Abstract
Although lymphocyte turnover in chronic human immunodeficiency virus and simian immunodeficiency virus (SIV) infection has been extensively studied, there is little information on turnover in acute infection. We carried out a prospective kinetic analysis of lymphocyte proliferation in 13 rhesus macaques inoculated with pathogenic SIV. A short-lived dramatic increase in circulating Ki-67+ lymphocytes observed at 1 to 4 weeks was temporally related to the onset of SIV replication. A 5- to 10-fold increase in Ki-67+ CD8+ T lymphocytes and a 2- to 3-fold increase in Ki-67+ CD3− CD8+ natural killer cells accounted for >85% of proliferating lymphocytes at peak proliferation. In contrast, there was little change in the percentage of Ki-67+ CD4+ T lymphocytes during acute infection, although transient increases in Ki-67− and Ki-67+ CD4+ T lymphocytes expressing CD69, Fas, and HLA-DR were observed. A two- to fourfold decline in CD4+ T lymphocytes expressing CD25 and CD69 was seen later in SIV infection. The majority of Ki-67+ CD8+ T lymphocytes were phenotypically CD45RA− CD49dhi Fashi CD25− CD69− CD28− HLA-DR− and persisted at levels twofold above baseline 6 months after SIV infection. Increased CD8+ T-lymphocyte proliferation was associated with cell expansion, paralleled the onset of SIV-specific cytotoxic T-lymphocyte activity, and had an oligoclonal component. Thus, divergent patterns of proliferation and activation are exhibited by CD4+ and CD8+ T lymphocytes in early SIV infection and may determine how these cells are differentially affected in AIDS.
Perturbations in T-lymphocyte homeostasis are a hallmark of lentivirus infection and are central to AIDS pathogenesis (10). The dynamics of T-lymphocyte turnover in lentivirus infection are complex and have largely been studied in chronic human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) infections. Shortened half-lives and increased turnover of CD4+ T lymphocytes and CD8+ T lymphocytes have been reported in chronically SIV-infected rhesus macaques and HIV-infected humans (13, 24, 32). Studies using expression of the Ki-67 antigen, a marker selectively expressed in dividing cells (2, 8, 34, 39), or telomere length to estimate cell division are consistent with increased turnover of CD8+ T lymphocytes (7, 28, 33, 42). However, estimates of CD4+ T-lymphocyte proliferation in HIV type 1 (HIV-1)-infected humans have differed widely, being increased (33, 36, 44) or normal (7, 28, 42). These discrepancies may in part be related to differences in stage of disease. In early stages of HIV infection, the fraction of Ki-67+ CD4+ T lymphocytes in peripheral blood and lymphoid tissue was the same as that in uninfected controls (7). In late stages of HIV-1 infection, a threefold increase in Ki-67+ CD4+ T lymphocytes was observed prior to treatment (33, 44), and this fraction declined to normal levels after 6 months of highly active antiretroviral therapy (44). In the cohort of HIV-1-infected individuals studied by Sachsenberg et al., the fraction of Ki-67+ CD4+ T lymphocytes was inversely correlated with CD4+ counts and the mean doubling time of CD4+ cells was two- to threefold shorter in patients with peripheral CD4+ counts of <200/μl than in HIV-infected individuals with CD4+ counts of >500/μl (33). The forces driving increased CD4+ and particularly CD8+ T-lymphocyte turnover in chronic HIV or SIV infection are not well understood but appear to be linked (13, 32, 33) and correlated with CD4+ T lymphocytopenia (33) or with generalized immune activation (12).
The early events following entry of a pathogen into its host are critical in determining the ultimate outcome of infection. In HIV and SIV infection, the level of set point plasma viremia is an important predictor of disease progression (20, 23). While the kinetics of antigen-specific cytotoxic T-lymphocyte (CTL) activity in acute SIV and HIV infection and the temporal association of this activity with a decline in plasma viral load have been well studied (15, 17), there are few data on the dynamics of T-lymphocyte proliferation in acute HIV or SIV infection. An understanding of the kinetics of T-lymphocyte proliferation and activation from the time that the lentivirus enters its host to the onset of immunodeficiency may shed light on the forces driving T-cell turnover in lentivirus infection and the basis for differences in turnover and depletion of CD4+ and CD8+ T lymphocytes.
In this study, we prospectively evaluated the kinetics of lymphocyte proliferation in rhesus macaques for 6 months after inoculation with pathogenic SIV, using expression of the Ki-67 antigen to identify proliferating cells. Ki-67, a nuclear antigen expressed in the G1, G2, S, and M phases but not the G0 phase of the cell cycle, has been widely used as a marker of proliferating or cycling cells (2, 8, 34, 39). By employing flow cytometric analysis with intracellular staining for the Ki-67 antigen, we were able to accurately delineate the phenotype of cells proliferating in response to SIV infection. Differential patterns of activation and proliferation of CD4+ and CD8+ T lymphocytes were observed in the first 6 months after SIV infection. Marked increases in proliferating CD8+ T lymphocytes and natural killer (NK) cells, but not CD4+ T lymphocytes, were seen in the first 4 weeks. Proliferating lymphocytes had a memory and activated phenotype. Increases in the number of activated cells were seen within both resting and proliferating subsets of CD4+ T lymphocytes in the first 4 weeks after SIV infection. A selective loss of activated CD4+ T lymphocytes, but not CD8+ T lymphocytes, was seen later in SIV infection.
MATERIALS AND METHODS
Animals.
Rhesus macaques used in the study were housed in the specific-pathogen-free colony at the New England Regional Primate Research Center. Specific-pathogen-free animals are free of type D retrovirus, simian T-lymphotropic virus type 1, SIV, and herpes B virus infections. Thirteen rhesus macaques were inoculated with pathogenic SIV, 2 with wild-type SIVmac251 (27 ng of p27) by the intravenous (i.v.) route and 11 with molecularly cloned SIVmac239 (8.5 ng of p27) by the intrarectal route. Seven rhesus macaques inoculated intrarectally with SIVmac239 had previously received a recombinant herpes simplex virus (HSV) vaccine expressing the envelope and Nef proteins of SIV (26a). The remaining six rhesus macaques were SIV naive and included one animal that had been vaccinated with a control recombinant HSV. Animals were evaluated once a week for the first 4 weeks after SIV infection and subsequently at 8, 12, 16 or 20, and 24 or 27 weeks after infection.
Animals were maintained in accordance with the guidelines of the Committee on Animals of the Harvard Medical School and the Guide for the Care and Use of Laboratory Animals (1).
Measurement of Ki-67+ lymphocytes in peripheral blood.
Immunophenotyping was done on lysates of freshly drawn whole blood. Surface staining of peripheral blood mononuclear cells (PBMC) was performed by standard procedures (15). Briefly, 100-μl volumes of whole blood were aliquoted into 12- by 75-mm polystyrene tubes and incubated with directly conjugated surface-staining antibodies for 30 min at 4°C. Cells were washed with phosphate-buffered saline containing 2% fetal calf serum (wash medium), incubated with 1 ml of 1× FACSLyse solution (Becton Dickinson Immunocytometry Systems [BDIS], San Jose, Calif.) for 10 min at room temperature to lyse erythrocytes, washed twice with wash medium, and incubated at room temperature for 40 min in the dark with 1 ml of ORTHO PermeaFix (Ortho Diagnostic Systems Inc., Raritan, N.J.). After being washed, permeabilized cells were incubated for 40 min at 4°C with monoclonal antibody (MAb) to Ki-67 antigen (clone MIB-1; Coulter, Miami, Fla.) conjugated to fluorescein isothiocyanate (FITC) or with the isotype control antibody mouse immunoglobulin G1 conjugated to FITC. Stained cells were fixed in 2% paraformaldehyde and kept overnight at 4°C prior to analysis on a FACSCalibur flow cytometer (BDIS). Data were analyzed using CellQuest software (BDIS). Isotype controls were used to set negative gates. Cells expressing high levels of Ki-67 and forming a distinct population were considered to be Ki-67+.
MAbs used for surface staining, in combination with intracellular staining for Ki-67 antigen, included CD3-phycoerythrin (PE) (clone SP34; PharMingen, San Diego, Calif.), CD20-peridinin chlorophyl protein (PerCP; BDIS), CD8-allophycocyanin (APC; PharMingen) or CD8-PerCP (BDIS), CD4-APC or CD4-PerCP (BDIS), CD45RA-APC (PharMingen), CD62L-selectin-PE (BDIS), CD3-APC or CD3-biotin (clone 6G12) with streptavidin red 613 (Gibco), CD25-PE (BDIS), CD28-PE (BDIS), HLA-DR–PE (BDIS), CD69-PE (PharMingen), Fas-PE (CALTAG, Burlingame, Calif.), and CD49d-PE (PharMingen). All antibodies with the exception of anti-CD3 (clone 6G12) were MAbs of antihuman specificity that cross-react with rhesus macaque antigens. Rhesus anti-CD3 (6G12) was kindly provided by Johnson Wong, Massachusetts General Hospital (16), and was custom biotinylated or conjugated with APC (Chromaprobe Inc., Mountain View, Calif.).
