Abstract
Background and Objective
The global prevalence of diabetes mellitus has markedly risen in recent years. Consequently, there has been a rise in the number of patients with diabetes undergoing cardiac surgery. Despite the existence of national and international guidelines to improve surgical outcomes in patients with diabetes, perioperative diabetes management optimisation remains inadequate resulting in these patients experiencing increased rates of surgical morbidity and mortality. This review aims to evaluate outcomes following cardiac surgery in patients with diabetes and assess strategies to enhance preoperative and perioperative optimization and postoperative outcomes.
Methods
A comprehensive literature search was performed for articles concerning perioperative management of diabetes in patients undergoing cardiac surgery as well as postoperative complications related to diabetes in addition to interventions utilised to optimize outcomes.
Key Content and Findings
Principle findings were extracted and synthesized. Patients with diabetes undergoing cardiac surgery exhibit increased perioperative complications, higher in-hospital mortality rates and inferior long-term survival. A key facilitator is specifically poor glycemic control, with glycated haemoglobin (HbA1c) serving as a predictive marker. However, measuring preoperative HbA1c is not routine, and there is no established threshold for deferring elective surgery. Preoperatively, emphasis should be placed on lowering the patient’s HbA1c through optimized medical management and continuous glucose monitoring. Intraoperatively, continuous insulin infusion therapy is recommended alongside postoperative continuation for critically ill patients. Prompt resumption of the patient’s routine medications post-surgery is also necessary.
Conclusions
Optimal glycemic control, both pre-, intra- and perioperatively, correlates with improved outcomes that are comparable to those without diabetes. Targeted efforts are warranted for patients with diabetes undergoing cardiac surgery to ensure long-term benefits for the patients and healthcare systems.
Keywords: Diabetes, cardiac surgery, glycated haemoglobin (HbA1c), perioperative management
Introduction
Background
Diabetes mellitus prevalence has sharply risen worldwide in recent years according to data released by the International Diabetes Federation (IDF) (1). As the prevalence increases, so does the number of patients requiring cardiac surgery who suffer from diabetes. After accounting for other cardiovascular risk factors such as smoking and hypercholesterolaemia, diabetes is found to nearly double the risk of cardiovascular disease (2). In fact, when compared to age- and sex-matched individuals without diabetes, diabetic patients have a 2- to 4-fold higher risk of developing cardiovascular disease and a 2- to 5-fold higher mortality rate from cardiovascular disease (3). Amongst all patients undergoing cardiac surgery, around 30% to 40% suffer from diabetes (4).
Rationale and knowledge gap
It is widely described in the literature that surgical morbidity and mortality are elevated in patients with diabetes including poor healing and wound complications, renal dysfunction and mortality (5). These complications lead to prolonged hospital stays, higher readmission rates and higher mortality (6).
Impaired wound healing
Diabetes can affect the body’s ability to heal and recover after surgery for a variety of reasons, including microbiological, cellular, metabolic, and structural variables (7). This includes immune system dysfunction caused by uncontrolled diabetes as well as vascular damage and cellular senescence. Inadequate synthesis of extracellular matrix, imbalance in cytokines and growth factors, reduced re-epithelialization, and changes in fibroblast migration and proliferation are all contributors for increased risk of postoperative wound infection (7). Moreover, diabetes can cause microvascular and macrovascular damage leading to poor blood flow to the heart and other organs as well as contribute to the impaired wound healing and higher risk of infection. For all of these reasons, diabetes may result in longer hospital stays and increased morbidity (6).
Metabolic instability and post-aggression metabolism due to surgical stress
Diabetes can lead to fluctuations in blood glucose levels, which can complicate anesthesia management and recovery (8). This response is part of the body’s natural reaction to injury and involves a series of physiological adaptations aimed at promoting healing and recovery. Key aspects of post-aggression metabolism include increased energy demand (9). Surgical stress leads to an increased metabolic rate, requiring more energy to support the healing process. This is often referred to as hypermetabolism. The body releases stress hormones such as cortisol, catecholamines (e.g., adrenaline), and glucagon to help mobilize energy stores and increase blood glucose levels to meet heightened energy demands (9). Insulin sensitivity may decrease, leading to higher blood glucose levels. The surgical stress triggers an inflammatory response, which can further influence metabolism and energy utilization. Understanding post-aggression metabolism is crucial for optimizing patient care after surgery, as it can influence recovery times, the risk of complications, and overall outcomes. Proper nutritional support and management of metabolic responses can help mitigate negative effects and promote healing (9,10).
Cardiovascular complications
Patients with diabetes often have additional cardiovascular risk factors, such as hypertension and dyslipidemia, which can complicate surgery and the recovery period. In addition, many patients with diabetes also have other comorbidities, such as obesity or kidney disease, which can further affect surgical outcomes (11).
Addressing these factors through careful preoperative management, intraoperative monitoring, and postoperative care can help improve outcomes for diabetic patients undergoing cardiac surgery.
In view of this observation, national and international organizations have released comprehensive guidelines to enhance surgical outcomes in patients with diabetes (12-14). The management of diabetes is evolving rapidly, with many new drugs now available and enhanced technology for blood glucose measurement and insulin delivery. However, despite this, many diabetics remain poorly optimised for surgery, with elevated glycated haemoglobin (HbA1c) levels, which inevitably leads to inferior outcomes and has associated costs to healthcare systems (6,15).
Objective
The aim of this paper is to review and summarise the most recent articles looking at the outcomes of diabetic patients following cardiac surgery, with a particular focus on considering strategies and interventions that can be implemented to optimise patients preoperatively and to improve postoperative outcomes. It will also give a schematic overview of the diabetic protocols pre-, intra-, and post-operatively in diabetic patients undergoing Cardiac Surgery. We present this article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1112/rc).
