and genetic investigations broaden our understanding of Ndufa3 gene mutations and their role in the development of Leigh syndrome.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10048-024-00782-8.
Keywords: Leigh syndrome, Mitochondrial disease, Ndufa3
Background
Mitochondria are semi-independent organelles in most eukaryotic organisms that act as the “powerhouses” by producing adenosine triphosphate (ATP). As the primary energy carrier, ATP is mainly produced through oxidative phosphorylation (OXPHOS) reactions within the inner mitochondrial membrane. OXPHOS comprises five protein complexes encoded by the cell nucleus and mitochondria, having vital roles in the mitochondrial electron transport chain and being a critical component of oxidative phosphorylation [1]. Any damage to the complex and its proteins can disrupt the electron transport chain, leading to impaired cell function and mitochondrial diseases, with an incidence rate of around 1 in 5000 live births [2].
Leigh syndrome is the most prevalent form of mitochondrial disease, characterized by progressive necrotic lesions in the thalamus, brainstem, and spinal cord. Children with Leigh syndrome inherit a gene or mitochondrial change that leads to nervous system cell death. So far, up to 100 genes have been linked to Leigh syndrome [3]. The inheritance patterns of Leigh syndrome vary and may include mitochondrial maternal inheritance, autosomal recessive, or dominant inheritance, with causative genes located in both mitochondrial and nuclear genomes [4]. With the advancements in next-generation sequencing technologies, mutations in nuclear genes contributing to mitochondrial diseases have been continuously identified [5–7].
During the assembly process of mitochondrial complex I and after the core subunit ND1 forms a 273KD complex with the Q module, subunits Ndufa3, Ndufa8, and Ndufa13 are recruited to form an intermediate product of about 283KD, which becomes Q/Pp-a. Ndufa8 transfers electrons from NADH to the respiratory chain; Ndufa13 encodes a subunit of the mitochondrial membrane respiratory chain NADH dehydrogenase (Complex I), which functions in the transfer of electrons from NADH to the respiratory chain, while Ndufa3 gene has an essential role in mitochondrial subunit assembly, stable group, and electron transport [8]. Mutations in Ndufa8 and Ndufa13 have been linked with several conditions. For example, in 2020, Tort et al. [9] reported structural and functional defects of mitochondrial respiratory chain complex 1 caused by Ndufa8 gene mutation in children with mitochondrial disease. Moreover, Gonzalez-Quintana et al. [10] found an association between the Ndufa13 gene mutation and Leigh syndrome. Furthermore, Alston and colleagues found that pathogenic bi-allelic mutations in Ndufa8 can cause Leigh syndrome with an isolated complex I deficiency. However, no association has been reported between Ndufa3 and Leigh syndrome or another type of neurologic regression.
Herein, we presented the first family with three affected siblings with mitochondrial disease caused by a mutation in the Ndufa3 gene.
Methods and results
Clinical presentation
A family suspected of having Leigh syndrome was examined in this study; the main clinical features are shown in Table S1.
First, we examined the proband, an 8-year-old male, born via cesarean section at 36 weeks; he is the second child from the second pregnancy. His birth weight was 2.6 kg. He did not experience perinatal asphyxia but presented with jaundice and received phototherapy. The patient had a normal birth history but developed a high muscle tone at 4 months of age. Compared to his peers, he gradually developed significant motor, language, and cognitive delays as he aged. He could control his head at 3 months, roll over at 4 months, sit independently at 8 months, crawl at 10 months, and stand-alone at around 2 years; he started speaking at 2. At the age of 7, he could walk a few steps independently, speak short phrases, and express basic needs. Also, at the age of 7, he fell while walking, which resulted in a deep chin wound healing slower than usual. Approximately 10 days after the injury, persistent extension and pain occurred in the left limb. After three months of rehabilitation therapy with some improvement, muscle tone significantly increased following cessation of rehabilitation therapy, prompting hospital admission for further evaluation.