TCR staining.
The T-cell receptor (TCR) variable beta-chain (Vβ) repertoire of proliferating CD3+ CD8+ T lymphocytes was determined by four-color flow cytometry. A panel of 19 antihuman TCR Vβ MAbs, which accounted for 15 to 36% of the repertoire of CD3+ CD8+ T lymphocytes in normal macaques (data not shown), was used. The panel consisted of the following antibodies conjugated to PE: Vβ1 (clone BL37.2), Vβ2 (clone MPB2D5), Vβ3 (clone JOV13), Vβ5 (clone MH3-2), Vβ5.1 (clone IMMU157), Vβ5.2 (clone 36213), Vβ7 (clone ZOE), Vβ8 (clone 56C5.2), Vβ11 (clone C21), Vβ12 (clone VER2.32), Vβ13.1 (clone IMMu222), Vβ13.6 (clone JU74.3), Vβ14 (clone CAS1.1.3), Vβ16 (clone TAMAYA 1.2), Vβ17 (clone E17.5F3.13), Vβ20 (clone ELL1.4), Vβ21.3 (clone IG125), Vβ22 (clone IMMU546), and Vβ23 (AF23). All antibodies with the exception of Vβ3 and Vβ5 were obtained from Coulter. Vβ3 and Vβ5 were obtained from PharMingen.
SIV CTL activity.
For antigen-specific stimulation, 1/10 the number of autologous fresh PBMC were infected at a multiplicity of infection of 5 PFU/cell with recombinant vaccinia virus vAbT388-6-1, expressing the Gag and Pol proteins of SIVmac251 and the Env protein of SIVmac239 (provided by D. Panicali, Therion Biologics, Cambridge, Mass.). After 90 min of incubation at 37°C, infected PBMC were mixed with the remaining PBMC at a responder-to-stimulator ratio of 10:1 in R-10 medium (15) and incubated at 37°C in a 5% CO2 incubator. Cells were fed with R-10 medium twice a week, and recombinant human interleukin-2 (kindly donated by M. Gately, Hoffman-LaRoche) was added at 10 IU per ml after 4 to 5 days. CTL activity was determined 10 to 14 days after in vitro stimulation by a standard 51Cr release assay as previously described (15). Bulk CTL activity was quantitated by calculation of lytic units (LU) per 106 PBMC, with 1 LU being defined as the number of effector cells required to induce 20% specific lysis of 104 target cells. Specific CTL activity was calculated by subtracting the LU values obtained with a control antigen from the LU values obtained in the presence of specific SIV proteins.
Kinetic analysis.
The kinetics of proliferation of different cell populations in peripheral blood were quantitated for the first 6 months of SIV infection. The Ki-67 antigen, a marker of proliferation, was used to estimate the proliferation rate, under the assumptions that all cells expressing this antigen are dividing and that cells that do not express the Ki-67 antigen are not dividing. A population balance on a given cell population yields the following expression for the rate of change in the population in peripheral blood:
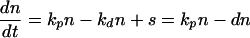 |
1 |
where n is the concentration of cells in the blood (per microliter), kp is the per-cell proliferation rate per day, kd is the per cell death rate per day, and s is the net source (influx minus outflow) of cells into the blood from other physiological compartments (lymph, tissue, etc.) (in number of cells per microliter of blood per day). In the absence of detailed information on cell death and redistribution, we have defined a per-cell disappearance rate, d (in inverse days), to represent the death rate minus the net redistribution rate (in-out) of cells into the blood:
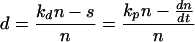 |
2 |
Thus, the disappearance rate accounts for the discrepancy between the expected increase in cell population due to proliferation and the actual change in the cell concentration. To express the population balance in terms of cell concentrations and not cell numbers, we assumed that the blood volume remained constant, and all rates were normalized by the blood concentration of the given cell type to convert to a per-cell basis.
We used the method of Sachsenberg et al. (33) to express the proliferation rate as kp = fKi67/T, where fKi67 is the fraction of cells in the blood expressing Ki-67 antigen and T is the cycle time of proliferating cells. Thus, we determined kp at all sample times, using the value T = 1.4 days. Although this approximation of the cycle time may differ between populations and vary over time, we used one value consistently to simplify the analysis. The rate of change in the cell population, dn/dt, was determined at the midpoint of each time interval as the average slope during that time interval (Δn/Δt). The values of n and kp at these time points were estimated by linear interpolation from the measured values. The disappearance rate, d, at the time point was then determined from equation 2. We verified that results obtained by using linear interpolation and average slopes matched those obtained with higher-order spline interpolations in a few test animal data sets.
Statistical analysis.
Statistical analysis was carried out with the program Statview (Abacus Concepts, Inc., Berkeley, Calif.). P values for differences between groups and time points were determined by the Mann-Whitney U test and the Wilcoxon signed-rank test, respectively. The relationship between variables was analyzed by simple linear-regression analysis and the Spearman rank correlation test.
RESULTS
Rapid appearance of proliferating lymphocytes in peripheral blood after SIV inoculation is temporally associated with onset of SIV replication.
The number and phenotype of Ki-67+ cells in peripheral blood were determined longitudinally in 13 rhesus macaques for 6 months after experimental SIV infection. Two SIV-naive rhesus macaques were inoculated i.v. with uncloned pathogenic SIVmac251, and 11 rhesus macaques were inoculated i.r. with cloned SIVmac239. Four of 11 macaques inoculated i.r. with SIVmac239 were SIV naive, while 7 animals had previously been immunized with a recombinant HSV expressing the envelope and Nef proteins of SIVmac239 (Table 1). Full details regarding immune responses to immunization and virologic and immunologic evaluation after challenge have been reported elsewhere (26a).
TABLE 1.
Grouping of rhesus macaques based on time of appearance of peak Ki-67 antigen expression in peripheral blood
| Group | Wk of peak Ki-67 expression in all lymphocytes | No. of animals (total n = 13) | Infecting SIV strain | Route of SIV inoculation | No. of animals with prior SIV immunizationa |
|---|---|---|---|---|---|
| A | 1 | 2 | SIVmac251 | i.v. | 0/2 |
| B | 2 | 7 | SIVmac239 | i.r.b | 4/7 |
| C | 4 | 2 | SIVmac239 | i.r. | 1/2 |
| D | NCc | 2 | SIVmac239 | i.r. | 2/2 |
Seven rhesus macaques had been immunized with an HSV recombinant expressing the envelope and Nef proteins of SIVmac239.
i.r., intrarectal.
NC, no change.
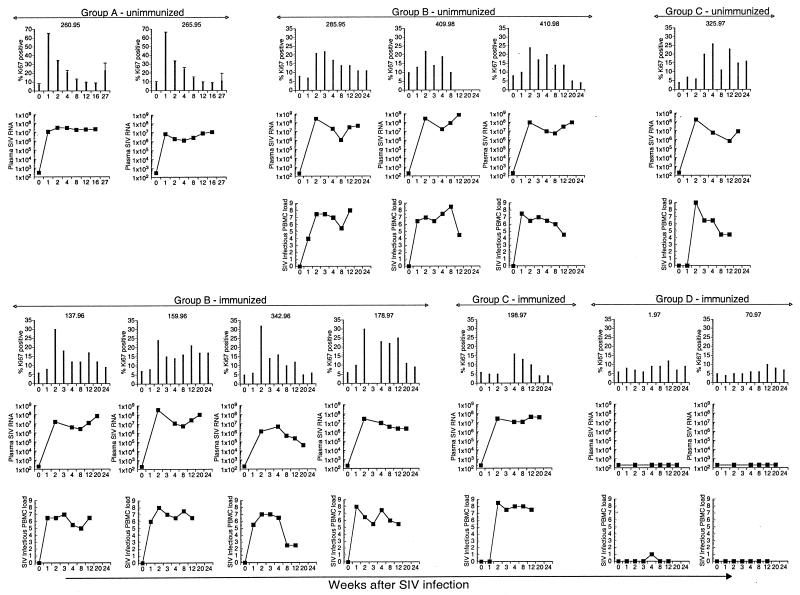
A three- to eightfold increase in the number of Ki-67+ lymphocytes was observed in 11 of 13 rhesus macaques in the first 3 weeks after SIV infection (Fig. 1). Based on the kinetics of appearance of peak elevations in circulating Ki-67+ lymphocytes, the animals were divided into four groups (Table 1). In groups A, B, and C, peak levels of Ki-67+ lymphocytes were detected 1, 2, and 4 weeks, respectively, after SIV infection (Table 1 and Fig. 1 and 2) and coincided with or immediately followed peak plasma SIV RNA levels (Fig. 1 and 2). The differences in kinetics of elevated Ki-67 antigen appeared to be due to differences in kinetics of SIV replication (Fig. 1 and 2). Thus, infectious SIV was first detected 1 week after SIV infection in group B and only 2 weeks after infection in group C, and this corresponded to elevated Ki-67 antigen being detected 2 weeks after SIV infection in group B and 3 to 4 weeks after SIV infection in group C (Fig. 1 and 2). Two animals (group D) that did not have detectable SIV viremia did not show increased numbers of Ki-67+ lymphocytes in their peripheral blood (Fig. 1 and 2). The magnitude of the increase in number of Ki-67+ lymphocytes was greater in animals inoculated with SIVmac251 by the i.v. route (group A) than in animals inoculated with SIVmac239 via the i.r. route (groups B and C) (P = 0.03; Mann-Whitney U test). However, the extent of the increase in Ki-67+ lymphocytes was not related to the height of peak SIV viremia or to the level of set point viremia (data not shown). Furthermore, within group B and group C animals, no differences in numbers of Ki-67+ lymphocytes were observed between immunized and SIV-naive animals (data not shown). The sharp increase in Ki-67+ cells in peripheral blood was short-lived and declined to levels roughly twofold above baseline by 12 weeks after SIV infection (Fig. 2 and Table 2). In all, these data suggest that SIV induces a significant though short-lived burst of proliferating circulating lymphocytes which is temporally related to initial SIV replication.