Methods
We conducted a comprehensive literature review on PubMed, Medline, and Google to identify English-language articles in the medical literature documenting postoperative complications related to diabetes as well as studies suggesting interventions to optimize the outcome for diabetic patients after cardiac surgery. Additional studies were identified by analyzing the reference lists of pertinent articles and by reading the most relevant international guidelines. The search strategy summary is highlighted in Table 1.
Table 1. The search strategy summary.
| Items | Specification |
|---|---|
| Date of search | 09/07/2024 |
| Databases and other sources searched | PubMed, Medline, and Google |
| Search terms used | Diabetes, cardiac surgery, perioperative management |
| Timeframe | From 1980 to June 2024 |
| Inclusion criteria | English language |
| Selection process | Selection conducted by 2 authors independently |
How diabetes and its control impact on outcomes following cardiac surgery
As alluded to above, diabetes mellitus is an important risk factor for coronary artery disease, which is found to have a more extensive and accelerated course (16,17). However, not only does diabetes increase the likelihood of a patient developing cardiovascular disease that requires surgical intervention, but also poor glycaemic control has been demonstrated to be associated with increased morbidity and mortality after surgery (18).
Patients with diabetes undergoing cardiac surgery are found to have a higher incidence of perioperative complications and inferior long-term survival. In addition, diabetics are found to have significantly higher in-hospital mortality rates (16,17,19). However, is this simply a consequence of diabetes itself? Probably not. There is accumulating evidence highlighting that poor glycaemic control is the issue. Hyperglycaemia is thought to be the factor that impacts on recovery and outcomes. Therefore, it follows that optimising glycaemic control preoperatively may result in improving outcomes, and reduce the cost involved with dealing with postoperative complications (20).
In this regard, there are several studies that demonstrate how poorly controlled diabetes correlates with poor surgical outcomes. From a biological perspective, hyperglycemia is found to be a risk factor for impaired wound healing (21,22), postoperative sepsis (23), endothelial dysfunction (24), cerebral ischemia (25) and postoperative renal insufficiency (19,26)—all of which can be conceptually linked to the increased morbidity and mortality observed. Furthermore, the stress of surgery itself causes metabolic disturbance that alters glucose homeostasis and can lead to hyperglycaemia even in a non-diabetic patient, but this is exacerbated in patients with diabetes who have impaired intrinsic glycaemic control as mentioned before.
Clinical studies verify the impact of diabetes on outcomes. Bucerius et al. (19) demonstrated how diabetes is an independent risk factor for postoperative delirium. In their cohort study, including 16,184 patients, it was found that 10.7% of patients with diabetes exhibited postoperative delirium, vs. 7.2% of patients without diabetes. Significant differences in the occurrence of postoperative stroke were also demonstrated (5.6% in the diabetes group vs. 4.1% in the non-diabetes group). Additionally, further case series have reported that diabetes mellitus was an independent predictor for sternal instability and wound infection (27).
More specifically, hyperglycaemia as a result of poor glycaemic control is said to be the important prognostic factor in surgical patients as opposed to diabetes itself. Anderson et al. (28) evidenced that preoperative hyperglycaemia doubled the mortality rate at one year for patients following CABG compared to those with normal preoperative blood glucose levels. Gandhi et al.’s (29) retrospective study of 409 patients undergoing cardiac surgery similarly found both preoperative and intraoperative hyperglycaemia to be distinct risk factors associated with adverse events, including mortality and the development of infectious, neurological, renal, cardiac and pulmonary complications in the first 30 days postoperatively.
There is clinical evidence to support the optimisation of glycaemic control. For example, Jin et al. (1) found that maintaining strict glycaemic control in diabetic patients undergoing cardiac surgery was associated with a lower risk of atrial fibrillation [odds ratio (OR) =0.48, 95% confidence interval (CI): 0.32–0.72; P=0.01] and sternal wound infection (OR =0.28, 95% CI: 0.14–0.54; P=0.01). Similarly, Omar et al. (30) described how better glycaemic control (target of 6.0 to 8.1 mmol/L), regardless of an associated diabetes diagnosis, led to a reduction in wound infection but also length of ventilation and intensive care stay.
Postoperative levels of glucose are also indicative. In a retrospective study of 291 patients undergoing coronary artery bypass grafting (CABG), McAlister et al. (31) demonstrated that the mean blood glucose level on the first postoperative day was an independent predictor of adverse surgical outcomes (death, myocardial infarction, stroke, and septic complications). The risk of adverse outcomes increased by 17% for every 1 mmol/L increase above 6.1 mmol/L. Similarly, Fish et al. (32) found that in CABG surgery, patients who had immediate postoperative glucose levels that were above 13.9 mmol/L experienced 10 times as many complications compared to patients with postoperative blood glucose below this threshold. Complications were defined as >9 days length of stay (upper decile), readmission within 60 days, or death. Patients meeting one or more of these criteria were regarded as having a single complication.
The above findings highlight potential areas where perioperative interventions can be made to improve outcomes. It can be predicted that careful glycaemic control during the perioperative period may lead to improved outcomes for patients undergoing cardiac surgery, with decreased in-hospital mortality and morbidity (33,34).
The role of HbA1c level in predicting surgical outcomes
Measuring a patients HbA1c is a reliable predictor of glycaemic control over a period of the last 2 to 3 months and is widely used as the marker of how well a patients diabetes is controlled (18,30). According to the American Diabetes Association, an HbA1c <7% (53 mmol/mol) corresponds to good glycaemic control for a patient with diabetes (35). Preoperative HbA1c testing in diabetic patients or those at risk of postoperative hyperglycaemia is beneficial as it highlights for the medical team patients who have poor control of their diabetes and provides an opportunity for intervention (18).