Physical examination (at 7 years) showed the patient is in a passive position with involuntary head movements. Horizontal nystagmus was observed in both eyes. The left limb was flexed in a back-extended position, with the left thumb adducted. Muscle tone was high; the patient was uncooperative during muscle strength examination, showing poor muscle coordination in all limbs. Bilateral knee reflexes and Achilles reflexes were slightly hyperactive, and the Babinski sign was negative on both sides.
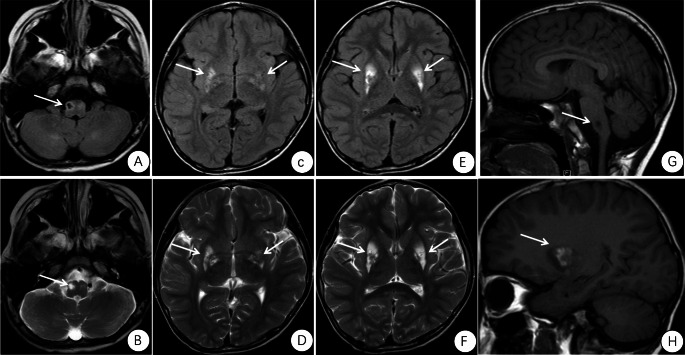
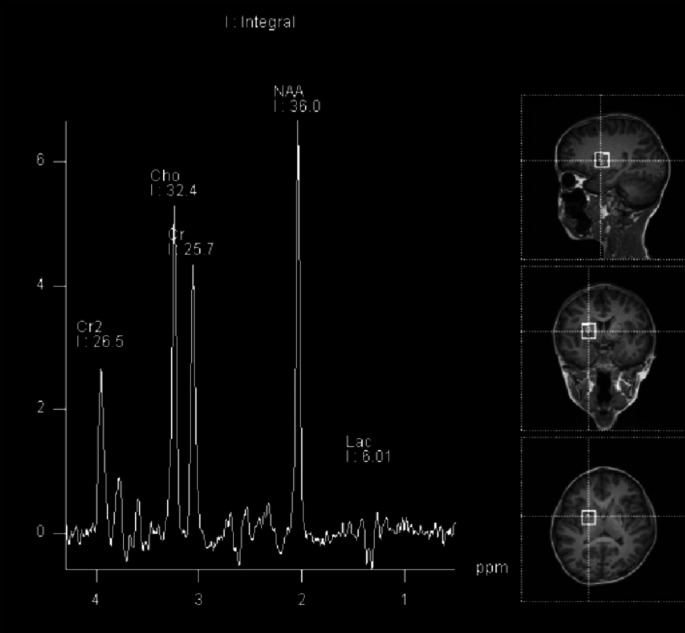
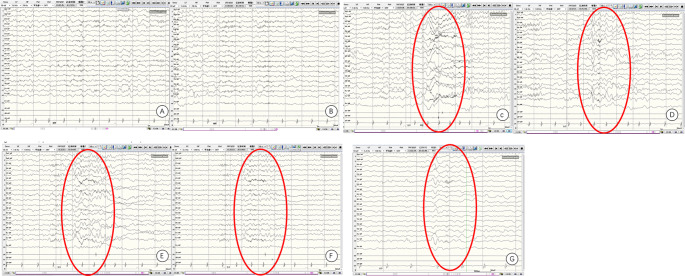
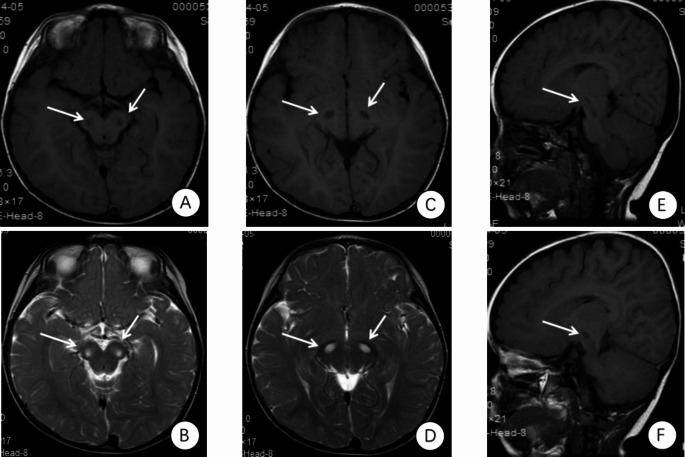
Cranial magnetic resonance imaging (MRI) findings of the proband revealed multiple symmetric abnormal signals within the cranial cavity. A hyperintense lesion was observed in the right medulla (Fig. 1). Magnetic resonance spectroscopy (MRS) indicated abnormal dual-peak lactate (Fig. 2). Video electroencephalography (EEG) showed slow background activity. During sleep, bilateral frontal 20–25 Hz low-amplitude β waves were detected. One cluster of sleep-related seizures was observed, accompanied by slight tremors in the lower limbs. Concurrent EEG displayed predominant 1 Hz high-amplitude δ slow waves with superimposed low-amplitude 30–35 Hz high-frequency fast waves in the right central, parietal, and midline regions (Cz). Short bursts lasted for 1 s (Fig. 3).
