Abstract
Follicular dendritic cell sarcoma is a rare mesenchymal neoplasm arising from follicular dendritic cells (FDC) of lymphoid follicles. While the majority of FDC sarcoma cases arise within lymph nodes, approximately 30% manifest in extranodal sites. Only 4 prior occurrences of intra-parotid FDC sarcomas have been documented. We are reporting a rare case of FDC of the parotid gland in a 65-year-old male with a questionable history of B-cell lymphoma. The patient underwent a right total parotidectomy and bilateral neck dissection. A diagnosis of follicular dendritic cell (FDC) sarcoma was made, with one positive intra-parotid node. The malignant cells expressed the characteristic markers for FDC sarcoma but with positivity of the melanocytic marker PRAME. This is a case of FDC sarcoma with an unusual extranodal localization in the parotid gland. Immunohistochemistry was useful in making a diagnosis although the positivity for the melanocytic marker PRAME was unusual and unreported before.
Keywords: Follicular dendritic cells, Sarcoma, Parotid gland, PRAME, Melanoma
Introduction
Soft tissue sarcomas are rare tumors comprising roughly 1% of malignancies in adults. Despite their rarity, they exhibit a substantial mortality rate, contributing to about 3–4% of cancer-related fatalities each year [1]. FDC sarcoma is an exceedingly uncommon form of sarcoma characterized by its low to intermediate malignant nature. It originates from follicular dendritic cells, yet instances of its occurrence in extranodal locations such as the mediastinum, gastrointestinal tract, liver, and spleen have also been documented [1–3]. Only four prior occurrences of intra-parotid FDC sarcomas have been documented [2].
Clinical history
Our patient is a 65-year-old male who presented with a right parotid mass and bilateral neck lymphadenopathy. He had an undocumented history of a cutaneous right cheek lesion that was previously biopsied and thought to represent B-cell lymphoma. On examination, there was a firm mass at the right parotid tail. There was also a palpable right neck lymphadenopathy in level 2A and level 3. Magnetic resonance imaging (MRI) of the neck showed a 5.1 × 4.5 × 8.3 cm enhancing heterogeneous T2 hyperintense lesion involving the right superficial parotid gland.
Results
Initially, an ultrasound-guided core biopsy was performed, which showed a poorly differentiated neoplasm, suggestive of FDC sarcoma. The patient then underwent right total parotidectomy and bilateral neck dissection. Sections of the parotid mass showed an infiltration of large cells with irregular nuclei, vesicular chromatin, prominent nucleoli, and moderate cytoplasm. A subset of the cells showed atypia with enlarged, highly irregular, and hyperchromatic nuclei. The malignant cells expressed CD21, CD23 (subset), CD35 (small subset), CXCL13 (subset), vimentin, fascin, and clusterin, suggestive of FDC origin (Fig. 1). The malignant cells also expressed CD4 and CD5 (subset) but were negative for all other T-cell markers (CD2, CD3, CD7, CD8, CD43, TIA-1, BF-1). Since a subset of FDC sarcomas can be associated with indolent T-lymphoblastic proliferations, TdT stain was performed and is negative. EBV was negative by in situ hybridization (EBER). Podoplanin (D2-40), which can be utilized as a marker for follicular dendritic cells was negative in our case. In addition, the malignant cells were positive for PRAME but negative for all other melanoma markers (S100, HMB45, Melan A, and SOX10). The infiltrate involved the parotid gland parenchyma and directly adjacent lymph nodes. Table 1 illustrates the different antibody clones used in the case. A next-generation sequencing (NGS) test was performed (Tempus 648 genes xT panel) and it detected 8 likely pathogenic somatic variants, including TP53, RB1, and FBXW7 loss-of-function variants. B-cell gene rearrangement studies by polymerase chain reaction (PCR) were performed but showed inconclusive results. Table 2 illustrates the different mutations detected along with their variant allele frequency (VAF). Taken together, the overall picture supports a diagnosis of follicular dendritic cell (FDC) sarcoma. A follow-up appointment was arranged with the Radiation Oncology department for further assessment and management.