Several studies and meta-analyses demonstrate how HbA1c level is correlated to an increased risk of myocardial infarction, stroke, and all-cause mortality in diabetic patients following CABG surgery (36-40). Moreover, HbA1c has also been associated with inferior outcomes following non-cardiac surgery (41-43).
According to Kim et al. (44), a preoperative HbA1c of greater than 7% (>53 mmol/mol) was identified as an independent risk factor for a short-term composite morbidity/mortality in diabetic patients undergoing CABG compared to patients in the normal HbA1c group (21% vs. 15%, P=0.04). This was despite patients in the high HbA1c group being younger, experiencing less blood loss, and needing fewer blood transfusions. Elevated preoperative HbA1c above 6.5% (48 mmol/mol) has also been associated with a higher incidence of postoperative delirium after cardiac surgery in diabetic patients (OR: 1.817, CI: 1.449–2.278; P=0.01) (45).
Lower levels of HbA1c in patients following cardiac surgery was associated with a lower risk of early and late mortality as well as a lower incidence of postoperative acute renal injury, neurologic symptoms, and wound infection when compared to higher levels, according to a study by Corazzari et al. (3). Following from these observations, Holt et al. (46) are conducting the Optimising Cardiac Surgery ouTcOmes in People with diabeteS (OCTOPuS) study in the UK on how to improve diabetic management in patients undergoing elective cardiac surgery and whether this improves the clinical outcomes. In their study, the aim is to optimise the preoperative HbA1c to less than 53 mmol/mol (<7%).
Despite all of the evidence presented above, a recent survey found that only 44% of cardiothoracic surgeons in the UK routinely measure preoperative HbA1c, and interestingly only 19% set a threshold below which they would not perform elective surgery (15). This could be explained by the fact that using HbA1c as an indicator of glycaemic control represents a challenge for cardiac surgical centres, possibly due to a lack of awareness or a more focus to prioritise other pre-operative tests to assess fitness for surgery and risk stratification (15). Indeed, glycaemic control does not feature in EuroSCORE II for example, however, it is part of the STS score.
Where preoperative HbA1c was measured by the cardiothoracic surgeons, there is a significant discrepancy about the threshold above which elective surgery would not be performed, ranging from 53 mmol/mol (7%) to 119 mmol/mol (12%) (15). In our centre, an upper limit of 69 mmol/mol (8.5%) is the threshold for performing elective cardiac surgery. This variability likely accounts for the absence of clear recommendations in national and international guidelines.
Putting all of the above together (summarized in Table 2), there is clearly good evidence that the inferior outcomes observed in patients with diabetes following cardiac surgery relates to poor glycaemic control in the pre and perioperative period rather than to the disease itself. Indeed, Navaratnarajah et al. (47) concluded that outcomes in patients with diabetes which is very well controlled have the potential to be normalised to those of patients without diabetes.
Table 2. Summary of the reported adverse clinical outcomes associated with impaired glucose control in patients undergoing cardiac surgery.
| Studies | Study objective | Study design & participants | Adverse clinical outcomes in patients with diabetes |
|---|---|---|---|
| Salomon et al. (17); The Journal of Thoracic and Cardiovascular Surgery, 1983 | To determine the short and long-term risks associated with diabetic patients undergoing coronary artery bypass grafting | Consecutive series of 3,707 patients (non-diabetic: n=3,295, diabetic treated with diet or oral medication: n=250, insulin dependent diabetic: n=162) undergoing isolated coronary artery bypass grafting | • Increased average number of grafts required due to more extensive diffuse coronary disease |
| • Increased perioperative mortality in both diabetic groups (5.1% for non-insulin-dependent diabetes, 4.5% for insulin-dependent diabetes) vs. non-diabetic patients (2.5%) | |||
| • Increased incidence of sternotomy complications (2% and 3% in the diabetic groups vs. 0.5% in the non-diabetic group) and renal insufficiency (3.1% and 3.0% in the diabetic groups vs. 0.1% in the non-diabetic group) | |||
| • Increased number of total hospital days (mean of 12.2 and 13.3 days in the diabetic groups vs. 10.7 days in the non-diabetic group) | |||
| • Lower long-term survival rate in diabetics | |||
| Bucerius et al. (19); The Journal of Thoracic and Cardiovascular Surgery, 2003 | To assess the impact of diabetes mellitus on cardiac surgery outcomes | Prospective evaluation of 16,184 patients undergoing cardiac surgery | Univariate analysis found that diabetes mellitus was an independent predictor of the following postoperative outcomes: |
| • Prolonged intensive care stay (23.9% vs. 17.7%) | |||
| • Sternal instability and/or infection (3.3% vs. 1.9%) | |||
| • Respiratory insufficiency (11.9% vs. 7.8%) | |||
| • Postoperative delirium (10.7% vs. 7.2%) | |||
| • Perioperative stroke (5.6% vs. 4.1%) | |||
| • Renal dysfunction (12.4% vs. 7.5%) | |||
| • Postoperative reintubation (8.2% vs. 5.9%) | |||
| Morricone et al. (26); Acta Diabetologica, 1999 |
To compare two homogeneous populations of patients undergoing cardiac surgery, only differing in the presence or absence of diabetes | Retrospective study of 700 patients: 350 with and 350 without diabetes | Univariate and multivariate logistic regression revealed diabetes an independent risk factor for: |
| • Increased total neurological complications (4 patients vs. 13 patients) | |||
| • Increased renal complications (5 patients vs. 20 patients) | |||
| • A higher re-opening rate (6 patients vs. 16 patients) | |||
| • More prolonged intensive care stay (188 patients vs. 223 patients) | |||
| • More blood transfusions (40 patients vs. 80 patients) | |||
| Gandhi et al. (29); Mayo Clinic Proceedings, 2005 | To estimate the relationship between intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients | Retrospective observational study of 409 consecutive patients | • The primary end point was a composite of death and various complications (infectious, neurological, renal, cardiac, and pulmonary) developing within 30 days postoperatively |