Fig. 1.
The proband’s cranial MRI examination. The first diagnosed patient showed multiple symmetric abnormal signals within the intracranial space, with hyperintense lesions on the right side of the medulla. (A) FLAIR: hypointense lesion on the right side of the medulla. (B) T2: hyperintense lesion on the right side of the medulla. (C) FLAIR. (D) T2. (E) FLAIR. (F) T2:hyperintense lesions in the basal ganglia. (G) T1: hypointense lesions on the right side of the medulla. (H) T1: hyperintense lesions in the basal ganglia
Fig. 2.
Abnormal MR spectroscopy showing lactic acid double peaks
Fig. 3.
Initial patient sleep EEG monitoring. During sleep, seizures occurred, characterized by slight tremors in the lower limbs. Simultaneous EEG recording showed predominant 1 Hz high-amplitude δ slow waves, superimposed with low-amplitude 30–35 Hz low-frequency and high-frequency fast waves in the right central, parietal, and midline areas (Cz) for a brief burst of 1-second
The proband received a cocktail therapy comprising vitamin B, coenzyme Q10, levodopa, and L-carnitine. Significant improvement in muscle tone was observed compared to before treatment. The proband was then discharged from the hospital. The last follow-up was conducted at the age of 8. After discharge from the hospital, the child stopped taking drugs due to vomiting after taking the medication (the medication was taken for about 1 month).
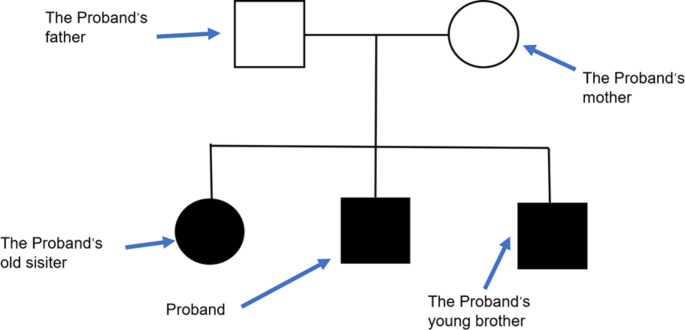
At present, the child is still undergoing rehabilitation treatment, and he has reduced muscle tension. He still has involuntary movements of the head, limbs, and torso and uses his right hand to eat, followed by intentional actions such as biting food, chewing food, other rhythmic movements, and quiet state light (video 1). No seizures were reported. Blood lactic acid was 5.98 mmol/L, blood pyruvate was 0.121 mmol/L, and blood lactic acid/pyruvate was 49.421. His parents are healthy and non-consanguineous (Fig. 4).
Fig. 4.