FIG. 1.
Longitudinal analysis of lymphocyte proliferation, plasma SIV RNA, and infectious SIV load in PBMC in individual rhesus macaques inoculated with pathogenic SIV. Data for seven animals immunized with an HSV recombinant expressing SIV proteins prior to SIV inoculation are shown in the bottom half of the figure. Data for six animals that were SIV naive prior to SIV inoculation (includes one animal, 285.95, that received a control HSV recombinant) are shown in the top panel. The animals were grouped on the basis of time of appearance of peak increase in Ki-67 expression in total lymphocytes in peripheral blood following SIV inoculation. This occurred at 1 week in group A, at 2 weeks in group B, and at 4 weeks after SIV inoculation in group C animals. In group D animals, there was no change in Ki-67 expression following SIV inoculation. Infectious SIV loads in PBMC were not available for the two group A animals.
FIG. 2.
Kinetics of plasma SIV viremia and its relationship to lymphocyte proliferation after acute SIV infection. Animals were grouped on the basis of time of appearance of peak increase in Ki-67+ lymphocytes after SIV infection. Means and standard errors for percentages of Ki-67+ lymphocytes (bars) and mean values for infectious SIV load in PBMC (14) and plasma SIV RNA (35) (lines) are shown. Viral load data for groups B to D are from Murphy et al. (26a).
TABLE 2.
Comparison of fraction of Ki-67+ cells and absolute cell counts before and after acute SIV infectiona
| % or no. of cells | Mean % or no. of cells ± SD
|
|||
|---|---|---|---|---|
| Day 0 (n = 11) | Peakb (n = 11) | Wk 12 (n = 10) | Wk 24 (n = 10) | |
| % of lymphocytes that are Ki-67+ | 6.9 ± 1.8 | 32.4 ± 17.0c | 15.6 ± 5.6c | 11.0 ± 6.1 |
| No. of Ki-67+ lymphocytes/μl | 302 ± 189 | 1,729 ± 1,377c | 751 ± 538 | 441 ± 373 |
| No. of lymphocytes/μl | 4,189 ± 2,057 | 5,136 ± 2,228 | 4,667 ± 2,745 | 3,827 ± 1,335 |
| % of CD3+ lymphocytes that are Ki-67+ | 5.2 ± 1.0 | 27.6 ± 9.9c | 11.5 ± 4.3c | 8.8 ± 4.8d |
| No. of Ki-67+ CD3+ cells/μl | 144 ± 64 | 984 ± 556c | 360 ± 300 | 204 ± 205 |
| No. of CD3+ cells/μl | 2,864 ± 1,309 | 3,464 ± 1,266 | 2,934 ± 1,524 | 2,288 ± 1,177 |
| % of CD3+ CD8+ lymphocytes that are Ki-67+ | 4.9 ± 2.0 | 42.5 ± 11.1c | 14.9 ± 4.9c | 10.3 ± 6.3d |
| No. of Ki-67+ CD3+ CD8+ cells/μl | 57 ± 37 | 862 ± 502c | 289 ± 252d | 147 ± 177 |
| No. of CD3+ CD8+ cells/μl | 1,233 ± 690 | 1,970 ± 905 | 1,759 ± 1,020 | 1,359 ± 871 |
| % of CD3+ CD4+ lymphocytes that are Ki-67+ | 5.3 ± 0.8 | 7.1 ± 6.5 | 7.3 ± 4.0 | 6.1 ± 4.6 |
| No. of Ki-67+ CD3+ CD4+ cells/μl | 88 ± 37 | 117 ± 123 | 76 ± 55 | 47 ± 32d |
| No. of CD3+ CD4+ cells/μl | 1,678 ± 654 | 1,529 ± 456 | 1,164 ± 610 | 904 ± 456d |
| % of NK cells that are Ki-67+ | 17.1 ± 9.8 | 49.6 ± 22.8c | 14.6 ± 5.3 | 10.1 ± 5.6 |
| No. of Ki-67+ NK cells/μl | 102 ± 94 | 495 ± 864c | 129 ± 107 | 42 ± 28d |
| No. of NK cells/μl | 640 ± 506 | 836 ± 1,061 | 823 ± 725 | 520 ± 392 |
| % of CD20+ lymphocytes that are Ki-67+ | 4.9 ± 1.3 | 3.8 ± 4.7 | 6.1 ± 3.4 | 2.6 ± 1.6 |
| No. of Ki-67+ CD20+ cells/μl | 48 ± 32 | 41 ± 75 | 79 ± 77 | 32 ± 23 |
| No. of CD20+ cells/μl | 820 ± 500 | 855 ± 495 | 1,145 ± 988 | 1,199 ± 609 |
Group D animals were excluded from this analysis.
Peak, time point after SIV infection at which the peak increase in Ki-67 expression was seen, i.e., week 1 for group A, week 2 for group B, and week 4 for group C animals.
P < 0.01 (Wilcoxon signed-rank test) versus day 0 value.
P < 0.05 (Wilcoxon signed-rank test) versus day 0 value.
CD8+ T lymphocytes and NK cells are the major cell populations contributing to the increase in proliferating lymphocytes after acute SIV infection.
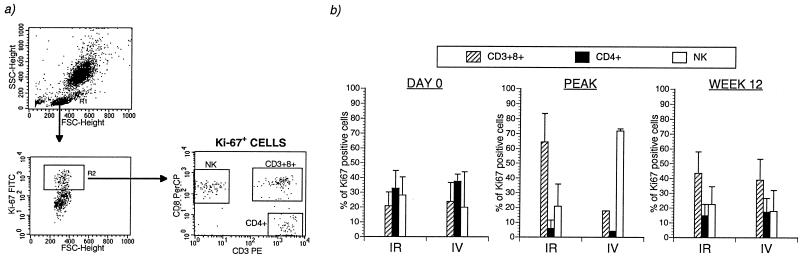
Four-color flow cytometry, combining staining of three cell surface markers with intracellular staining for Ki-67 antigen, was used to delineate the phenotypes of proliferating cell populations in acute SIV infection (Fig. 3a). The acute increase in proliferating lymphocytes evident 1 to 4 weeks after SIV infection in groups A to C was almost entirely due to an increase in number of Ki-67+ CD8+ T lymphocytes and CD3− CD8+ NK cells (Fig. 3b). A 7- to 10-fold increase in the fraction of Ki-67+ CD8+ T lymphocytes and a 2- to 3-fold increase in the fraction of Ki-67+ NK cells were detected 1 to 4 weeks after SIV infection (Fig. 3b). At peak increase, 49.6% ± 22.8% of the NK cells (mean ± standard deviation [SD]) and 42.5% ± 11.1% of the CD8+ T lymphocytes (mean ± SD) expressed Ki-67, and the magnitude of the increase above the baseline level was highly significant (Table 2). The increase in Ki-67+ NK cells was short-lived, with cell numbers returning to baseline levels within 12 weeks after SIV infection (Table 2). In contrast, the number of Ki-67+ CD8+ T lymphocytes remained significantly elevated at two- to fourfold above baseline values 12 and 24 weeks after SIV infection (Table 2).
FIG. 3.