| • Higher initial, mean, and maximal intraoperative glucose concentrations were associated with patients experiencing the primary endpoint (P=0.01). This remained the case for mean and maximal glucose levels after conducting multivariable analysis | |||
| • A 20-mg/dL increase in the mean intraoperative glucose level was associated with an increase of more than 30% in outcomes by logistic regression analyses (adjusted odds ratio, 1.34; 95% confidence interval, 1.10–1.62) | |||
| Halkos et al. (38); The Annals of Thoracic Surgery, 2008 | To determine whether diabetes mellitus and HbA1c were independent risk factors for reduced long-term survival, adjusted for 29 covariates | Prospective study of 3,201 patients | • Patients with HbA1c >7% had a lower unadjusted 5-year survival (P=0.01) compared to those with HbA1c <7% |
| • Patients with diabetes mellitus had a lower unadjusted 5-year survival compared with patients without diabetes (P=0.01) | |||
| • After multivariable adjustment, a higher HbA1c was linked to reduced long-term survival for each unit increase in HbA1c (hazard ratio 1.15, P=0.01) | |||
| Sato et al. (40); The Journal of Clinical Endocrinology & Metabolism, 2010 | To determine the relationship between preoperative glycemic control, intraoperative insulin sensitivity, and adverse events after cardiac surgery | Prospective cohort study of 143 nondiabetic and 130 diabetic patients. Diabetic were grouped based on their HbA1c, good (<6.5%) or poor (>6.5%) glycemic control | • Diabetic patients with poor glycemic control were more likely to have major complications (P=0.01) and minor infections (P=0.01). They required more blood products and had an increased length of stay in the intensive care (P=0.03) and in the hospital (P=0.01) than non-diabetic patients |
| • For every 1 mg/kg/min decrease in insulin sensitivity, incidence of major complications increased (P=0.01) | |||
| Kim et al. (44); The Journal of Thoracic and Cardiovascular Surgery, 2020 | To determine the impact of preoperative HbA1c level in patients with diabetes mellitus undergoing off-pump coronary artery bypass on perioperative glycemic variability and short-term outcome | Retrospective study of 703 patients. Patients were divided into 2 groups: HbA1c <7.0% or HbA1c ≥7.0%. Primary outcome was a composite of postoperative permanent stroke, prolonged ventilation, deep sternal wound infection, renal failure, reoperation, and 30-day mortality | • There was an increased incidence of postoperative morbidity and mortality in patients with HbA1c ≥7.0% (21% vs. 15%, P=0.04) |
| • Multivariable logistic regression analysis revealed preoperative HbA1c ≥7.0% was independently associated with a composite of postoperative morbidity and mortality | |||
| Kotfis et al. (45); Neuropsychiatric Disease and Treatment, 2019 | To determine whether postoperative delirium in cardiac surgery is associated with diabetes mellitus or increased preoperative HbA1c | Prospective cohort study of 3,178 patients [1,010 (31.8%) were diabetic]. Delirium assessment was performed twice a day during the first 5 days after the operation | • Patients with delirium were more often diabetic (42.03% vs. 29.86%, P=0.01) and on oral diabetic medications (34.66% vs. 24.07%, P=0.01) |
| • More delirious than non-delirious patients had increased preoperative HbA1c (≥6%) (44.54% vs. 33.04%, P=0.01), significance reached only nondiabetic patients (20.44% vs. 14.86%, P=0.018) | |||
| • Univariate analysis revealed that diabetes was a risk factor for developing postoperative delirium (OR: 1.703, 95% CI: 1.401–2.071, P=0.01), but only for patients on oral diabetic medications (OR: 1.617, 95% CI: 1.319–1.983, P=0.01) | |||
| • There was an association between HbA1c and postoperative delirium (OR: 1.269, 95% CI: 1.161–1.387, P=0.01) | |||
| • Multivariate analysis controlled for diabetes showed that postoperative delirium was associated with preoperative HbA1c level |
HbA1c, glycated haemoglobin; OR, odds ratio; CI, confidence interval.
In the context of elective surgeries at least, there is a real opportunity for preoperative intervention and it could be argued that not doing so exposes patients at an unnecessary higher risk of harm. But what exactly can be done? Furthermore, optimisation of glycaemic control during the perioperative period should be possible for all diabetic patients regardless of urgency. However, there is a lack of studies in the literature to determin the best time interval to optimise diabetes preoperatively in particular in patients needing urgent Cardiac Surgery (i.e., patients with unstable angina, critical aortic stenosis, infective endocarditis, etc.). Further studies will be needed to determin when the harm of waiting for surgery will carry more risks than the adverse outcome due to a poorly controlled diabetes.
Potential strategies for intervention
Preoperative optimisation
Given the association between HbA1c and outcomes following cardiac surgery and the link between glycemic control in the perioperative period and outcomes, there are a number of strategies that can be adopted to optimize patients pre and perioperatively which are likely to have a positive effect on patient outcomes following cardiac surgery. Perioperative hyperglycaemia, whether the cause is known diabetes, undiagnosed diabetes or stress hyperglycaemia, is a risk factor for increased complications, length of stay and mortality across all surgical specialties (48).
In the preoperative period, the principal aim should be to lower the HbA1c of the patient. There are many strategies that can be employed to achieve this which are discussed below. Principally, it requires improved medical management. For type 2 diabetes, there are now a number of new medications that have been introduced in the last decade or so which have revolutionized the management of this condition (49). Regulatory authorities now require cardiovascular safety data before approving new antidiabetic medications, therefore there has been an increased focus on the cardiovascular effects of these new drugs (50).