Patient pedigree
The proband’s older sister is the first child of the first pregnancy, delivered via cesarean section at 36 weeks with a birth weight of 2.8 kg. She had a normal birth history. She exhibited growth and developmental delays around 6 months of age. She could independently sit at around 1 year, having low muscle tone. She was evaluated at 1 year of age at our hospital. Cranial MRI (bilateral medial peduncle lesion, Fig. 5) and genetic metabolic screening (tandem mass spectrometry) in blood and urine were performed, showing no abnormalities. Her cognitive development was stagnant after 7 years of age. Now, the patient is 11 years old. Because of low muscle tone, she cannot walk and stand independently, but she can sit. Obvious involuntary head movements were observed in a sitting position, and paroxysmal horizontal nystagmus and isotropia were also detected, which were very obvious when the patient was nervous (video 2). Blood lactic acid was 5.48 mmol/L, blood pyruvate was 0.115 mmol/L, and blood lactic acid/pyruvate was 47.652.
Fig. 5.
The cranial MRI of the proband’s sister. Brain stem lesion. (A, C, E, F) T1: brain stem lesion; (B, D) T2: brain stem lesion
The proband’s younger brother is the third child of the second pregnancy, delivered via cesarean section at 36 weeks with a birth weight of 2.8 kg. He did not experience perinatal asphyxia but had jaundice and received phototherapy. He also experienced developmental delays from a young age; he started walking and speaking at the age of 2.5, although he currently has unclear speech and can express basic needs. He can walk unaided but often struggles. He walks with a wide gate, drags his left lower limb, has bilateral postural incoordination involuntary limb tremors (video 3), and has an intellectual level better than the older children. Blood lactic acid was 4.22 mmol/L, blood pyruvate was 0.089 mmol/L, and blood lactic acid/pyruvate was 47.416.
Whole exome sequencing
Peripheral blood samples (2 ml) were collected from the proband, his parents, and brothers, using EDTA as an anticoagulant. The samples were sent to Beijing Maikino Medical Laboratory for genetic testing. Genomic DNA was extracted from white blood cells isolated from peripheral blood using the QIAamp DNA extraction kit (Qiagen, Shanghai, China). The whole genome DNA was fragmented to 150–200 bp by enzyme digestion, and biotin capture probes were employed (Makino, Beijing, China). The gene’s exome and flanking 20 bp regions were enriched to construct the target genome library. Double-ended sequencing was performed using the DNBSEQ-T7 sequencer (BGI, Shenzhen, China), with reads of about 150 bp. Data quality control ensured an average sequencing depth of > 100X, and coverage required that the sequencing depth of 20X was > 95%. The FASTQ file was aligned to the Human Reference Genome (hg19) using BWA software. Sentieon software parameters were utilized to detect SNP and Indel variations. ANNOVAR was employed for SNP/Indel variation annotation. Hazard prediction was performed by association with 1000 genomes, ESP6500, dbSNP, EXAC, HGMD, and REVEL, as well as MutationTaster, SIFT, PolyPhen-2, SPIDEX, and dbscSNV. The pathogenicity of the variants was assessed according to the American Society for Medical Genetics and Genomics (ACMG) guidelines. Association analysis was carried out along the family line to screen for common mutations among the three children, emerging or complex heterozygous variants.