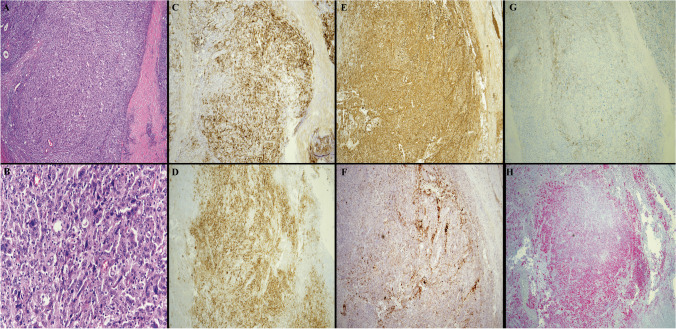
Fig. 1.
The composite picture represents parotid gland involvement by follicular dendritic cell sarcoma. A H&E stain, low-power magnification (4 ×) of the sarcoma cells with rare unremarkable glands noted. B H&E stain, high-power magnification (40 ×) showing the large sarcoma cells, mostly with one centrally placed nucleoli and open chromatin. Scattered large highly atypical malignant cells are noted. Few interspersed small lymphocytes are also seen. C CD21 expression by the sarcoma cells (4 ×). D CD23 expression (4 ×). E Clusterin expression (4 ×). F CXCL13 expression (4 ×). G CD35 partial expression (4 ×). H PRAME expression (4 ×)
Table 1.
List of immunohistochemical markers used and their antibody clones
| Immunohistochemical stains | Antibody clones |
|---|---|
| BF-1 | Invitrogen (8A3) |
| CD2 | Cell Marque (MRQ-11) |
| CD3 | Roche (2GV6) |
| CD4 | Cell Marque (SP35) |
| CD5 | Roche (SP19) |
| CD7 | Cell Marque (MRQ-56) |
| CD8 | Cell Marque (C8/144B) |
| CD21 | Dako (1F8) |
| CD23 | Roche (SP23) |
| CD35 | Dako (Ber-MAC-DRC) |
| CD43 | Roche (L60) |
| CD45 | Agilent (2B11 + PD7/26) |
| CD68 | Roche (KP-1) |
| Anti-Clusterin | Millipore (41D) |
| CXCL 13 | R & D Systems (53610) |
| EMA | Cell Marque (E29) |
| Fascin | Cell Marque 55 K-2 |
| HMB-45 | Cell Marque (HMB-45) |
| Melan-A | Roche (A103) |
| Podoplanin | Cell Marque (D2-40) |
| PRAME | Abcam (EPR20330) |
| S100 | Roche (Polyclonal) |
| SOX-10 | Cell Marque (EP268) |
| Tia-1 | Biocare (TIA1) |
| Tdt | Agilent (EP266) |
| Vimentin | Roche (V9) |
Table 2.
Summary of tumor genomic profiles of our case by NGS
| Tumor-specific gene mutation | Variant allele fraction (VAF) |
|---|---|
| TP53: c.560-1_560delinsAA, p.G187D, NM_000546 | 22.2% |
| ATR: c.481A > T p.R161*, NM_001184 | 21.6% |
| KDM6A: c.571C > T p.Q191*, NM_001291415 | 21.4% |
| TERT: c.-146C > T, NM_198253 | 20.1% |
| RB1: c.1547G > A p.W516*, NM_000321 | 17.2% |
| FBXW7: c.881del p. S294fs, NM_033632 | 12.5% |
| NOTCH1: c.1255 + 1G > A, NM_017617 | 10.1% |
| FHIT: c.250-1G > A, NM_001166243 | 8.1% |
Discussion
Follicular dendritic cells are a specialized type of dendritic cells that are largely restricted to lymphoid follicles. They form dense three-dimensional meshworks within benign follicles, which maintain the follicular architecture [4]. FDC sarcoma is a neoplastic proliferation of cells showing morphologic and immunophenotypic features of follicular dendritic cells [5]. The etiology of that neoplastic transformation is unknown although it may evolve in situations in which there is FDC hyperplasia and overgrowth [4]. It usually occurs de novo; however, it can sometimes occur in association with hyaline vascular Castleman disease, whether simultaneously or as a succeeding event [4]. It presents as a painless solid mass, usually nodal (mainly cervical lymph nodes) but it can also involve extra nodal sites, such as tonsils, spleen, skin, and gastrointestinal tract [4, 6, 7]. A new variant has been recently described: EBV-positive inflammatory follicular dendritic cell tumor and is reported to occur exclusively in the liver and spleen, exhibit more interspersed lymphoplasmacytic infiltrate, and express EBV by in situ hybridization [8]. Overall, FDC sarcoma is considered a low-grade sarcoma that has a significant recurrence rate in nearly half the cases, and it also can metastasize [3]. Surgical resection remains the best treatment for these tumors.