Two new classes of drugs, sodium-glucose cotransporter 2 inhibitors (SGLT2 inhibitors) and glucagon-like peptide-1 receptor agonists (GLP-1 receptor agonists) have been shown to reduce events in patients with both diabetes and cardiovascular disease (51). Empagliflozin and canagliflozin, both SGLT2 inhibitors, demonstrated a significant reduction in a composite of cardiovascular death, non-fatal myocardial infarction and non-fatal stroke in the EMPA-REG OUTCOME trial and the CANVAS program, respectively in patient populations with type 2 diabetes and an increased cardiovascular risk (49). It is thought the beneficial mechanism of action is driven by haemodynamic/metabolic mechanisms of action (51). SGLT2 inhibitors act on the SGLT-2 proteins expressed in the renal proximal convoluted tubules to reduce the reabsorption of filtered glucose, decrease the renal threshold for glucose, and promote urinary glucose excretion (52). Moreover, SGLT2 inhibitors cause a mild diuretic effect (due to osmotic diuresis from glucose in the urine), leading to a reduction in blood volume and consequently in blood pressure (52). SGLT2 inhibitors have been shown to provide cardiovascular and renal protective effects, potentially due to mechanisms beyond just glucose lowering, such as reduced preload and afterload on the heart and improved cardiac function. By inhibiting the SGLT-2-dependent glucose and sodium reabsorption, they inhibit the renin-angiotensin-aldosterone system and therefore the reduced afterload (caused by arterial vasodilatation) and preload (caused by natriuresis and diuresis) is cardioprotective (52). SGLT2 inhibitors are now recommended with a Class I indication for patients with heart failure even without diabetes (53). They have shown to reduce myocardial infarction, stroke, and cardiovascular death in patients with type 2 diabetes (54) and to decrease the risk of cardiovascular hospitalization and death for heart failure in both patients with heart failure and low ejection fraction (55) as well as heart failure with preserved ejection fraction (56).
Also Liraglutide and semaglutide, GLP-1 receptor agonists, have also been shown to reduce cardiovascular-related death and/or cardiovascular events (51).
However, many diabetic patients are not on these medications and the preoperative period provides an opportunity for patients to be reviewed by their medical diabetic team so that their medication can be reviewed and optimised.
Continuous glucose monitoring is becoming routine in the care of patients with type 1 diabetes and has revolutionised their treatment. The majority of patients with type 2 diabetes periodically measure HbA1c and often inconsistently perform self-monitoring of their blood glucose (57). Continuous glucose monitoring is now also increasingly available for patients with poorly controlled type 2 diabetes. Having this continuous data encourages patients to aim for improved glycaemic control pre- and postoperatively (57). In particular, the ease of use and the frequency of data provided has the potential to revolutionize the perioperative glycemic control as described by Scrimgeour et al. (58) who looked at the use of continuous glucose monitoring also in the intensive care unit after Cardiac Surgery. There are still some limitations in terms of reliability of the data provided in the immediate postoperative period (59), but with the improvement in technology it is believed that it may transform the perioperative glycaemic management to reduce adverse outcomes related to diabetes (58).
The OCTOPuS pilot study aimed to evaluate the feasibility of implementing a manualized intervention to improve management of diabetes in patients prior to cardiothoracic surgery. The 8–12-week outpatient intervention involved an initial assessment along with a management plan comprising seven components: HbA1c management, hypertension management, lipid management, weight management, smoking cessation, exercise and support from spouses, other relatives or friends. The aim was for the OCTOPuS practitioner to implement this intervention by working with the patient via fortnightly telephone consultations after listing for surgery so that they could attain optimal clinical status prior to undergoing surgery. This intervention resulted in 9 out of the 13 participants starting a new medication (two started insulin, five started SGLT2 inhibitors and two started a GLP-1 receptor agonist). The median HbA1c was 10 mmol/mol lower [interquartile range (IQR), −13 to −3] after intervention than at baseline. A 2.5 kg reduction in body weight (IQR, −5 to −1 kg) and a reduction in waist circumference of −4.1 cm (−5.3, −2.0) were also seen. However, there were no changes observed in blood pressure or lipid level (6,46). The potential impact of this on outcomes is significant.
Five other studies looked at the impact of preoperative diabetes management on postoperative outcomes across different surgical specialties (39,59-62). The goals of the preoperative management of diabetes are summarized in Table 3.
Table 3. Goals of the preoperative period (39,46,59-62).
| Target | Preoperative management strategy |
|---|---|
| Lowering HbA1c | Emphasis on lifestyle modification |
| Monitoring glucose | |
| Optimizing diabetic medications with involvement from the diabetic and nutritional team. Particularly, use of SGLT2 inhibitors or GLP-1 receptor agonists that have demonstrated cardiovascular benefits | |
| Blood pressure | Consider starting antihypertensive medications (with preference of ACEi and ARBs) |
| Lipids | Start statin therapy when indicated |
| Weight | Input from dietitians and nutritional team for all patients with a BMI >25 kg/m2 |
| Smoking | Smoking cessation |
| Exercise | Specific exercise advice and possible prehabilitation programme |
| Support from spouses, other relatives, or friends | Involvement from these supporters in the initial and follow-up stages of the intervention |
HbA1c, glycated haemoglobin; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index.
To help achieve these goals, some cardiac surgical centres have in-house preoperative optimization programmes to facilitate these efforts, as relying on patients primary care physicians and local hospitals can prove challenging due to capacity and competing requests. The in-house diabetic optimization programs are typically multidisciplinary, involving coordination between endocrinologists, anesthesiologists, cardiologists, cardiac surgeons, and nursing staff (18,63-65). The key components and strategies commonly employed in such programs are:
Preoperative assessment: it includes a comprehensive review of the patient’s diabetic history, current medications, recent HbA1c levels, and any diabetes-related complications that can impact the surgical outcomes (e.g., nephropathy, neuropathy, cardiovascular disease).
