Abstract
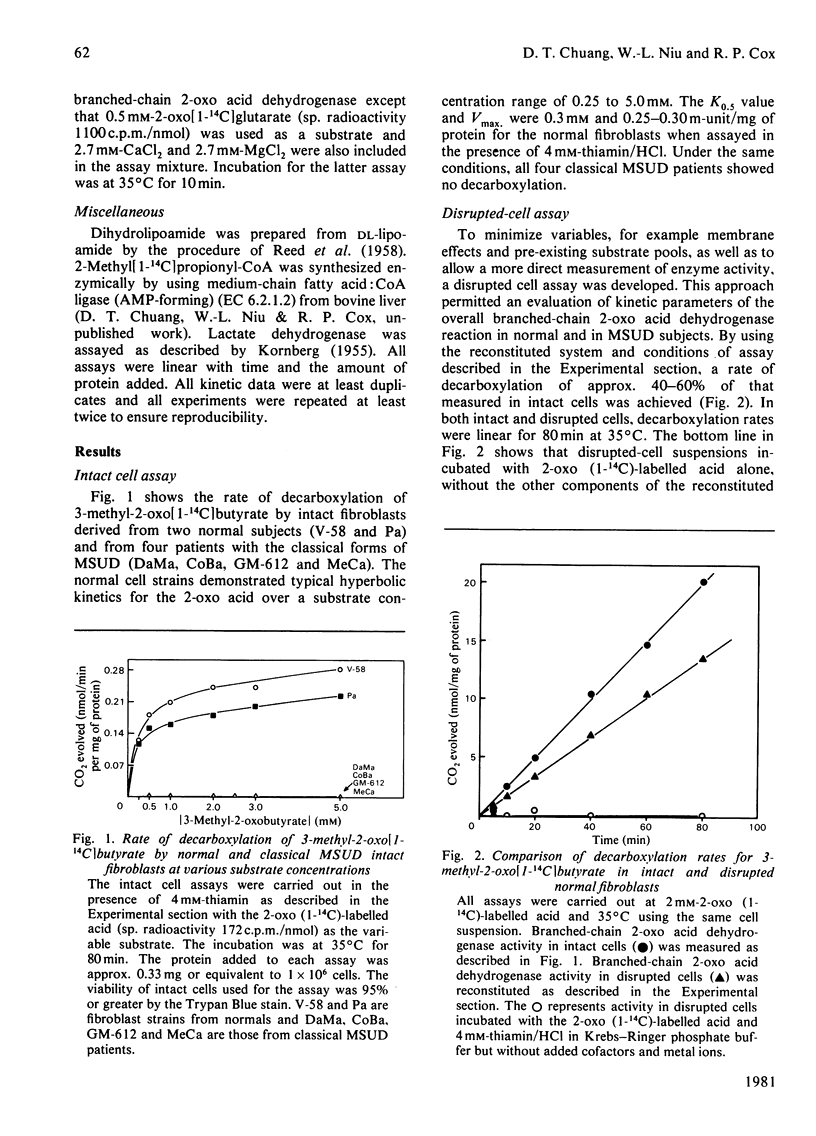
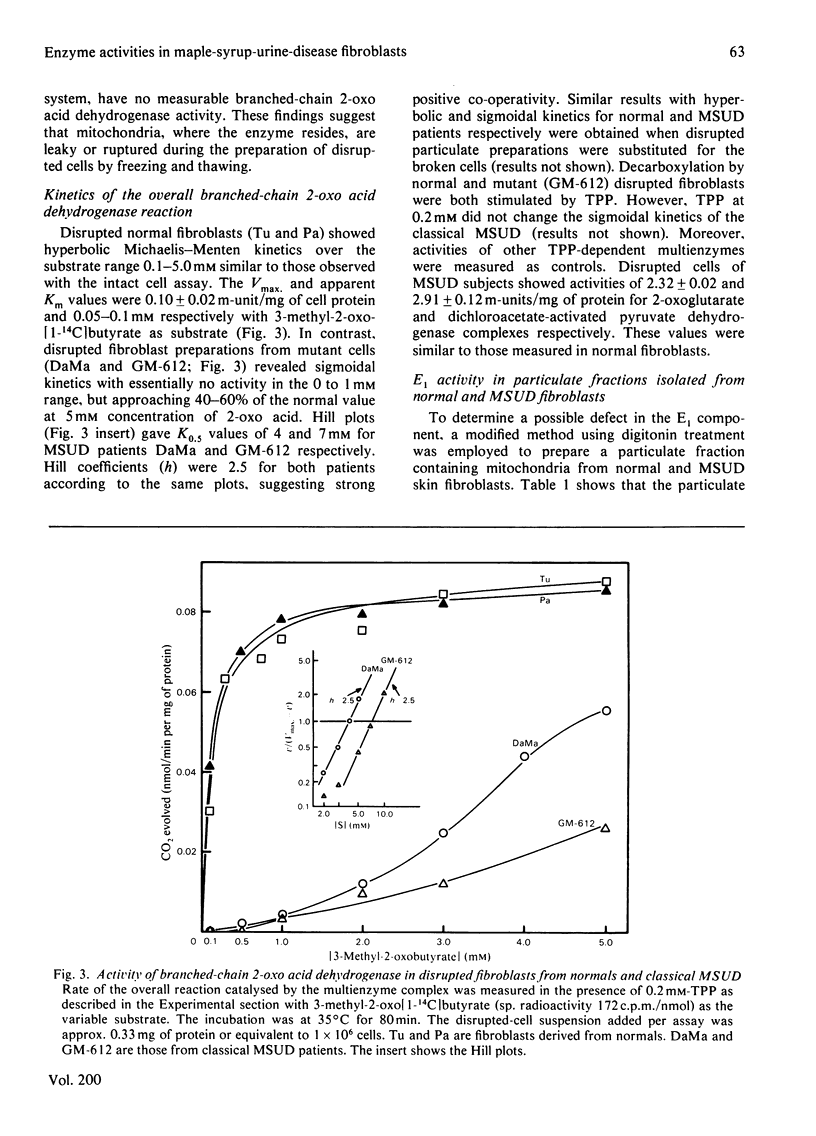
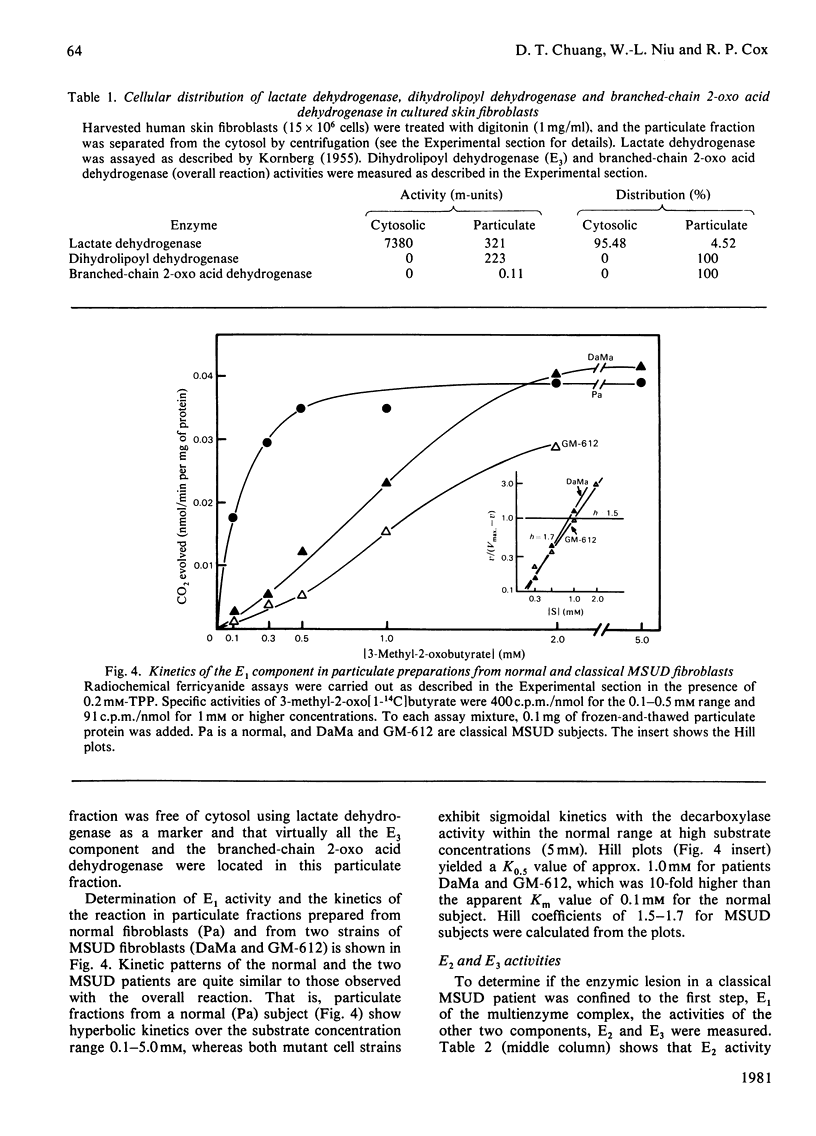
1. Comparisons of the activity and kinetics of the branched-chain 2-oxo acid dehydrogenase in cultured skin fibroblasts from normal and classical maple-syrup-urine-disease (MSUD) subjects provide a kinetic explanation for the enzyme defect. 2. In the intact cell assays, normal fibroblasts demonstrated hyperbolic kinetics with 3-methyl-2-oxo[1-14C]butyrate as a substrate. Intact fibroblasts from four classical MSUD patients showed no decarboxylation over a substrate concentration range of 0.25 to 5.0 mM, and thiamin (4 mM) was without effect. 3. The overall reaction of the multienzyme complex was efficiently reconstituted by using a disrupted-cell system. Normals again showed typical hyperbolic kinetics at the 2-oxo acid concentrations of 0.1 to 5 mM. The Vmax. and apparent Km values were 0.10 +/- 0.02 m-unit/mg of protein and 0.05-0.1 mM respectively, with 3-methyl-2-oxobutyrate. In contrast, classical MSUD patients exhibited sigmoidal kinetics (Hill coefficient, 2.5) with activity approaching 40-60% of the normal value at 5 mM substrate. The K0.5 values from the Hill plots for MSUD patients were 4-7 mM. 4. The E1 (branched-chain 2-oxo acid decarboxylase) component of the multienzyme complex was measured in disrupted-particulate preparations. Normals again showed hyperbolic kinetics with the 2-oxo acid, whereas MSUD preparations exhibited sigmoidal kinetics with the activity of E1 strictly dependent on substrate concentration. Apparent Km or K0.5 were 0.1 and 1.0 mM for normal and MSUD subjects respectively. 