CD8+ T lymphocytes and NK cells are the dominant proliferating cells in acute SIV infection. (a) A representative plot from one rhesus macaque 2 weeks after SIV infection. Individual cell subsets within the small-lymphocyte gate were analyzed for intracellular Ki-67 antigen as shown. The small-lymphocyte gate included >91% of all lymphocytes. The definition of CD4+ T lymphocytes as CD3+ CD8− was validated by concurrent three-color phenotyping with MAb to CD3, CD4, and CD8 (data not shown). FSC, forward scatter; SSC, side scatter. (b) Longitudinal analysis of fractions of Ki-67 antigen-positive cells in different lymphocyte subsets in 13 rhesus macaques after SIV infection. Mean and standard error for the fraction of Ki-67+ cells in each lymphocyte subset are shown.
A twofold increase in percentage of Ki-67+ CD4+ T lymphocytes was seen in two macaques in group A and in two macaques in group C (Fig. 3b). However, no increase was seen in group B, which comprised the majority of macaques inoculated i.r. with SIVmac239 (Fig. 3b). Taken as a whole, the increase in Ki-67+ CD4+ T lymphocytes did not reach statistical significance in the first 6 months after SIV infection (Table 2). Similarly, the fraction of Ki67+ cells in circulating B lymphocytes did not show an increase after SIV infection (Table 2).
Even though the dramatic increase in Ki-67+ cell numbers after SIV infection was transient, an analysis of cells making up the total pool of Ki-67+ lymphocytes in peripheral blood at later time points revealed a dominant component of CD8+ T lymphocytes (Fig. 4). Prior to SIV infection, the proliferating cells in peripheral blood consisted of roughly equal parts CD4+ T lymphocytes, CD8+ T lymphocytes, and NK cells (Fig. 4b). At the time of peak proliferation, >80% of the total pool of Ki-67+ lymphocytes were derived from NK cells or CD8+ T lymphocytes, and CD4+ T lymphocytes contributed <5% to the total pool of Ki-67+ lymphocytes (Fig. 4b). At later time points, when the contribution of NK cells had declined to baseline levels, CD8+ T lymphocytes accounted for >40% of the total pool of cells expressing Ki-67 in peripheral blood (Fig. 4), indicating persistent CD8+ T-lymphocyte proliferation following SIV infection.
FIG. 4.
Composition of the total pool of Ki-67+ cells in peripheral blood before and after pathogenic SIV infection. (a) A representative plot illustrating analysis of Ki-67+ cells. FSC, forward scatter; SSC, side scatter. (b) Composition of Ki-67+ cells during SIV infection in two macaques inoculated intravenously (IV) with SIVmac251 and in nine macaques inoculated intrarectally (IR) with SIVmac239. Peak refers to the time of maximal expression of Ki-67 after SIV infection. The two group D animals are excluded from analysis.
Kinetics of turnover of lymphocyte subsets in acute SIV infection.
Significant differences in the proliferation rates (kp) of CD4+ T lymphocytes, CD8+ T lymphocytes, and NK cells were observed in the first 6 months after SIV infection (Fig. 4). While the baseline kp values for CD4+ and CD8+ T lymphocytes were similar, a rapid and significant increase in kp was seen in CD8+, but not CD4+, T lymphocytes. Although the baseline kp values for NK cells were three- to sevenfold higher, the kinetics of proliferation of NK cells and CD8+ T lymphocytes paralleled each other closely in the first 4 weeks after SIV infection (Fig. 5).
FIG. 5.
Differing kinetics of proliferation for CD4+ and CD8+ T lymphocytes in acute SIV infection. kp is the proliferation rate calculated by the formula kp = fKi67/T, and d refers to the net disappearance rate calculated with the formula d = (kdn − s)/n = [kpn − (dn/dt)]/n.
In spite of the dramatic fluctuations in proliferation rate, the corresponding variation in cell concentration revealed a net disappearance rate (d) that rapidly mimicked the change in proliferation kinetics (Fig. 5). The calculated disappearance rate reflects both death of lymphocytes and the net migration of lymphocytes out of the circulation. A lag between proliferation and disappearance was evident for CD8+ T lymphocytes, but not NK cells, at the time of peak kp, and this was reflected in an increase in the number of CD8+ T lymphocytes in peripheral blood (Fig. 5 and Table 2). At later time points, small differences in the balance between proliferation and disappearance of any cell type accounted for exponential changes in cell concentration (data not shown).
We used linear regression analysis to further examine the relationship of lymphocyte proliferation to cell number and SIV viral load. In the first 4 weeks after SIV infection, CD8+ T-lymphocyte proliferation was directly correlated to peripheral CD8+ T-lymphocyte counts, but there was no correlation with CD4+ T lymphocytopenia or SIV viral load (Fig. 6a and b and data not shown). This relationship persisted up to 6 months after SIV infection (data not shown), which suggests that a sustained increase in CD8+ T-lymphocyte turnover is associated with cell expansion initiated immediately after SIV infection. CD4+ T-lymphocyte proliferation showed a weak direct correlation with CD4+ T lymphocytopenia which was significant only in the first 4 weeks after SIV infection (Fig. 6c and data not shown). Even though the early effects of SIV infection on CD4+ and CD8+ T-lymphocyte proliferation were very divergent, the two processes appear to be linked since there was a significant, albeit weak, positive correlation between the fractions of proliferating CD4+ and CD8+ T lymphocytes (R2 = 0.187) (Fig. 6d).
FIG. 6.
Relationship between proliferating CD4+ and CD8+ T lymphocytes and cell counts in the first 4 weeks after SIV infection. Plots of linear-regression analysis are shown. The dotted lines depict 95% confidence bands for means. P values were generated by the software Statview, using analysis of variance.
Proliferating CD8+ and CD4+ T lymphocytes have a memory phenotype and are activated.
The phenotype of T lymphocytes expressing Ki-67 in four SIV-naive macaques inoculated intrarectally with SIVmac239 was investigated extensively, using a panel of antibodies to delineate naive, memory, and activated subsets of T lymphocytes.
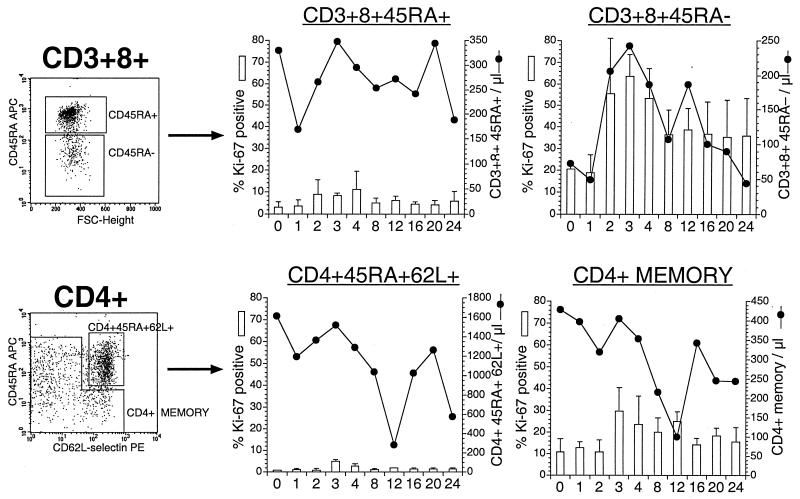
A threefold increase in Ki-67+ cells was seen in both the CD45RA+ and CD45RA− fractions of CD8+ T lymphocytes (Fig. 7). The percentage of Ki-67+ cells in CD45RA+ CD8+ T lymphocytes (mean ± SD) increased from 3% ± 2.4% at baseline to 8.3% ± 1.3% 3 weeks after SIV infection (P = 0.07; Wilcoxon signed-rank test). However, the bulk of the increase in Ki-67+ CD8+ T lymphocytes was largely due to cycling in the CD45RA− memory subset, since there was an increase from 20.5% ± 2.4% at baseline to 63.8% ± 9.8% 3 weeks after SIV infection (P = 0.07; Wilcoxon signed-rank test).
FIG. 7.
Longitudinal analysis of proliferation of naive and memory CD4+ and CD8+ T lymphocytes after SIV infection. Representative plots with delineation of naive and memory CD4+ and CD8+ T lymphocytes are shown. CD45RA was used to designate CD3+ CD8+ T lymphocytes as having a naive (CD45RA+) or memory (CD45RA−) phenotype. CD45RA and CD62L-selectin were used to delineate naive and memory CD4+ T lymphocytes. The fractions of Ki-67+ naive and memory CD4+ and CD8+ T lymphocytes (shown as columns) and their absolute cell counts (shown as lines) at corresponding times after SIV infection are depicted. Data are means of values from four animals. Error bars indicate the standard error for percent Ki-67 expression. FSC, forward scatter.