Glycemic control optimization: to establish individualized glycemic targets for the perioperative period and adjust oral hypoglycemic agents and insulin regimens as necessary as described above.
Nutritional and lifestyle optimization: to provide tailored nutritional advice to optimize glucose control. This includes dietary adjustments to stabilize blood glucose levels and, if feasible, to implement a preoperative exercise program to improve insulin sensitivity and overall cardiovascular fitness.
Patient education and self-management: to educate patients on the importance of glycemic control before surgery and how to monitor blood glucose levels, and to encourage them to regularly monitor their blood glucose levels and report any significant deviations from target ranges.
Multidisciplinary coordination: it involves an endocrinologist in the preoperative planning, especially for patients with poorly controlled diabetes or complex cases. Regular communication among the surgical, anesthesia, cardiology, and diabetes care teams is foundamental to ensure that the glycemic management plan is cohesive and adjusted for the patient’s surgical and medical needs.
Preoperative blood glucose stabilization: for patients with significant hyperglycemia, to initiate an intravenous insulin infusion protocol to stabilize blood glucose levels before surgery, to address any electrolyte disturbances, such as hypokalemia or hyperkalemia, that can be exacerbated by insulin therapy.
Day of surgery protocols: to manage preoperative fasting periods carefully, including potential adjustments in insulin dosing to prevent hypoglycemia.
Postoperatively: the patients are then followed up in the immediate post-operative period while still inpatients and subsequently referred to the local diabetic team at discharge (see below).
The model of having such a service in-house enables focused optimization and in the long run may potentially be cost effective for the cardiac surgical centre through reducing patient complications and postoperative length of stay. There are several studies published in the literature from different centers around the world supporting the evidence that having an in-house optimization program plays a crucial role in keeping a tight perioperative glycemic control with the results of a lower incidence of postoperative complications and improved surgical outcomes (66-70).
Intraoperative optimisation
Given the evidence supporting that intraoperative glycaemic control may impact on postoperative morbidity and mortality, effort should be made to ensure optimisation of glycaemic control during surgery.
The importance of intraoperative use of insulin in diabetic patients undergoing CABG was reported by Lazar et al. (71) and consists of a modified glucose, insulin, and potassium solution to maintain a blood glucose level between 120 and 180 mg/dL. When the anaesthesia was induced, the combination of glucose, insulin, and potassium was commenced, and it was continued for 12 hours in the intensive care unit. In this study, strict glycaemic control was associated to a higher cardiac index, less need for vasopressors, lower incidence of infections (1% vs. 10%, P=0.03) and atrial fibrillation (16.6% vs. 42%; P=0.01) when compared with standard therapy. This led to a reduction of in-hospital stay (6.5±0.1 vs. 9.2±0.3 days; P=0.01) and survival advantage at 2 years (P=0.04) with lower incidence of recurrent angina (5% versus 19%; P=0.01).
Furnary et al. utilised an automated continuous intravenous insulin (CII) protocol to demonstrate that blood glucose levels that were controlled within a predetermined target range (between 100–150 mg/dL) during the perioperative period resulted in CABG mortality and rates of deep sternal wound infection (2.5% and 0.8% respectively) being normalized to those of non-diabetic patients. The protocol was initiated in the operating room and continued up to 2 days postoperatively. It involved prescribing insulin initiation, infusion and titration rates and glucose testing frequency requirements. Meanwhile, hyperglycaemia was independently predictive of increasing mortality (P<0.0001), deep sternal wound infection (P=0.02), and length of stay (P=0.01) (33).
Continuous insulin infusions are preferable compared with intermittent intravenous insulin boluses or subcutaneous insulin injections for glycaemic control (18). The best approach for perioperative insulin therapy is by intravenous administration. It enables rapid titration, making glycaemic control easier to manage (72). All diabetic patients undergoing cardiac surgery should receive continuous insulin infusion intraoperatively as well as for at least 24 hours postoperatively to maintain blood glucose levels ≤10 mmol/L (180 mg/dL) (level of evidence B) (18). Patients receiving intravenous insulin should have blood glucose monitored every 30 to 60 minutes and potassium level should be maintained between 4 and 5.5 mequiv/L (18).
Postoperative optimisation
Clinical guidelines including The Joint British Diabetes Societies, recommend the use of IV CII for the management of hyperglycaemia in critically ill patients even those without a history of diabetes (12,73-75). This is supported by findings from van den Berghe et al. (76) in which utilising intensive insulin therapy postoperatively reduced morbidity and mortality among critically ill patients, even if they had no prior diagnosis of diabetes. Additionally, Hruska et al. found that administration of a continuous insulin drip protocol with target blood glucose concentrations of 120–160 mg/dL in the immediate postoperative period resulted in a decrease in the incidence of would infection in surgical patients with diabetes (77).
Once patients progress to the ward from the critical area, it is again important to ensure optimal glycaemic control. Patients should be reinstated on their diabetic treatment as soon as possible. To aid this, most hospitals have a diabetic specialist nursing team who should be able to support ward nurses and the medical teams optimising treatment for patients—particularly helping patients who have hyperglycaemia as changes to diabetic medication doses and introduction of other medications can be performed whilst patients are in hospital and it often provides a good opportunity for these changes to be made whilst patients are being supervised with regular blood glucose monitoring.
It will be important though to ensure any changes are communicated with the patient’s primary care team and it would be advisable for patients who were found to have poor control whilst an inpatient to have early review by their local diabetic team to ensure that further treatment optimisation can be performed in the community. It is also important to counsel the patient and relatives appropriately, especially in case new medications were introduced.