5. Measurements of E2 (dihydrolipoyl transacylase) and E3 (dihydrolipoyl dehydrogenase) in MSUD preparations showed them to be in the normal range. 6. The above data suggest a defect in the E1 step of branched-chain 2-oxo acid dehydrogenase in classical MSUD patients.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bremer J., Davis E. J. The effect of acylcarnitines on the oxidation of branched chain alpha-keto acids in mitochondria. Biochim Biophys Acta. 1978 Mar 30;528(3):269–275. doi: 10.1016/0005-2760(78)90016-4. [DOI] [PubMed] [Google Scholar]
- Butterworth P. J., Tsai C. S., Eley M. H., Roche T. E., Reed L. J. A kinetic study of dihydrolipoyl transacetylase from bovine kidney. J Biol Chem. 1975 Mar 10;250(5):1921–1925. [PubMed] [Google Scholar]
- CIECIURA S. J., MARCUS P. I., PUCK T. T. Clonal growth in vitro of epithelial cells from normal human tissues. J Exp Med. 1956 Oct 1;104(4):615–628. doi: 10.1084/jem.104.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANCIS J., JANSEN V., HUTZLER J., LEVITZ M. THE METABOLISM OF LEUCINE IN TISSUE CULTURE OF SKIN FIBROBLASTS OF MAPLE-SYRUP-URINE DISEASE. Biochim Biophys Acta. 1963 Nov 8;77:523–524. doi: 10.1016/0006-3002(63)90536-5. [DOI] [PubMed] [Google Scholar]
- Dancis J., Hutzler J., Cox R. P. Maple syrup urine disease: branched-chain keto acid decarboxylation in fibroblasts as measured with amino acids and keto acids. Am J Hum Genet. 1977 May;29(3):272–279. [PMC free article] [PubMed] [Google Scholar]
- Dancis J., Hutzler J., Rokkones T. Intermittent branched-chain ketonuria. Variant of maple-syrup-urine disease. N Engl J Med. 1967 Jan 12;276(2):84–89. doi: 10.1056/NEJM196701122760204. [DOI] [PubMed] [Google Scholar]