Although analysis of the total CD4+ T-lymphocyte population did not reveal a significant increase in Ki-67 expression, evaluation of the memory subset revealed a two- to threefold increase (11% ± 6% at baseline to 29.8% ± 11%; P = 0.07 [Wilcoxon signed-rank test]) 3 weeks after SIV infection (Fig. 7). Since the naive CD45RA+ CD62L+ population constituted >70% of the total CD4+ T lymphocytes in peripheral blood and <5% of them were cycling, elevations in number of Ki-67+ CD4+ T lymphocytes were masked when total CD4+ T lymphocytes were analyzed (Fig. 7 and data not shown).
The relationships between proliferating naive and memory T lymphocytes and their numbers in peripheral blood were different for CD4+ and CD8+ T lymphocytes (Fig. 7). A significant positive correlation was observed between the percentage of Ki-67+ CD3+ CD8+ CD45RA− lymphocytes and the total number of CD3+ CD8+ CD45RA− lymphocytes in peripheral blood (R2 = 0.173; P = 0.02). In contrast, the increase in Ki-67+ memory CD4+ T lymphocytes was not associated with an increase in the total number of memory CD4+ T lymphocytes in peripheral blood. This may reflect ongoing destruction of CD4+ T lymphocytes, since activated memory CD4+ cells are targets for productive SIV infection. Alternatively, it may also indicate redistribution of proliferating CD4+ T lymphocytes.
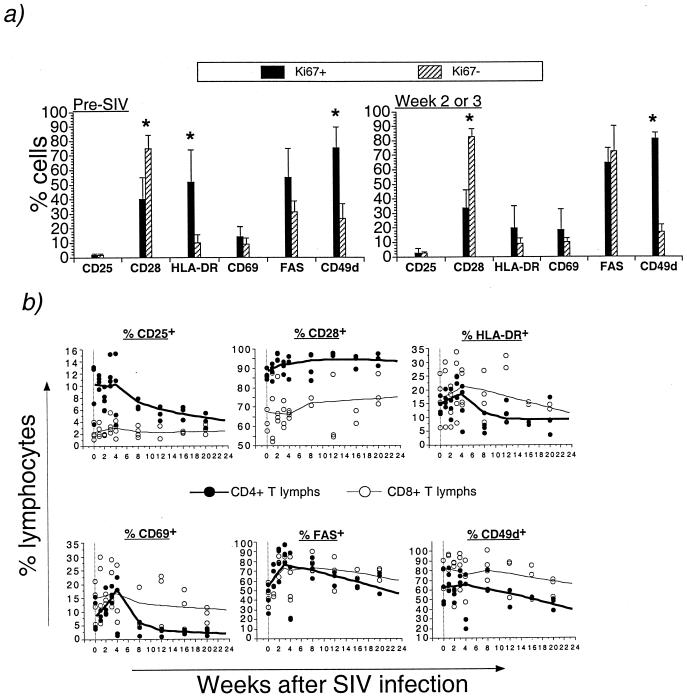
The phenotype of proliferating CD8+ T lymphocytes in acute SIV infection was further characterized by using a panel of antibodies specific for molecules upregulated following lymphocyte activation or the costimulatory molecule CD28. Prior to SIV infection, the majority of Ki-67+ CD8+ T lymphocytes had the phenotype CD25− CD69− HLA-DR+ CD49dhi Fashi; roughly half were CD28− and half were CD28+ (Fig. 8a). In contrast, the majority of Ki-67− (resting) CD8+ T lymphocytes had the phenotype CD28+ HLA-DR− Fasneg/lo CD49dneg/lo (Fig. 8a). The differences in the proportions of CD28+, HLA-DR+, and CD49d+ cells between the Ki-67+ and Ki-67− CD8+ T lymphocytes were statistically significant (P < 0.05; Mann-Whitney U test). Following SIV infection, and at the time of peak increase in proliferation, the phenotype of Ki-67+ and Ki-67− CD8+ T lymphocytes was altered, with a two- to threefold increase in Fas expression among Ki-67− CD8+ T lymphocytes (P = 0.07; Wilcoxon signed-rank test) and a decline in HLA-DR+ in Ki-67+ CD8+ T lymphocytes (P = 0.07; Wilcoxon signed-rank test) (Fig. 8a).
FIG. 8.
Longitudinal phenotypic analysis of T lymphocytes in acute SIV infection. (a) Differential expression of activation and costimulatory molecules on Ki-67+ and Ki-67− CD8+ T lymphocytes prior to and after SIV infection. Asterisks indicate a statistically significant difference between Ki-67+ and Ki-67− cells for that surface marker (Mann-Whitney U test). (b) Bivariate scattergram plots and Lowess lines of data from four rhesus macaques showing changes in the fraction of CD4+ and CD8+ T lymphocytes (lymphs) expressing the indicated molecules in the first 6 months after intrarectal SIVmac239 inoculation. One rhesus macaque died of AIDS 10 weeks after SIV infection.
Longitudinal phenotypic analysis following acute SIV infection showed several differences between CD4+ and CD8+ T lymphocytes (Fig. 8b and Table 3). A rapid increase and subsequent decline in CD4+ T lymphocytes expressing CD69, Fas, and HLA-DR was observed in acute SIV infection (Fig. 8b), and there was a significant negative correlation between time after SIV infection and CD4+, but not CD8+, T lymphocytes expressing CD25, CD69, HLA-DR, or CD49d (Table 3).
TABLE 3.
Longitudinal phenotypic analysis of T lymphocytes in acute SIV infectiona
| Phenotype | % of CD4+ or CD8+ cells expressing phenotype at wk post-SIV infection
|
Rb,c | P valuec | |||
|---|---|---|---|---|---|---|
| 0 | 3 | 12 | 24 | |||
| CD4+ CD25+ | 9.2 ± 4.6 | 11.9 ± 2.3 | 5.4 ± 1.2 | 4.4 ± 1.1 | −0.614 | <0.0001 |
| CD4+ CD28+ | 86.7 ± 2.6 | 90.8 ± 2.8 | 97.2 ± 0.7 | 93.0 ± 2.2 | 0.415 | 0.0141 |
| CD4+ CD69+ | 9.3 ± 5.9 | 16.2 ± 2.6 | 2.6 ± 1.5 | 1.7 ± 0.9 | −0.649 | <0.0001 |
| CD4+ HLA-DR+ | 13.2 ± 3.0 | 21.4 ± 3.7 | 11.0 ± 4.1 | 8.7 ± 4.7 | −0.475 | 0.005 |
| CD4+ Fas+ | 41.0 ± 13.2 | 85.4 ± 7.6 | 61.0 ± 7.2 | 35.1 ± 22 | −0.166 | 0.3252 |
| CD4+ CD49d+ | 57.8 ± 17.0 | 69.8 ± 7.1 | 51.3 ± 10.0 | 34.7 ± 6.7 | −0.488 | 0.0039 |
| CD8+ CD25+ | 2.2 ± 1.2 | 3.4 ± 1.5 | 1.9 ± 1.2 | 2.2 ± 0.8 | 0.006 | 0.9724 |
| CD8+ CD28+ | 68.6 ± 12.7 | 65.6 ± 6.4 | 65.6 ± 18.3 | 74.3 ± 2.4 | 0.315 | 0.0624 |
| CD8+ CD69+ | 13.7 ± 6.6 | 19.8 ± 8.2 | 16.2 ± 10.1 | 11.6 ± 1.2 | −0.255 | 0.1321 |
| CD8+ HLA-DR+ | 16.1 ± 8.4 | 20.7 ± 12.1 | 22.9 ± 11.1 | 11.8 ± 6.2 | −0.154 | 0.3609 |
| CD8+ Fas+ | 43.2 ± 10.8 | 85.0 ± 7.7 | 66.1 ± 17.7 | 54.8 ± 2.6 | 0.070 | 0.6768 |
| CD8+ CD49d+ | 76.5 ± 12.5 | 82.1 ± 16.9 | 73.7 ± 20.8 | 67.7 ± 14.0 | −0.269 | 0.1109 |
Data points included 0, 1, 2, 3, 4, 8, 12, 20, and 24 weeks after SIV infection.
Spearman rank correlation for changes in fraction of cells expressing the indicated molecule with time after SIV infection.
R and P values calculated from all data points in the first 6 months after intrarectal SIVmac239 inoculation of four rhesus macaques.
An increase in Fas expression observed in both CD4+ and CD8+ T lymphocytes 2 to 3 weeks after SIV infection was largely a result of an increase in Fas-positive cells within the Ki-67− population of T lymphocytes (Table 3 and data not shown). As observed in CD8+ T lymphocytes, Fas-positive cells among Ki-67− CD4+ T lymphocytes increased from 28% ± 13% (mean ± SD) prior to SIV infection to 67% ± 20% 2 to 3 weeks after SIV infection (P = 0.07; Wilcoxon signed-rank test). Activated CD4+ T lymphocytes expressing CD25, CD69, HLA-DR, or CD49d were also present in comparable proportions within both the Ki-67− and Ki-67+ cell pools (data not shown).