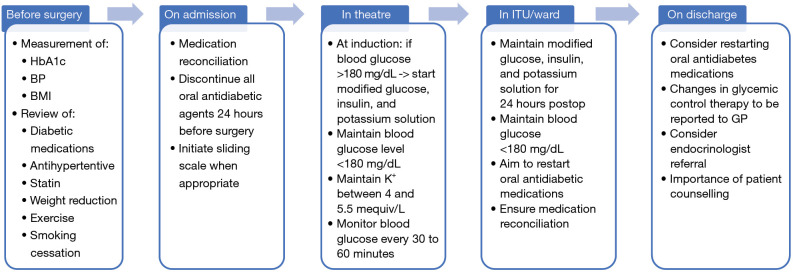
We summarized the aforementioned aspects of the suggested management of perioperative diabetes in Figure 1.
Figure 1.
Recommended pathway of perioperative management of diabetes. HbA1c, hemoglobin A1c; BP, blood pressure; BMI, body mass index; GP, general practitioner; ITU, intensive therapy unit.
Conclusions
Diabetes is becoming an increasing comorbidity for patients undergoing cardiac surgery. The evidences presented here clearly demonstrate that glycaemic control correlates with outcomes, including preoperatively and perioperatively. Additionally, it has been shown that diabetic patients with good glycaemic control can achieve similar outcomes to patients without diabetes after cardiac surgery. As such, effort should be expended on optimising diabetic patients. This includes preoperative optimisation for elective patients where real opportunity exits to help patients make important changes that can significantly improve glycaemic control as well as improved glycaemic control whilst patients are inpatients in the perioperative period.
In this paper we have presented the evidence that supports these statements and have explored strategies that can be employed by cardiac surgical centres to improve the glycaemic control of diabetic patients. These will inevitably lead to improved outcomes of these patients experiencing less morbidity and mortality.
More than that though, there is a real opportunity to make an impact on these patients’ glycaemic control which can last long beyond the perioperative period—hopefully for the remainder of the patient’s life, which may lead to reduced incidence of diabetic complications and therefore significant potential cost savings for the health care system long term.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1112/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1112/coif). J.M.A serves as an unpaid editorial board member of Journal of Thoracic Disease from October 2023 to September 2025. The other authors have no conflicts of interest to declare.
References
- 1.Jin X, Wang J, Ma Y, et al. Association Between Perioperative Glycemic Control Strategy and Mortality in Patients With Diabetes Undergoing Cardiac Surgery: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) 2020;11:513073. 10.3389/fendo.2020.513073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao Kondapally Seshasai S, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829-41. 10.1056/NEJMoa1008862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corazzari C, Matteucci M, Kołodziejczak M, et al. Impact of preoperative glycometabolic status on outcomes in cardiac surgery: Systematic review and meta-analysis. J Thorac Cardiovasc Surg 2022;164:1950-1960.e10. 10.1016/j.jtcvs.2021.05.035 [DOI] [PubMed] [Google Scholar]
- 4.Raza S, Blackstone EH, Sabik JF, 3rd. The diabetes epidemic and its effect on cardiac surgery practice. J Thorac Cardiovasc Surg 2015;150:783-4. 10.1016/j.jtcvs.2015.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biancari F, Giordano S. Glycated Hemoglobin and the Risk of Sternal Wound Infection After Adult Cardiac Surgery: A Systematic Review and Meta-Analysis. Semin Thorac Cardiovasc Surg 2019;31:465-7. 10.1053/j.semtcvs.2019.02.029 [DOI] [PubMed] [Google Scholar]
- 6.Holt RIG, Dritsakis G, Barnard-Kelly K, et al. The Optimising Cardiac Surgery ouTcOmes in People with diabeteS (OCTOPuS) randomised controlled trial to evaluate an outpatient pre-cardiac surgery diabetes management intervention: a study protocol. BMJ Open 2021;11:e050919. 10.1136/bmjopen-2021-050919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swoboda L, Held J. Impaired wound healing in diabetes. J Wound Care 2022;31:882-5. 10.12968/jowc.2022.31.10.882 [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Chen K, Li X, et al. Postoperative adverse events in patients with diabetes undergoing orthopedic and general surgery. Medicine (Baltimore) 2019;98:e15089. 10.1097/MD.0000000000015089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Şimşek T, Şimşek HU, Cantürk NZ. Response to trauma and metabolic changes: posttraumatic metabolism. Ulus Cerrahi Derg 2014;30:153-9. 10.5152/UCD.2014.2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasenboehler E, Williams A, Leinhase I, et al. Metabolic changes after polytrauma: an imperative for early nutritional support. World J Emerg Surg 2006;1:29. 10.1186/1749-7922-1-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manoharan P, Nadarajah R, Suganthan N. Assessment of Additional Risk Factors for Cardiovascular Disease and Awareness Among Adult Patients With Diabetes Mellitus: A Cross-Sectional Study From Northern Sri Lanka. Cureus 2022;14:e30047. 10.7759/cureus.30047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhatariya K, Levy N, Kilvert A, et al. NHS Diabetes guideline for the perioperative management of the adult patient with diabetes. Diabet Med 2012;29:420-33. 10.1111/j.1464-5491.2012.03582.x [DOI] [PubMed] [Google Scholar]