- Dancis J., Hutzler J., Snyderman S. E., Cox R. P. Enzyme activity in classical and variant forms of maple syrup urine disease. J Pediatr. 1972 Aug;81(2):312–320. doi: 10.1016/s0022-3476(72)80301-9. [DOI] [PubMed] [Google Scholar]
- Danner D. J., Elsas L. J., 2nd Subcellular distribution and cofactor function of human branched chain alpha-ketoacid dehydrogenase in normal and mutant cultured skin fibroblasts. Biochem Med. 1975 May;13(1):7–22. doi: 10.1016/0006-2944(75)90135-0. [DOI] [PubMed] [Google Scholar]
- Danner D. J., Lemmon S. K., Besharse J. C., Elsas L. J., 2nd Purification and characterization of branched chain alpha-ketoacid dehydrogenase from bovine liver mitochondria. J Biol Chem. 1979 Jun 25;254(12):5522–5526. [PubMed] [Google Scholar]
- Danner D. J., Lemmon S. K., Elsas L. J., 2nd Substrate specificity and stabilization by thiamine pyrophosphate of rat liver branched chain alpha-ketoacid dehydrogenase. Biochem Med. 1978 Feb;19(1):27–38. doi: 10.1016/0006-2944(78)90004-2. [DOI] [PubMed] [Google Scholar]
- Koike M., Koike K. Structure, assembly and function of mammalian alpha-keto acid dehydrogenase complexes. Adv Biophys. 1976:187–227. [PubMed] [Google Scholar]
- Lau K. S., Fatania H. R., Randle P. J. Inactivation of rat liver and kidney branched chain 2-oxoacid dehydrogenase complex by adenosine triphosphate. FEBS Lett. 1981 Apr 6;126(1):66–70. doi: 10.1016/0014-5793(81)81034-4. [DOI] [PubMed] [Google Scholar]
- Lyons L. B., Cox R. P., Dancis J. Complementation analysis of maple syrup urine disease in heterokaryons derived from cultured human fibroblasts. Nature. 1973 Jun 29;243(5409):533–535. doi: 10.1038/243533a0. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Mackall J. C., Lane M. D. Role of pyruvate carboxylase in fatty acid synthesis: alterations during preadipocyte differentiation. Biochem Biophys Res Commun. 1977 Dec 7;79(3):720–725. doi: 10.1016/0006-291x(77)91171-8. [DOI] [PubMed] [Google Scholar]