The rapid increase in proliferating CD8+ T lymphocytes includes an antigen-specific component.
Many acute viral infections are associated with a rapid, short-lived massive expansion of T lymphocytes which can largely be accounted for by expansion of antigen-specific CD8+ T lymphocytes (3, 5, 26). In SIV-infected rhesus macaques, the increase in Ki-67+ CD8+ T lymphocytes was dependent on SIV replication since it did not occur in group D animals that had undetectable levels of SIV replication (Fig. 2). Furthermore, the kinetics of detection of in vitro SIV-specific CTL activity paralleled the appearance of elevated numbers of Ki-67+ CD8+ T lymphocytes in peripheral blood (data not shown), and the magnitude of the increase was directly proportional to the strength of Gag-specific CTL activity assessed by measurement of lytic units (R2 = 0.208; P = 0.003). This supported the contribution of an antigen-specific component to the elevated number of Ki-67+ CD8+ T lymphocytes.
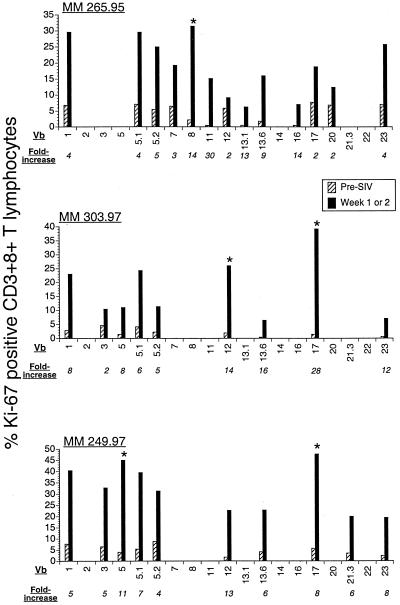
To determine whether proliferation of CD8+ T lymphocyte was oligoclonal or polyclonal, we investigated the increase in the fraction of proliferating cells within individual TCR Vβ subsets of CD8+ T lymphocytes at the peak of proliferation. In three rhesus macaques, prior to SIV infection, the panel of 19 antihuman TCR Vβ MAbs accounted for 17 to 36% of the TCR Vβ repertoire of CD8+ T lymphocytes (data not shown). One to 2 weeks after SIV infection, there was a global increase in proliferation of all detectable Vβ subsets of CD8+ T lymphocytes (Fig. 9). However, the magnitude of proliferation differed widely among individual TCR Vβ subsets, and a >25% proliferating fraction associated with an eightfold or greater increase in proliferation above baseline was seen in fewer than 3 of the 19 TCR Vβ subsets (Fig. 9). Together, these data suggest that during acute SIV infection there is polyclonal and oligoclonal proliferation of CD8+ T lymphocytes and that SIV-specific CD8+ T lymphocytes are likely to contribute to the expansion of proliferating CD8+ T lymphocytes.
FIG. 9.
Oligoclonal proliferation of CD8+ T lymphocytes in acute SIV infection. Proliferating fractions of 19 TCR Vβ (Vb) subsets were determined by flow cytometry before and at the peak of proliferation after SIV infection in three rhesus macaques. Asterisks denote Vβ subsets with >25% proliferating fraction and a more than eightfold increase in proliferation following SIV infection. MM, Macacamulatta.
DISCUSSION
Despite accumulating data on the kinetics of T-cell turnover in chronic HIV and SIV infection, there is a lack of information with regard to acute infection. In this study we used the proliferation marker Ki-67 to study the kinetics of T-lymphocyte turnover during acute SIV infection. Juvenile rhesus macaques inoculated with pathogenic SIV demonstrated a rapid and short-lived 7- to 10-fold increase in proliferating CD8+ T lymphocytes and a 2- to 3-fold increase in proliferating NK cells within 3 weeks of infection. The kinetics and magnitude of proliferation differed among individual animals, and these differences appeared to be related to differences in kinetics of SIV replication. Little or no increase in CD4+ T-lymphocyte proliferation was observed, and when present it was primarily confined to the memory subset. In all, these findings are consistent with the primary responses elicited by infections with viruses such as Epstein-Barr virus, polyomavirus, and lymphocytic choriomeningitis virus, which induce a large expansion of CD8+ T lymphocytes that is disproportionate to the increase in CD4+ T lymphocytes (3, 4, 11, 22, 26, 37).
The use of bromodeoxyuridine or [2H]glucose, although well-suited for examination of T-cell turnover under steady-state conditions (13, 24, 32), has limitations in the setting of acute viral infections. Since assessments using these techniques generally require 3 to 14 days of drug administration, results reflect changes in T-cell turnover occurring over an extended period of time. Moreover, they only allow examination of the fraction of lymphocytes that take up label during the period of drug administration and, hence, are not suited for longitudinal studies after acute viral infection. In contrast, analysis of Ki-67 expression allows rapid assessment of changes in the proliferation of different lymphocyte fractions on a daily basis. The cell cycle specificity of the Ki-67 antigen has been widely studied in vitro in tumor cell lines and in mitogen-stimulated lymphocytes (2, 8, 21, 34). These studies have demonstrated that the Ki-67 antigen is not expressed in G0 but is expressed to different levels in the G1, S, G2, and M phases of the cell cycle, with maximal expression commonly seen in the G2/M phase. A sizable proportion of Ki-67+ cells in peripheral blood are in the G1 stage of the cell cycle (39; M. A. DeMaria, personal communication), and it is not known whether all these cells are committed to undergoing division. Ki-67+ cells in G1 could be cells that have entered the cell cycle from G0, or they might represent cells that have just completed mitosis (21). If all Ki-67+ cells in G1 do not divide, measurement of Ki-67 may overestimate the size of the proliferating cell population. Conversely, since expression of Ki-67 antigen can decline in late G1 or early S phase of the cell cycle and not all Ki-67neg and Ki-67lo cells are noncycling (2), underestimating the extent of proliferation is also possible. Despite these caveats, results regarding turnover of T lymphocytes obtained by analysis of Ki-67 expression (12, 33) have generally correlated well with those obtained using bromodeoxyuridine (24, 32) and [2H]glucose (13) measurements of T-lymphocyte turnover during chronic lentivirus infection and support its validity for use in measuring in vivo proliferation.
The rapidity of the host response and the fact that proliferating cells are almost exclusively NK cells and CD8+ T lymphocytes suggest triggering of both innate and antigen-specific immune responses. Both antigen-specific and bystander proliferation have been proposed to contribute to increases in CD8+ T-cell proliferation observed after viral infection (37). With the advent of sensitive techniques like tetramer technology, it has become possible to accurately quantitate the contribution of antigen-specific CD8+ T lymphocytes in virus-induced proliferation. In many primary viral infections, virus-specific CD8+ T lymphocytes have been shown to constitute as much as 40 to 70% of the total pool of activated CD8+ T lymphocytes (5, 22, 26). In acute and chronic HIV infections, there is evidence for large oligoclonal expansions of antigen-specific CD8+ T lymphocytes (9, 29). In our study, the kinetics of lymphocyte proliferation after SIV infection paralleled those described for other primary virus infections, and it is likely that the proliferating CD8+ T lymphocytes had a significant antigen-specific component. We have indirect evidence suggesting that there is a significant antigen-specific component at the height of proliferation, although the precise extent of this remains to be determined. First, the increase in Ki-67+ CD8+ T lymphocytes paralleled the onset of SIV-specific CTL activity. Second, the majority of proliferating CD8+ T lymphocytes were activated and CD28−, a phenotype frequently reported in virus-specific memory cells. Analysis of telomere lengths and recent functional studies have shown that the CD28− subset of CD8+ T lymphocytes consists of oligoclonal antigen-specific memory populations that contain large proportions of functional virus-specific (cytomegalovirus and HIV) memory CTL precursors (19, 25, 41). Finally, in a small number of animals, the disproportionate proliferation of a few Vβ TCR subsets suggested the occurrence of oligoclonal proliferation of CD8+ T lymphocytes, consistent with what has been observed in acute HIV and SIV infections (6, 29).