Histologically, these tumors can be difficult to diagnose, as the morphological spectrum is broad and often causes confusion. Cytological atypia is present only in a subset of cases and mitotic figures are common but highly variable in number. By immunohistochemistry, FDCs express CD21, CD23, CD35, CXCL13, and clusterin. They also usually express vimentin, fascin, HLA-DR, and EMA and variably positive for CD68, S100, and CD45 [1, 4]. Clusterin staining is reported to be highly sensitive (100%) and specific (93%) and along with CD21 and CD23, constitute the essential stains required to establish a definitive diagnosis [9]. PRAME stain exhibits diffuse positivity in most melanomas, while typically presenting as negative or showing limited and focal immunoreactivity in nevi [10]. Variable degrees of PRAME staining have been sporadically observed in other malignant tumors, including most synovial sarcomas, myxoid liposarcomas, and malignant peripheral nerve sheath tumors (MPNST) [10]. Other neoplasms such as seminomas and carcinomas of various origins including endometrial, serous ovarian, mammary ductal, lung, and renal showed an intermediate proportion of cases and variable extent of tumor cells positive for PRAME protein expression [10]. To our knowledge, PRAME positivity has not been reported in FDC sarcoma before. In our case, PRAME is positive but all other melanoma markers (S100, HMB45, Melan A, and SOX10) are negative. Few FDC sarcoma cases with aberrant phenotype have been reported before including a case of intra-abdominal FDC sarcoma with pleomorphic features and aberrant expression of neuroendocrine markers [11], an unusual case of FDC sarcoma of the omentum with pleomorphic morphology and aberrant cytokeratin expression [12], another case with aberrant T-cell antigen expression [13], and a clinicopathologic study of 15 FDC cases with expression of MDM2, somatostatin receptor 2A, and PD-L1 [14].
Although genetic drivers for tumorigenesis in FDC are largely unknown, recent genomic profiling studies have revealed several recurrent gene alterations in FDC sarcoma, including BRAF V600E mutation [15] and loss-of-function variants in tumor suppressor genes involved in the regulation of NF-κB pathway and cell cycle, such as NFKBIA, CYLD, CDKN2A, and RB1 genes [16]. In addition, genomic profiling for one patient with primary esophageal follicular dendritic cell sarcoma revealed pathogenic variants in multiple genes, including CHEK2, FAT1, TP53, DPYD, ERBB2IP, FBXW7, KMT2D, PPP2R1A, and TSC2 [17]. The NGS results for this patient identified loss-of-function pathogenic variants in RB1 (p.W516*), TP53 (p.G187D), and FBXW7 (p.S294fs), which have been reported previously in FDC sarcoma patients, supporting the FDC sarcoma diagnosis.
In conclusion, we report a case of FDC sarcoma with an unusual extranodal localization in the parotid gland. Furthermore, the aberrant positive expression of the melanocytic marker PRAME has not been reported before. All other melanocytic markers were negative in our case and the characteristic FDC markers are positive.
Funding
This study was not supported by any funding.
Declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study formal consent is not required.