- Odessey R. Direct evidence for the inactivation of branched-chain oxo-acid dehydrogenase by enzyme phosphorylation. FEBS Lett. 1980 Dec 1;121(2):306–308. doi: 10.1016/0014-5793(80)80369-3. [DOI] [PubMed] [Google Scholar]
- Odessey R. Reversible ATP-induced inactivation of branched-chain 2-oxo acid dehydrogenase. Biochem J. 1980 Oct 15;192(1):155–163. doi: 10.1042/bj1920155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker P. J., Randle P. J. Active and inactive forms of branched-chain 2-oxoacid dehydrogenase complex in rat heart and skeletal muscle. FEBS Lett. 1980 Apr 7;112(2):186–190. doi: 10.1016/0014-5793(80)80176-1. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Randle P. J. Partial purification and properties of branched-chain 2-oxo acid dehydrogenase of ox liver. Biochem J. 1978 Jun 1;171(3):751–757. doi: 10.1042/bj1710751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit F. H., Yeaman S. J., Reed L. J. Purification and characterization of branched chain alpha-keto acid dehydrogenase complex of bovine kidney. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4881–4885. doi: 10.1073/pnas.75.10.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REED L. J., KOIKE M., LEVITCH M. E., LEACH F. R. Studies on the nature and reactions of protein-bound lipoic acid. J Biol Chem. 1958 May;232(1):143–158. [PubMed] [Google Scholar]
- SANADI D. R., LANGLEY M., WHITE F. alpha-Ketoglutaric dehydrogenase. VII. The role of thioctic acid. J Biol Chem. 1959 Jan;234(1):183–187. [PubMed] [Google Scholar]