The magnitude of increase in numbers of proliferating cells as determined by the fraction of Ki-67+ cells did not translate into a comparable increase in circulating cell number. Thus, a sevenfold increase in the number of Ki-67+ CD8+ T lymphocytes was associated with only a threefold increase in the number of CD8+ T lymphocytes in peripheral blood, and in the instance of NK cells there was no detectable change in cell number. A discordance between the number of cycling memory lymphocytes and the actual number of functional memory CTL precursors (CTLp) has been observed after other viral infections (38). Mice infected with influenza A virus showed continued cycling of virus-specific CTLp over a 7-day period, and yet during this period, CTLp frequencies were considerably lower than was anticipated from the rate of cycling (38). In the SIV-infected macaques, fluctuations in proliferation rates of different lymphocyte subsets were associated with rapid changes in rates of disappearance that closely paralleled proliferation kinetics. We do not know whether this “disappearance” was due to cell death, cell redistribution, or both. The maintenance of steady-cell numbers despite considerable proliferation may reflect ongoing activation-induced apoptosis or redistribution of cells from the blood into tissue sites of SIV replication. In our study, at the height of increase in number of Ki-67+ lymphocytes, more than 80% of cycling (Ki-67+) and resting (Ki-67−) T lymphocytes were Fas positive, and Fas-mediated apoptosis might have contributed to the increased level of cell death. Increased levels of apoptotic cells, as evidenced by increased annexin binding, have been demonstrated in tetramer-positive CD8+ T lymphocytes after acute SIV infection (18). Disappearance of cells from the circulation due to redistribution is another mechanism that could result in discordance between the rate of proliferation and the degree of observed cell expansion. Significant redistribution of T lymphocytes is a common occurrence in HIV and SIV infections, and the degree of trapping in peripheral lymphoid tissues increases with disease progression and increased viral replication (27, 30, 31, 44).
The kinetics of T-lymphocyte proliferation in the first 6 months after SIV infection differ in several respects from those reported in chronic HIV and SIV infection (13, 32, 33). In contrast to chronic infection, the magnitude of CD8+ T-lymphocyte proliferation was far in excess of CD4+ T-lymphocyte proliferation during acute infection. Furthermore, in acute infection, proliferation of CD8+ T lymphocytes was directly correlated to peripheral CD8+, but not CD4+, T-lymphocyte counts while proliferation of CD4+ T lymphocytes was directly correlated with CD4+ T lymphocytopenia, and neither was correlated with viral load. This is unlike the situation in chronic HIV infection, for which a significant positive correlation between viral load and CD4+ T-lymphocyte proliferation was observed and both CD4+ and CD8+ T-lymphocyte proliferation were related to the extent of CD4+ lymphocytopenia (33). Similar to chronic SIV infection, we did observe a direct, albeit weak, correlation between the numbers of proliferating CD4+ and CD8+ T lymphocytes, suggesting that in spite of the differences in magnitude, the two processes are linked from very early in SIV infection. In a recent study of T-lymphocyte proliferation in HIV-infected individuals on highly active antiretroviral therapy, Hazenberg et al. demonstrated that the increased proliferation of naive and memory CD4+ and CD8+ T lymphocytes appears to be driven by generalized immune activation rather than being a homeostatic response (12).
In this study we also analyzed the kinetics of T-lymphocyte activation and the fate of activated T lymphocytes in the first 6 months after SIV infection. An increase in numbers of activated CD4+ T lymphocytes, particularly those expressing CD69, was seen at the peak of lymphocyte proliferation. Surprisingly, expression of multiple activation markers was observed in both Ki-67− and Ki-67+ cells. This is of interest in view of the recent observation that replication-competent SIV is found in Ki-67− and Ki-67+ compartments of CD4+ T lymphocytes (43). Although a large number of cells are activated, depending on the degree of activation, only a subset may be susceptible target cells for productive infection (10). Among the activated CD4+ T lymphocytes, we observed a higher peak and a disproportionate decline in numbers of CD69-positive cells, suggesting that they were productively infected (40). In contrast to that of CD4+ T lymphocytes, activation of CD8+ T lymphocytes was less evident, and up to 6 months after SIV infection there was no loss of activated CD8+ T lymphocytes.
In conclusion, the SIV-macaque model has allowed us to prospectively evaluate disturbances in T-lymphocyte proliferation from the onset of lentivirus infection. We have shown that perturbations in T-lymphocyte dynamics are initiated very early in SIV infection and differ qualitatively and quantitatively for CD4+ and CD8+ T lymphocytes. The first detectable change is a consistent dramatic increase in CD8+ T-lymphocyte proliferation at the peak of viral replication that is associated with an expansion of circulating CD8+ T lymphocytes. The increase in proliferating CD8+ T lymphocytes is sustained later in infection, albeit at lower levels. The alterations in CD4+ T lymphocytes are characterized by a variable but generally weak proliferative response. Instead, a rapid increase and then a decline in the number of activated cells are seen within both the proliferating and resting subsets of CD4+ T lymphocytes. Future longitudinal studies to determine how these early perturbations are related to the rate of progression to AIDS may help in elucidating mechanisms leading to immunodeficiency after lentivirus infection.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants RR00168, AI38559, AI43890, CA83248, and CA56591.
We gratefully acknowledge Ron Desrosiers and David Knipe for providing blood samples from animals from the herpesvirus recombinant vaccine study (supported by AI38131) and for review of the manuscript, John Shiver for suggestions regarding SIV-specific CTL assays, Jeff Lifson for plasma SIV RNA measurements, Rafick Sekaly for advice regarding rhesus TCR Vβ-specific antibodies, and MaryAnn DeMaria and Michael Rosenzweig for helpful discussions and assistance with flow cytometry.
REFERENCES
- 1.Anonymous. Guide for the care and use of laboratory animals. Washington, D.C.: The Institute of Laboratory Animal Resources, National Research Council; 1996. pp. 86–123. [Google Scholar]
- 2.Bruno S, Darzynkiewicz Z. Cell cycle dependent expression and stability of the nuclear protein detected by Ki-67 antibody in HL-60 cells. Cell Prolif. 1992;25:31–40. doi: 10.1111/j.1365-2184.1992.tb01435.x. [DOI] [PubMed] [Google Scholar]
- 3.Butz E A, Bevan M J. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callan M F, Steven N, Krausa P, Wilson J D, Moss P A, Gillespie G M, Bell J I, Rickinson A B, McMichael A J. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat Med. 1996;2:906–911. doi: 10.1038/nm0896-906. [DOI] [PubMed] [Google Scholar]
- 5.Callan M F, Tan L, Annels N, Ogg G S, Wilson J D, O'Callaghan C A, Steven N, McMichael A J, Rickinson A B. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus in vivo. J Exp Med. 1998;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Z W, Kou Z C, Lekutis C, Shen L, Zhou D, Halloran M, Li J, Sodroski J, Lee-Parritz D, Letvin N L. T cell receptor V beta repertoire in an acute infection of rhesus monkeys with simian immunodeficiency viruses and a chimeric simian-human immunodeficiency virus. J Exp Med. 1995;182:21–31. doi: 10.1084/jem.182.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleury S, de Boer R J, Rizzardi G P, Wolthers K C, Otto S A, Welbon C C, Graziosi C, Knabenhans C, Soudeyns H, Bart P A, Gallant S, Corpataux J M, Gillet M, Meylan P, Schnyder P, Meuwly J Y, Spreen W, Glauser M P, Miedema F, Pantaleo G. Limited CD4+ T-cell renewal in early HIV-1 infection: effect of highly active antiretroviral therapy. Nat Med. 1998;4:794–801. doi: 10.1038/nm0798-794. [DOI] [PubMed] [Google Scholar]
- 8.Gerdes J, Lemke H, Baisch H, Wacker H H, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 9.Gorochov G, Neumann A U, Kereveur A, Parizot C, Li T, Katlama C, Karmochkine M, Raguin G, Autran B, Debre P. Perturbation of CD4+ and CD8+ T-cell repertoires during progression to AIDS and regulation of the CD4+ repertoire during antiviral therapy. Nat Med. 1998;4:215–221. doi: 10.1038/nm0298-215. [DOI] [PubMed] [Google Scholar]