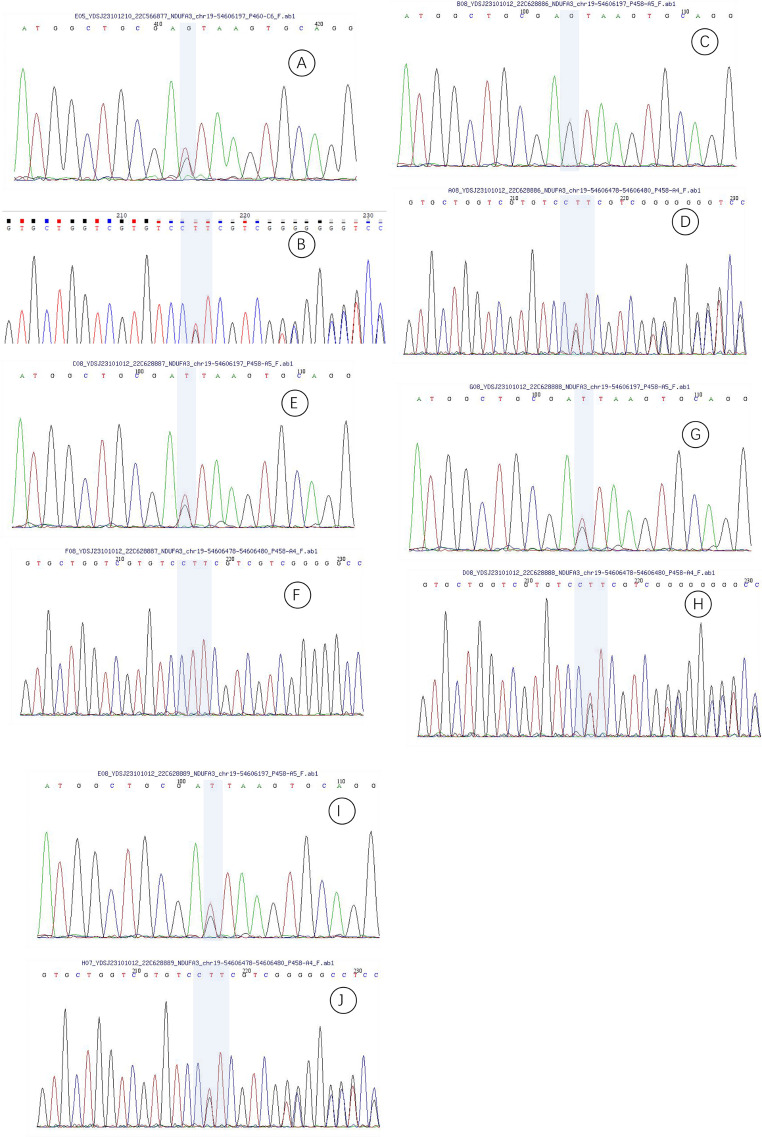
Compound heterozygous mutations were identified in the Ndufa3 gene in proband’s samples (transcript NM_004542), with mutation sites c.10 + 1G > T and c.66_68delCTT (p.22_23delSFinsS). Both parents were heterozygous carriers of these mutations. Siblings were also found with compound heterozygous mutations (Fig. 6).
Fig. 6.
First-Generation Sequencing Profiles. (A) Sequencing profile of the proband’s mutation site c.10 + 1G > T. (B) Sequencing profile of the proband’s mutation site c.66_68delCTT. (C) Sequencing profile of the proband’s father’s mutation site c.10 + 1G > T. (D) Sequencing profile of the proband’s father’s mutation site c.66_68delCTT. (E) Sequencing profile of the proband’s mother’s mutation site c.10 + 1G > T. (F) Sequencing profile of the proband’s mother’s mutation site c.66_68delCTT. (G) Sequencing profile of the proband’s sister’s mutation site c.10 + 1G > T. (H) Sequencing profile of the proband’s sister’s mutation site c.66_68delCTT. (I) Sequencing profile of the proband’s brother’s mutation site c.10 + 1G > T. (J) Sequencing profile of the proband’s brother’s mutation site c.66_68delCTT
Minigene Experiment (c.10 + 1G > T)
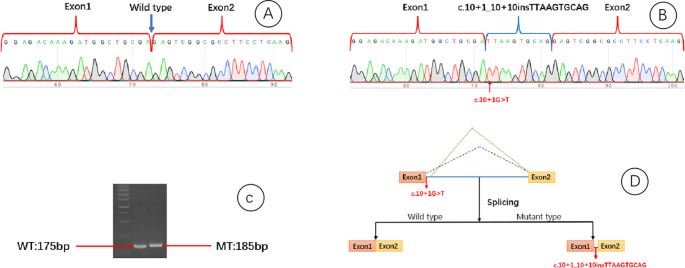
To validate the potential splicing effect caused by the c.10 + 1G > T mutation, an in vitro minigene splicing assay was conducted (Figure S1). Wild-type (WT) and mutant-type (MT) Ndufa3 plasmids were constructed, containing exon1, exon2, and intron1. The WT construct was amplified from normal human genomic DNA using the following primers: Ndufa3 - F5’AAGCTTGGTACCGAGCTCGGATCCGCTGTCGCCGCCGCGGAGACAAAGATGG3’; Ndufa3 - R5’TTAAACGGGCCCTCTAGACTCGAGCGAGGCCCCCGACGACGAAGGACACGAC3’. The amplified product was cloned into the pMini-CopGFP vector (Beijing Hitrobio Biotechnology Co., Ltd., Beijing, China) at the BamHI/XhoI restriction sites using the ClonExpress II One Step Cloning Kit (Vazyme, Nanjing, China). WT was validated by Sanger sequencing. MT was obtained by site-directed mutagenesis of WT using the following primers: Ndufa3 - MT-F: 5’ GGCTGCGATTAAGTGCAGGTGCCGGTGGCGCA3’ and Ndufa3 - MT-R: 5’ TGCACTTAATCGCAGCCATCTTTGTCTCCGCG3’. MT was validated by Sanger sequencing. WT and MT plasmids were transfected into HEK293T cells. After 48 h, total RNA was