Consent for publication
For this type of study consent for publication is not required.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Makis W, Hudson EW, Chiu B (2017) Recurrent follicular dendritic cell sarcoma of the parotid gland imaged with 18F-FDG PET/CT. Nucl Med Mol Imaging 51:354–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McClelland E, Bashyam A, Derbyshire S, Di Palma S (2018) Follicular dendritic cell sarcoma presenting as a painless lump in the parotid. BMJ Case Reports 2018. 10.1136/bcr-2018-224301 [DOI] [PMC free article] [PubMed]
- 3.Qu C, Tian X, Ma Y, Xie X, Wang M, Dong Y, Zhang J, Liu P, Yang Y (2020) Multidisciplinary diagnosis and treatment of recurrent follicular dendritic cell sarcoma in abdomen: a case report. Medicine 99:e23588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rezk SA, Nathwani BN, Zhao X, Weiss LM (2013) Follicular dendritic cells: origin, function, and different disease-associated patterns. Hum Pathol 44:937–950 [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Hisijos N, Omman R, Pambuccian S, Mirza K (2019) Follicular dendritic cell sarcoma or not? A series of 5 diagnostically challenging cases. Clin Med Insights Oncol 13:1179554919844531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan JK, Tsang WY, Ng CS, Tang SK, Yu HC, Lee AW (1994) Follicular dendritic cell tumors of the oral cavity. Am J Surg Pathol 18:148–157 [DOI] [PubMed] [Google Scholar]
- 7.Hollowood K, Stamp G, Zouvani I, Fletcher CD (1995) Extranodal follicular dendritic cell sarcoma of the gastrointestinal tract. Morphologic, immunohistochemical and ultrastructural analysis of two cases. Am J Clin Pathol 103:90–97 [DOI] [PubMed] [Google Scholar]
- 8.Cheuk W, Chan JK, Shek TW, Chang JH, Tsou MH, Yuen NW, Ng WF, Chan AC, Prat J (2001) Inflammatory pseudotumor-like follicular dendritic cell tumor: a distinctive low-grade malignant intra-abdominal neoplasm with consistent Epstein-Barr virus association. Am J Surg Pathol 25:721–731 [DOI] [PubMed] [Google Scholar]
- 9.Grogg KL, Lae ME, Kurtin PJ, Macon WR (2004) Clusterin expression distinguishes follicular dendritic cell tumors from other dendritic cell neoplasms: report of a novel follicular dendritic cell marker and clinicopathologic data on 12 additional follicular dendritic cell tumors and 6 additional interdigitating dendritic cell tumors. Am J Surg Pathol 28:988–998 [DOI] [PubMed] [Google Scholar]
- 10.Lezcano C, Jungbluth AA, Busam KJ (2021) PRAME immunohistochemistry as an Ancillary Test for the assessment of melanocytic lesions. Surg Pathol Clin 14:165–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padilla-Rodríguez AL, Bembassat M, Lazaro M, Ortiz-Hidalgo C (2007) Intra-abdominal follicular dendritic cell sarcoma with marked pleomorphic features and aberrant expression of neuroendocrine markers: report of a case with immunohistochemical analysis. Appl Immunohistochem Mol Morphol 15(3):346–352 [DOI] [PubMed] [Google Scholar]
- 12.Fratoni S, Niscola P, Cupelli L et al (2014) An unusual case of follicular dendritic cell sarcoma of the omentum with pleomorphous morphology and aberrant cytokeratin expression. J Hematopathol 7:177–180 [Google Scholar]
- 13.Yamada Y, Haga H, Hernandez M, Kubota KC, Orii F, Nagashima K, Matsuno Y (2009) Follicular dendritic cell sarcoma of small intestine with aberrant T-cell marker expression. Pathol Int 59(11):809–812 [DOI] [PubMed] [Google Scholar]
- 14.Agaimy A, Michal M, Hadravsky L, Michal M (2016) Follicular dendritic cell sarcoma: clinicopathologic study of 15 cases with emphasis on novel expression of MDM2, somatostatin receptor 2A, and PD-L1. Ann Diagn Pathol 23:21–28 [DOI] [PubMed] [Google Scholar]
- 15.Go H, Jeon YK, Huh J, Choi SJ, Choi YD, Cha HJ, Kim HJ, Park G, Min S, Kim JE (2014) Frequent detection of BRAF(V600E) mutations in histiocytic and dendritic cell neoplasms. Histopathology 65:261–272 [DOI] [PubMed] [Google Scholar]
- 16.Griffin GK, Sholl LM, Lindeman NI, Fletcher CD, Hornick JL (2016) Targeted genomic sequencing of follicular dendritic cell sarcoma reveals recurrent alterations in NF-kappaB regulatory genes. Modern: Pathol Off J United States Can Acad Pathol, Inc 29:67–74 [DOI] [PubMed] [Google Scholar]
- 17.Ren W, Sun Q, Wu PY, Huang B, Yang J, Yan J, Liu BR (2018) Profiles of genomic alterations in primary esophageal follicular dendritic cell sarcoma: a case report. Medicine 97:e13413 [DOI] [PMC free article] [PubMed] [Google Scholar]