- 10.Grossman Z, Herberman R B. T-cell homeostasis in HIV infection is neither failing nor blind: modified cell counts reflect an adaptive response of the host. Nat Med. 1997;3:486–490. doi: 10.1038/nm0597-486. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton-Easton A M, Christensen J P, Doherty P C. Turnover of T cells in murine gammaherpesvirus 68-infected mice. J Virol. 1999;73:7866–7869. doi: 10.1128/jvi.73.9.7866-7869.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hazenberg M D, Stuart J W, Otto S A, Borleffs J C, Boucher C A, de Boer R J, Miedema F, Hamann D. T-cell division in human immunodeficiency (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART) Blood. 2000;95:249–255. [PubMed] [Google Scholar]
- 13.Hellerstein M, Hanley M B, Cesar D, Siler S, Papageorgopoulos C, Wieder E, Schmidt D, Hoh R, Neese R, Macallan D, Deeks S, McCune J M. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat Med. 1999;5:83–89. doi: 10.1038/4772. [DOI] [PubMed] [Google Scholar]
- 14.Johnson R P, Lifson J D, Czajak S C, Cole K S, Manson K H, Glickman R, Yang J, Montefiori D C, Montelaro R, Wyand M S, Desrosiers R C. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: inverse relationship of degree of protection with level of attenuation. J Virol. 1999;73:4952–4961. doi: 10.1128/jvi.73.6.4952-4961.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaur A, Grant R M, Means R E, McClure H, Feinberg M, Johnson R P. Diverse host responses and outcomes following simian immunodeficiency virus SIVmac239 infection in sooty mangabeys and rhesus macaques. J Virol. 1998;72:9597–9611. doi: 10.1128/jvi.72.12.9597-9611.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawai T, Wong J, MacLean J, Cosimi A B, Wee S. Characterization of a monoclonal antibody (6G12) recognizing the cynomolgus monkey CD3 antigen. Transplant Proc. 1994;26:1845–1846. [PubMed] [Google Scholar]
- 17.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuroda M J, Schmitz J E, Charini W A, Nickerson C E, Lifton M A, Lord C I, Forman M A, Letvin N L. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J Immunol. 1999;162:5127–5133. [PubMed] [Google Scholar]
- 19.Lewis D E, Yang L, Luo W, Wang X, Rodgers J R. HIV-specific cytotoxic T lymphocyte precursors exist in a CD28− CD8+ T cell subset and increase with loss of CD4 T cells. AIDS. 1999;13:1029–1033. doi: 10.1097/00002030-199906180-00005. [DOI] [PubMed] [Google Scholar]
- 20.Lifson J D, Nowak M A, Goldstein S, Rossio J L, Kinter A, Vasquez G, Wiltrout T A, Brown C, Schneider D, Wahl L, Lloyd A L, Williams J, Elkins W R, Fauci A S, Hirsch V M. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J Virol. 1997;71:9508–9514. doi: 10.1128/jvi.71.12.9508-9514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez F, Belloc F, Lacombe F, Dumain P, Reiffers J, Bernard P, Boisseau M R. Modalities of synthesis of Ki67 antigen during the stimulation of lymphocytes. Cytometry. 1991;12:42–49. doi: 10.1002/cyto.990120107. [DOI] [PubMed] [Google Scholar]
- 22.Lukacher A E, Moser J M, Hadley A, Altman J D. Visualization of polyoma virus-specific CD8+ T cells in vivo during infection and tumor rejection. J Immunol. 1999;163:3369–3378. [PubMed] [Google Scholar]
- 23.Mellors J W, Rinaldo C R, Jr, Gupta P, White R M, Todd J A, Kingsley L A. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 24.Mohri H, Bonhoeffer S, Monard S, Perelson A S, Ho D D. Rapid turnover of T lymphocytes in SIV-infected rhesus macaques. Science. 1998;279:1223–1227. doi: 10.1126/science.279.5354.1223. [DOI] [PubMed] [Google Scholar]
- 25.Monteiro J, Batliwalla F, Ostrer H, Gregersen P K. Shortened telomeres in clonally expanded CD28− CD8+ T cells imply a replicative history that is distinct from their CD28+ CD8+ counterparts. J Immunol. 1996;156:3587–3590. [PubMed] [Google Scholar]
- 26.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 26a.Murphy C G, Lucas W T, Means R E, Czajak S, Hale C L, Lifson J D, Kaur A, Johnson R P, Knipe D M, Desrosiers R C. Vaccine protection against simian immunodeficiency virus by recombinant strains of herpes simplex virus. J Virol. 2000;74:7745–7754. doi: 10.1128/jvi.74.17.7745-7754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pakker N G, Notermans D W, de Boer R J, Roos M T, de Wolf F, Hill A, Leonard J M, Danner S A, Miedema F, Schellekens P T. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat Med. 1998;4:208–214. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 28.Palmer L D, Weng N-P, Levine B L, June C H, Lane H C, Hodes R J. Telomere length, telomerase activity, and replicative potential in HIV infection: analysis of CD4+ and CD8+ T cells from HIV-discordant monozygotic twins. J Exp Med. 1997;185:1381–1386. doi: 10.1084/jem.185.7.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pantaleo G, Demarest J F, Soudeyns H, Graziosi C, Denis F, Adelsberger J W, Borrow P, Saag M S, Shaw G M, Sekaly R P. Major expansion of CD8+ T cells with a predominant V beta usage during the primary immune response to HIV. Nature. 1994;370:463–467. doi: 10.1038/370463a0. [DOI] [PubMed] [Google Scholar]
- 30.Roederer M. Getting to the HAART of T cell dynamics. Nat Med. 1998;4:145–146. doi: 10.1038/nm0298-145. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg Y J, Janossy G. The importance of lymphocyte trafficking in regulating blood lymphocyte levels during HIV and SIV infections. Semin Immunol. 1999;11:139–154. doi: 10.1006/smim.1999.0169. [DOI] [PubMed] [Google Scholar]
- 32.Rosenzweig M, DeMaria M A, Harper D M, Friedrich S, Jain R K, Johnson R P. Increased rates of CD4+ and CD8+ T lymphocyte turnover in simian immunodeficiency virus-infected macaques. Proc Natl Acad Sci USA. 1998;95:6388–6393. doi: 10.1073/pnas.95.11.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sachsenberg N, Perelson A S, Yerly S, Schockmel G A, Leduc D, Hirschel B, Perrin L. Turnover of CD4+ and CD8+ T lymphocytes in HIV-1 infection as measured by Ki-67 antigen. J Exp Med. 1998;187:1295–1303. doi: 10.1084/jem.187.8.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwarting R, Gerdes J, Niehus J, Jaeschke L, Stein H. Determination of the growth fraction in cell suspensions by flow cytometry using the monoclonal antibody Ki-67. J Immunol Methods. 1986;90:65–70. doi: 10.1016/0022-1759(86)90384-4. [DOI] [PubMed] [Google Scholar]
- 35.Suryanarayana K, Wiltrout T A, Vasquez G M, Hirsch V M, Lifson J D. Plasma SIV RNA viral load determination by real-time quantification of product generation in reverse transcriptase-polymerase chain reaction. AIDS Res Hum Retrovir. 1998;14:183–189. doi: 10.1089/aid.1998.14.183. [DOI] [PubMed] [Google Scholar]
- 36.Tenner-Racz K, Stellbrink H J, van Lunzen J, Schneider C, Jacobs J P, Raschdorff B, Grosschupff G, Steinman R M, Racz P. The unenlarged lymph nodes of HIV-1-infected, asymptomatic patients with high CD4 T cell counts are sites for virus replication and CD4 T cell proliferation. The impact of highly active antiretroviral therapy. J Exp Med. 1998;187:949–959. doi: 10.1084/jem.187.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tough D F, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 38.Tripp R A, Lahti J M, Doherty P C. Laser light suicide of proliferating virus-specific CD8+ T cells in an in vivo response. J Immunol. 1995;155:3719–3721. [PubMed] [Google Scholar]
- 39.Tsurusawa M, Ito M, Zha Z, Kawai S, Takasaki Y, Fujimoto T. Cell-cycle-associated expressions of proliferating cell nuclear antigen and Ki-67 reactive antigen of bone marrow blast cells in childhood acute leukemia. Leukemia. 1992;6:669–674. [PubMed] [Google Scholar]
- 40.Veazey R S, Tham I C, Mansfield K G, DeMaria M, Forand A E, Shvetz D E, Chalifoux L V, Sehgal P K, Lackner A A. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4+ T cells are rapidly eliminated in early SIV infection in vivo. J Virol. 2000;74:57–64. doi: 10.1128/jvi.74.1.57-64.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weekes M P, Carmichael A J, Wills M R, Mynard K, Sissons J G. Human CD28− CD8+ T cells contain greatly expanded functional virus-specific memory CTL clones. J Immunol. 1999;162:7569–7577. [PubMed] [Google Scholar]
- 42.Wolthers K C, Bea G, Wisman A, Otto S A, de Roda Husman A-M, Schaft N, de Wolf F, Goudsmit J, Coutinho R A, van der Zee A G J, Meyaard L, Miedema F. T cell telomere length in HIV-1 infection: no evidence for increased CD4+ T cell turnover. Science. 1996;274:1543–1547. doi: 10.1126/science.274.5292.1543. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Z-Q, Schuler T, Zupancic M, Wietgrefe S, Staskus K A, Reimann K A, Reinhart T A, Rogan M, Cavert W, Miller C J, Veazey R S, Notermans D, Little S, Danner S A, Richman D D, Havlir D, Wong J, Jordan H L, Schacker T W, Racz P, Tenner-Racz K, Letvin N L, Wolinsky S, Haase A T. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Z-Q, Notermans D W, Sedgewick G, Cavert W, Wietgrefe S, Zupancic M, Gebhard K, Henry K, Boies L, Chen Z, Jenkins M, Mills R, McDade H, Goodwin C, Schuwirth C M, Danner S A, Haase A T. Kinetics of CD4+ T cell repopulation of lymphoid tissues after treatment of HIV-1 infection. Proc Natl Acad Sci USA. 1998;95:1154–1159. doi: 10.1073/pnas.95.3.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]