extracted from the cells using TRIzol reagent (Cowin Biotech Co., Jiangsu, China). Reverse transcription polymerase chain reaction (RT-PCR) was performed using primers 5’GGCTAACTAGAGAACCCACTGCTTA3’ and 5’CCCCCGACGACGAAGGACC3’. Gel electrophoresis and Sanger sequencing were used to analyze PCR fragments and determine gene isoforms. Finally, the nucleotide sequence was translated into a protein sequence using the Expasy-translate tool to analyze the impact of the mutation on the translation process.
Investigators concluded the following: ① the wild-type plasmid transcribed mRNA sequence aligns with expectations, containing complete exon 1 and exon 2 (Fig. 7A); ② the mutant-type plasmid transcribes one mRNA product: retention of a 10-bp sequence in the 1st intron, denoted as NM_004542:c.10 + 1_10 + 10insTTAAGTGCAG (Fig. 7B). Without undergoing nonsense-mediated decay (NMD), a frameshift mutation results in a truncated protein (Fig. 7C), represented as p.Arg4IlefsTer59. Overall analysis suggested that the NM_004542:c.10 + 1G > T mutation causes the retention of a 10-bp sequence in the 1st intron (Fig. 7D).
Fig. 7.
Minigene experimental results. (A) Wild-type plasmid transcription mRNA sequence matches the expected pattern, including complete exon1 and exon2. (B) Mutant plasmid transcribes a single mRNA product: intron 1 retention with a 10 bp sequence. (C) In the absence of nonsense-mediated decay (NMD), a frameshift results in the formation of a truncated protein. (D) NM_004542: The c.10 + 1G > T mutation leads to intron 1 retention with a 10 bp sequence
Conservation analysis and Gene Expression
The transcriptional sites of the Ndufa3 gene NM_004542, specifically c.10 + 1G > T and c.66_68delCTT, exhibit substantial conservation across various species (Figure S2). The gene expression profile of Ndufa3 reveals significant activity during pivotal developmental stages, spanning embryonic, fetal, infant, and adult phases (Figure S3, source: https://gdap.org.cn/). Ndufa3 manifests predominant expression in particular tissues (Figure S4, source: https://gdap.org.cn/), with noteworthy presence in bone marrow, muscles, pituitary gland, prostate, salivary glands, skin, and blood. Additionally, expression is discernible in the brain, highlighting its importance in neural tissues.
