Abstract
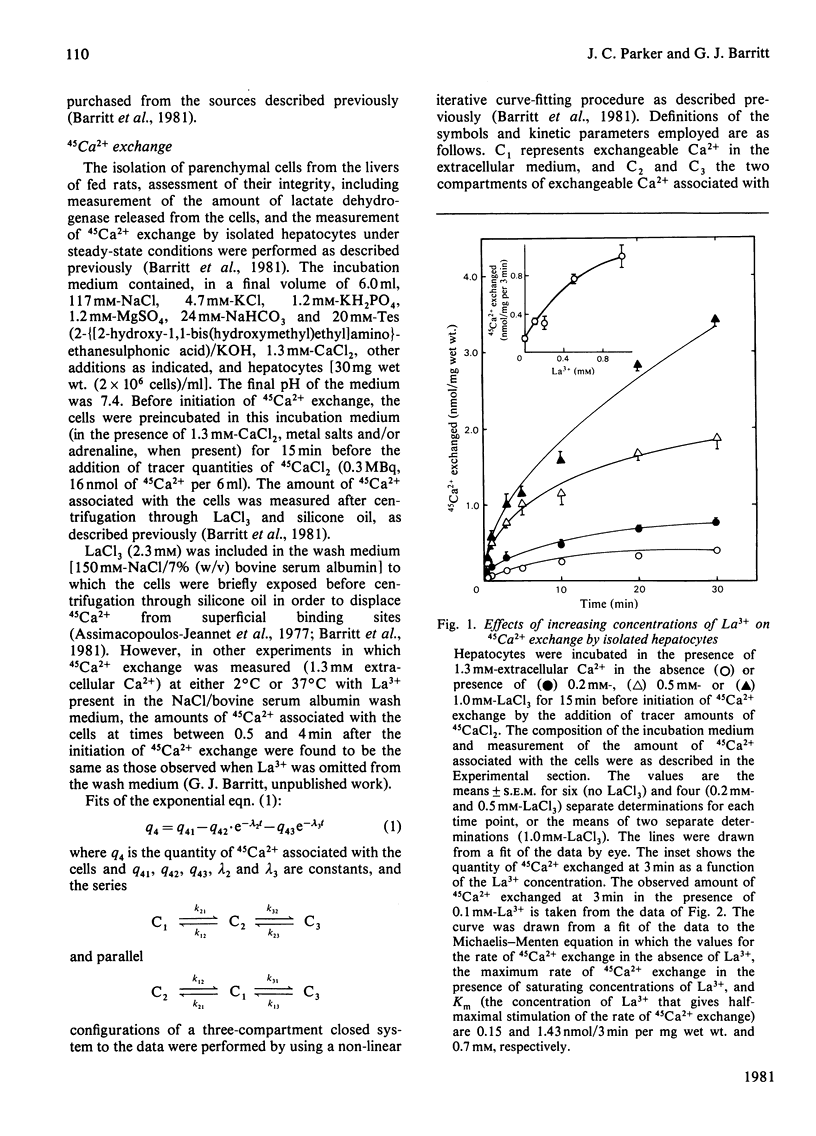
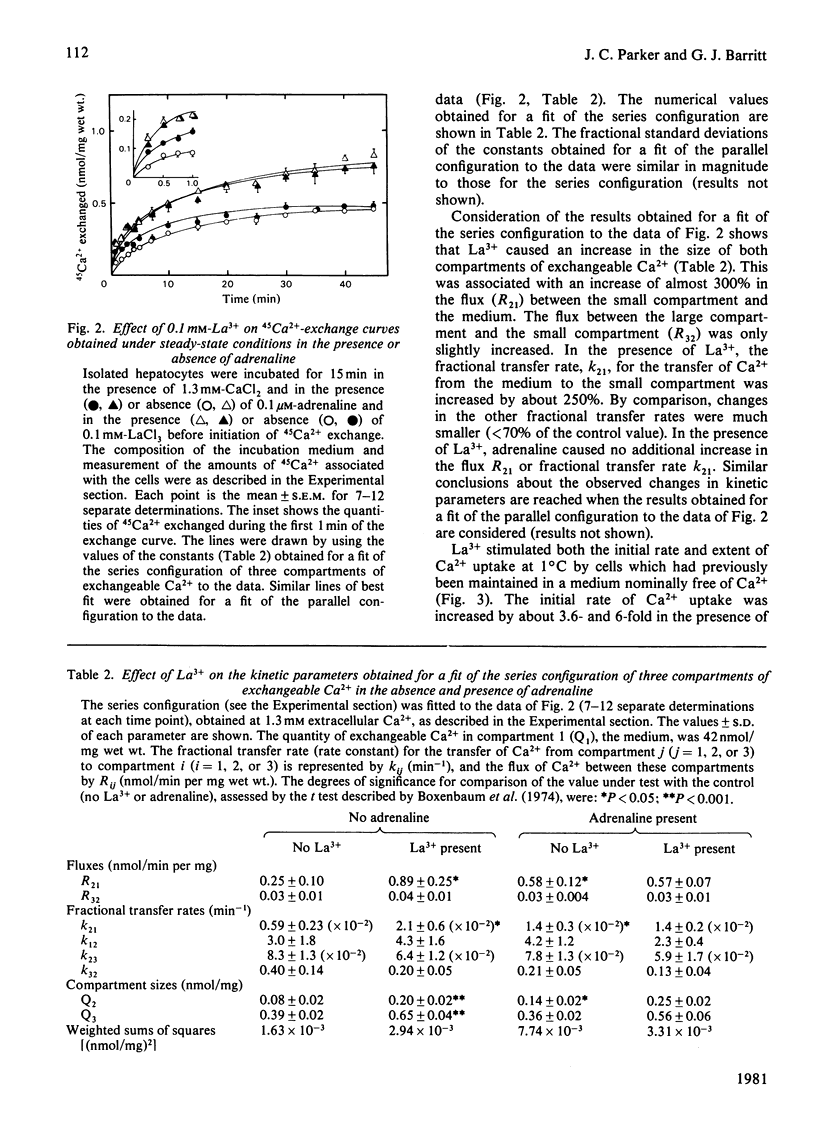
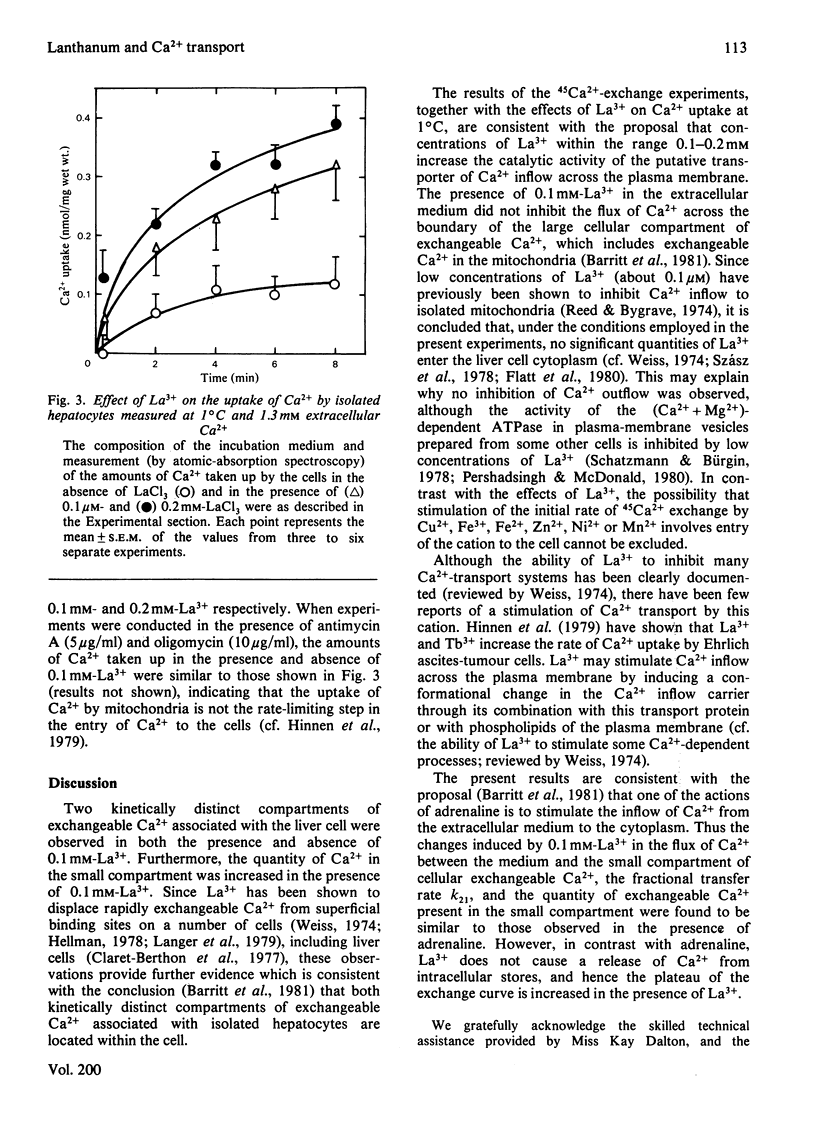
LaCl3 stimulated the initial rate of 45Ca2+ exchange measured under steady-state conditions in isolated liver cells. Cu2+ greater than La3+ = Fe3+ greater than Fe2+ = Zn2+ greater Ni2+ greater than Mn2+ also stimulated 45Ca2+ exchange. Compartmental analysis of 45Ca2+-exchange curves obtained in the presence or absence of La3+, and in the presence or absence of adrenaline, showed that the predominant effect of La3+ is to stimulate the inflow of Ca2+ to the cell from the medium. No evidence for an inhibition of Ca2+ outflow from the cell was obtained. In the presence of La3+, adrenaline caused no further stimulation of Ca2+ inflow to the cell. In the absence of adrenaline, La3+ increased the uptake of Ca2+ (measured by atomic-absorption spectroscopy) by isolated hepatocytes incubated at 1 degree C. The proposal that La3+ stimulates Ca2+ inflow to the liver cell by inducing a conformational change in the Ca2+-inflow transporter of the plasma membrane is briefly discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assimacopoulos-Jeannet F. D., Blackmore P. F., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. Studies on role of calcium in alpha-adrenergic activation of phosphorylase. J Biol Chem. 1977 Apr 25;252(8):2662–2669. [PubMed] [Google Scholar]
- Baker P. F. The regulation of intracellular calcium in giant axons of Loligo and Myxicola. Ann N Y Acad Sci. 1978 Apr 28;307:250–268. doi: 10.1111/j.1749-6632.1978.tb41956.x. [DOI] [PubMed] [Google Scholar]