- Sakurai Y., Fekuyoshi Y., Hamada M., Hayakawa T., Koike M. Mammalian alpha-keto acid dehydrogenase complexes. VI. Nature of the multiple forms of pig heart lipoamide dehydrogenase. J Biol Chem. 1970 Sep 10;245(17):4453–4462. [PubMed] [Google Scholar]
- Seegmiller J. E., Westall R. G. The enzyme defect in maple syrup urine disease (branched chain ketoaciduria). J Ment Defic Res. 1967 Dec;11(4):288–294. doi: 10.1111/j.1365-2788.1967.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Sheu K. F., Hu C. W., Utter M. F. Pyruvate dehydrogenase complex activity in normal and deficient fibroblasts. J Clin Invest. 1981 May;67(5):1463–1471. doi: 10.1172/JCI110176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf D. A., Parks J. K. Friedreich's ataxia: I. Normal pyruvate dehydrogenase complex activity in platelets. Ann Neurol. 1978 Oct;4(4):366–368. doi: 10.1002/ana.410040412. [DOI] [PubMed] [Google Scholar]
- Sullivan S. G., Dancis J., Cox R. P. Modulation of branched-chain alpha-keto acid decarboxylase activity in rat liver mitochondria by hypophysectomy. Arch Biochem Biophys. 1976 Sep;176(1):225–234. doi: 10.1016/0003-9861(76)90160-0. [DOI] [PubMed] [Google Scholar]
- Wendel U., Wentrup H., Rüdiger H. W. Maple syrup urine disease: analysis of branched chain ketoacid decarboxylation in cultured fibroblasts. Pediatr Res. 1975 Sep;9(9):709–717. doi: 10.1203/00006450-197509000-00005. [DOI] [PubMed] [Google Scholar]
- Wendel U., Wöhler W., Goedde H. W., Langenbeck U., Passarge E., Rüdiger H. W. Rapid diagnosis of maple syrup urine disease (branched chain ketoaciduria) by micro-enzyme assay in leukocytes and fibroblasts. Clin Chim Acta. 1973 May 30;45(4):433–440. doi: 10.1016/0009-8981(73)90046-6. [DOI] [PubMed] [Google Scholar]