Discussion
The Ndufa3 gene is located on chromosome 19q13.42 and codes for a chain of 84 amino acids. During the formation of mitochondrial complex I, the core subunit ND1 interacts with the Q module, resulting in a complex weighing 273 kDa. Following this, additional subunits, namely Ndufa3, Ndufa8, and Ndufa13, join, forming an intermediate product approximately 283 kDa in size, called Q/Pp-a [8, 11]. Ndufa3 has a pivotal role in assembling mitochondrial complex I. Ndufa13, which is also implicated in this process, has been associated with various clinical manifestations, including Leigh syndrome, early-onset hypotonia, motor impairments, sensory deficits, and instability of mitochondrial complex I. A previous study [9] reported mutations in the Ndufa8 gene in a pair of siblings; a sister presented with delayed motor and language development, along with seizures, while her brother experienced mild language development delay with bradycardia and severe pulmonary hypertension with right ventricular dilation during anesthesia induction prior to a urethral surgery. Moreover, Yatsuka et al. [12] presented a case of a child with homozygous Ndufa8 mutations, characterized by seizures, microcephaly, and developmental delays, with a bleak prognosis. Still, no pathogenic mutations in the Ndufa3 gene have been reported.
Here, we present a family with three affected members, all displaying symptoms since early childhood, primarily marked by abnormalities in muscle tone and delays in motor and language development, albeit relatively mild. The initial case (second child of the second pregnancy experienced) showed worsened muscle tone issues post-trauma, persisting for about a year before gradual recovery. Imaging data unveiled lesions in the basal ganglia and brainstem, consistent with a diagnosis of Leigh syndrome. Genetic analysis revealed a compound heterozygous mutation in the family, with the c.66_68delCTT mutation resulting in the loss of one amino acid. Minigene experiments further confirmed that the splicing mutation c.10 + 1G > T leads to the retention of a 10-bp sequence in the 1st intron, forming a truncated protein without undergoing nonsense-mediated decay. Conservation analysis affirmed that both mutation regions are highly conserved across various species.
The Ndufa3 gene is primarily expressed during embryonic, fetal, infant, and adult stages. The three affected individuals in this family are in their adolescence, representing a period of low Ndufa3 expression, which implies that cognitive and motor development in these individuals may progress slowly.
The primary sites of Ndufa3 gene expression include the bone marrow, muscles, pituitary gland, prostate, salivary glands, skin, and blood. The initial case, experiencing delayed wound healing after skin injury, may be attributed to the high expression of Ndufa3 in muscles and skin. So far, no significant damage in other organs has been observed in the affected individuals.
The exact pathogenic mechanism of Ndufa3 remains incompletely understood. Studies [13] have indicated that the expression of the Ndufa3 gene rises following vigorous physical activity, potentially linked to its role in oxidative phosphorylation and energy metabolism processes. Furthermore, obesity resulting from ovarian removal surgery may involve decreased expression of mitochondrial energy metabolism genes, including Ndufa3 [14]. Research suggests that Ndufb5, Ndufs7, and Ndufa3 are pivotal in regulating oxidative stress in MCAO/reperfusion-induced brain injury [15].
Conclusion
This is the first study that identified a mutation in the Ndufa3 gene in multiple members of the same family with Leigh syndrome. The clinical manifestations in the three affected family members (three children) were relatively mild, predominantly impacting the central nervous system, skin, and muscles. A cocktail therapy approach has shown efficacy in their management. This study broadens the genetic phenotype spectrum of Leigh syndrome. The pathogenesis associated with Ndufa3 is thought to be linked to its influence on the function of mitochondrial complex I; however, the precise mechanism remains elusive and warrants further investigation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Author contributions
BG L, WJ W and SZ S contributed to the study conception and design. LH W, X W, C L, YK D and BC L collected the data and performed the data analysis. JT H, X W, C L, YK D and BC L contributed to the interpretation of the data and the completion of figures and tables. All authors contributed to the drafting of the article and final approval of the submitted version.
Funding
None.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Ethical approval (2021 − 152) was obtained by the Ethics Committee of Children’s Hospital of Hebei Province l. All patients signed the informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bao-Guang Li and Wen-Juan Wu contributed equally to this work.