- Barritt G. J., Parker J. C., Wadsworth J. C. A kinetic analysis of the effects of adrenaline on calcium distribution in isolated rat liver parenchymal cells. J Physiol. 1981 Mar;312:29–55. doi: 10.1113/jphysiol.1981.sp013614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxenbaum H. G., Riegelman S., Elashoff R. M. Statistical estimations in pharmacokinetics. J Pharmacokinet Biopharm. 1974 Apr;2(2):123–148. doi: 10.1007/BF01061504. [DOI] [PubMed] [Google Scholar]
- Claret-Berthon B., Claret M., Mazet J. L. Fluxes and distribution of calcium in rat liver cells: kinetic analysis and identification of pools. J Physiol. 1977 Nov;272(3):529–552. doi: 10.1113/jphysiol.1977.sp012058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatt P. R., Boquist L., Hellman B. Calcium and pancreatic beta-cell function. The mechanism of insulin secretion studied with the aid of lanthanum. Biochem J. 1980 Aug 15;190(2):361–372. doi: 10.1042/bj1900361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foden S., Randle P. J. Calcium metabolism in rat hepatocytes. Biochem J. 1978 Mar 15;170(3):615–625. doi: 10.1042/bj1700615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnen R., Miyamoto H., Racker E. Ca2+ translocation in Ehrlich ascites tumor cells. J Membr Biol. 1979 Sep 14;49(4):309–324. doi: 10.1007/BF01868989. [DOI] [PubMed] [Google Scholar]
- Katzung B. G., Reuter H., Porzig H. Lanthanum inhibits Ca inward current but not Na-Ca exchange in cardiac muscle. Experientia. 1973 Sep 15;29(9):1073–1075. doi: 10.1007/BF01946727. [DOI] [PubMed] [Google Scholar]
- Keppens S., Vandenheede J. R., De Wulf H. On the role of calcium as second messenger in liver for the hormonally induced activation of glycogen phosphorylase. Biochim Biophys Acta. 1977 Feb 28;496(2):448–457. doi: 10.1016/0304-4165(77)90327-0. [DOI] [PubMed] [Google Scholar]