References
- 1.Sazanov LA (2015) A giant molecular proton pump: structure and mechanism of respiratory complex I. Nat Rev Mol Cell Biol 16:375–388 [DOI] [PubMed] [Google Scholar]
- 2.Schaefer AM, Taylor RW, Turnbull DM et al (2004) The epidemiology of mitochondrial disorders–past, present and future. Biochim Biophys Acta 1659:115–120 [DOI] [PubMed] [Google Scholar]
- 3.Subspecialty Group of Neurology, the Society of Pediatrics, Chinese Medical Association (2023) Specialty Committee of Neurology, Futang Research Center of Pediatric Development; Editorial Board, Chinese Journal of Pediatrics. [Expert consensus on the diagnosis and treatment of Leigh syndrome (2023)]. Zhonghua Er Ke Za Zhi 61(12):1077–1085 [DOI] [PubMed] [Google Scholar]
- 4.Brandt U (2006) Energy converting NADH:quinone oxidoreductase (complex I). Annu Rev Biochem 75:69–92 [DOI] [PubMed] [Google Scholar]
- 5.Kashani A, Thiffault I, Dilenge ME et al (2014) A homozygous mutation in the NDUFS1 gene presents with a mild cavitating leukoencephalopathy. Neurogenetics 15:161–164 [DOI] [PubMed] [Google Scholar]
- 6.Perrier S, Gauquelin L, Tétreault M et al (2018) Recessive mutations in NDUFA2 cause mitochondrial leukoencephalopathy. Clin Genet 93:396–400 [DOI] [PubMed] [Google Scholar]
- 7.Liu Z, Zhang L, Ren C et al (2022) Whole genome and exome sequencing identify NDUFV2 mutations as a new cause of progressive cavitating leukoencephalopathy. J Med Genet 59:351–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guerrero-Castillo S, Baertling F, Kownatzki D et al (2017) The Assembly pathway of mitochondrial respiratory chain complex I. Cell Metab 25(1):128–139 [DOI] [PubMed] [Google Scholar]
- 9.Tort F, Barredo E, Parthasarathy R et al (2020) Biallelic mutations in NDUFA8 cause complex I deficiency in two siblings with favorable clinical evolution. Mol Genet Metab 131(3):349–357 [DOI] [PubMed] [Google Scholar]
- 10.González-Quintana A, García-Consuegra I, Belanger-Quintana A et al (2020) Novel NDUFA13 mutations Associated with OXPHOS Deficiency and Leigh Syndrome: a Second Family Report. Genes (Basel) 11(8):855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rak M, Rustin P (2014) Supernumerary subunits NDUFA3, NDUFA5 and NDUFA12 are required for the formation of the extramembrane arm of human mitochondrial complex I. FEBS Lett 588:1832–1838 [DOI] [PubMed] [Google Scholar]
- 12.Yatsuka Y, Kishita Y, Formosa LE et al (2020) A homozygous variant in NDUFA8 is associated with developmental delay, microcephaly, and epilepsy due to mitochondrial complex I deficiency. Clin Genet 98:155–165 [DOI] [PubMed] [Google Scholar]
- 13.Ghosh M, Cho HW, Park JW et al (2016) Comparative transcriptomic analyses by RNA-seq to elucidate differentially expressed genes in the muscle of Korean Thoroughbred horses. Appl Biochem Biotechnol 180:588–608 [DOI] [PubMed] [Google Scholar]
- 14.Luo ZH, Liu ZW, Mao Y et al (2020) Cajanolactone A, a stilbenoid from cajanus cajan, prevents ovariectomy-induced obesity and liver steatosis in mice fed a regular diet. Phytomedicine 78:153290 [DOI] [PubMed] [Google Scholar]
- 15.Ma Q, Wang C, Wang M et al (2021) Investigation of brain damage mechanism in middle cerebral artery occlusion/reperfusion rats based on i-TRAQ quantitative proteomics. Exp Brain Res 239:1247–1260 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.