- Langer G. A., Frank J. S., Nudd L. M. Correlation of calcium exchange, structure, and function in myocardial tissue culture. Am J Physiol. 1979 Aug;237(2):H239–H246. doi: 10.1152/ajpheart.1979.237.2.H239. [DOI] [PubMed] [Google Scholar]
- Pershadsingh H. A., McDonald J. M. A high affinity calcium-stimulated magnesium-dependent adenosine triphosphatase in rat adipocyte plasma membranes. J Biol Chem. 1980 May 10;255(9):4087–4093. [PubMed] [Google Scholar]
- Reed K. C., Bygrave F. L. Accumulation of lanthanum by rat liver mitochondria. Biochem J. 1974 Feb;138(2):239–252. doi: 10.1042/bj1380239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Sooranna S. R., Evans C. J. Insulin-like actions of nickel and other transition-metal ions in rat fat-cells. Biochem J. 1976 Feb 15;154(2):349–357. doi: 10.1042/bj1540349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzmann H. J., Bürgin H. Calcium in human red blood cells. Ann N Y Acad Sci. 1978 Apr 28;307:125–147. doi: 10.1111/j.1749-6632.1978.tb41939.x. [DOI] [PubMed] [Google Scholar]
- Schimmel R. J. Calcium antagonists and lipolysis in isolated rat epididymal adipocytes: effects of tetracaine, manganese, cobaltous and lanthanum ions and D600. Horm Metab Res. 1978 Mar;10(2):128–134. doi: 10.1055/s-0028-1093458. [DOI] [PubMed] [Google Scholar]
- Szász I., Sarkadi B., Schubert A., Gárdos G. Effects of lanthanum on calcium-dependent phenomena in human red cells. Biochim Biophys Acta. 1978 Sep 22;512(2):331–340. doi: 10.1016/0005-2736(78)90257-2. [DOI] [PubMed] [Google Scholar]