- 13.Lazar HL, McDonnell M, Chipkin SR, et al. The Society of Thoracic Surgeons practice guideline series: Blood glucose management during adult cardiac surgery. Ann Thorac Surg 2009;87:663-9. 10.1016/j.athoracsur.2008.11.011 [DOI] [PubMed] [Google Scholar]
- 14.O'Neill F, Carter E, Pink N, et al. Routine preoperative tests for elective surgery: summary of updated NICE guidance. BMJ 2016;354:i3292. 10.1136/bmj.i3292 [DOI] [PubMed] [Google Scholar]
- 15.Luthra S, Salhiyyah K, Dritsakis G, et al. Diabetes management during cardiac surgery in the UK: A survey. Diabet Med 2021;38:e14388. 10.1111/dme.14388 [DOI] [PubMed] [Google Scholar]
- 16.Morris JJ, Smith LR, Jones RH, et al. Influence of diabetes and mammary artery grafting on survival after coronary bypass. Circulation 1991;84:III275-84. [PubMed] [Google Scholar]
- 17.Salomon NW, Page US, Okies JE, et al. Diabetes mellitus and coronary artery bypass. Short-term risk and long-term prognosis. J Thorac Cardiovasc Surg 1983;85:264-71. [PubMed] [Google Scholar]
- 18.Arthur CPS, Mejía OAV, Lapenna GA, et al. Perioperative Management of the Diabetic Patient Referred to Cardiac Surgery. Braz J Cardiovasc Surg 2018;33:618-25. 10.21470/1678-9741-2018-0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bucerius J, Gummert JF, Walther T, et al. Impact of diabetes mellitus on cardiac surgery outcome. Thorac Cardiovasc Surg 2003;51:11-6. 10.1055/s-2003-37280 [DOI] [PubMed] [Google Scholar]
- 20.Lehwaldt D, Kingston M, O'Connor S. Postoperative hyperglycaemia of diabetic patients undergoing cardiac surgery - a clinical audit. Nurs Crit Care 2009;14:241-53. 10.1111/j.1478-5153.2009.00350.x [DOI] [PubMed] [Google Scholar]
- 21.Marhoffer W, Stein M, Maeser E, et al. Impairment of polymorphonuclear leukocyte function and metabolic control of diabetes. Diabetes Care 1992;15:256-60. 10.2337/diacare.15.2.256 [DOI] [PubMed] [Google Scholar]
- 22.McMurry JF, Jr. Wound healing with diabetes mellitus. Better glucose control for better wound healing in diabetes. Surg Clin North Am 1984;64:769-78. 10.1016/s0039-6109(16)43393-1 [DOI] [PubMed] [Google Scholar]
- 23.Rayfield EJ, Ault MJ, Keusch GT, et al. Infection and diabetes: the case for glucose control. Am J Med 1982;72:439-50. 10.1016/0002-9343(82)90511-3 [DOI] [PubMed] [Google Scholar]
- 24.Hempel A, Maasch C, Heintze U, et al. High glucose concentrations increase endothelial cell permeability via activation of protein kinase C alpha. Circ Res 1997;81:363-71. 10.1161/01.res.81.3.363 [DOI] [PubMed] [Google Scholar]
- 25.Pulsinelli WA, Levy DE, Sigsbee B, et al. Increased damage after ischemic stroke in patients with hyperglycemia with or without established diabetes mellitus. Am J Med 1983;74:540-4. 10.1016/0002-9343(83)91007-0 [DOI] [PubMed] [Google Scholar]
- 26.Morricone L, Ranucci M, Denti S, et al. Diabetes and complications after cardiac surgery: comparison with a non-diabetic population. Acta Diabetol 1999;36:77-84. 10.1007/s005920050149 [DOI] [PubMed] [Google Scholar]
- 27.Furnary AP, Zerr KJ, Grunkemeier GL, et al. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg 1999;67:352-60; discussion 360-2. 10.1016/s0003-4975(99)00014-4 [DOI] [PubMed] [Google Scholar]
- 28.Anderson RE, Brismar K, Barr G, et al. Effects of cardiopulmonary bypass on glucose homeostasis after coronary artery bypass surgery. Eur J Cardiothorac Surg 2005;28:425-30. 10.1016/j.ejcts.2005.05.025 [DOI] [PubMed] [Google Scholar]
- 29.Gandhi GY, Nuttall GA, Abel MD, et al. Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients. Mayo Clin Proc 2005;80:862-6. 10.4065/80.7.862 [DOI] [PubMed] [Google Scholar]
- 30.Omar AS, Salama A, Allam M, et al. Association of time in blood glucose range with outcomes following cardiac surgery. BMC Anesthesiol 2015;15:14. 10.1186/1471-2253-15-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McAlister FA, Man J, Bistritz L, et al. Diabetes and coronary artery bypass surgery: an examination of perioperative glycemic control and outcomes. Diabetes Care 2003;26:1518-24. 10.2337/diacare.26.5.1518 [DOI] [PubMed] [Google Scholar]
- 32.Fish LH, Weaver TW, Moore AL, et al. Value of postoperative blood glucose in predicting complications and length of stay after coronary artery bypass grafting. Am J Cardiol 2003;92:74-6. 10.1016/s0002-9149(03)00472-7 [DOI] [PubMed] [Google Scholar]
- 33.Furnary AP, Wu Y, Bookin SO. Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project. Endocr Pract 2004;10 Suppl 2:21-33. 10.4158/EP.10.S2.21 [DOI] [PubMed] [Google Scholar]
- 34.Doenst T, Wijeysundera D, Karkouti K, et al. Hyperglycemia during cardiopulmonary bypass is an independent risk factor for mortality in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg 2005;130:1144. 10.1016/j.jtcvs.2005.05.049 [DOI] [PubMed] [Google Scholar]
- 35.American Diabetes Association . 8. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018;41:S73-85. 10.2337/dc18-S008 [DOI] [PubMed] [Google Scholar]
- 36.Rollins KE, Varadhan KK, Dhatariya K, et al. Systematic review of the impact of HbA1c on outcomes following surgery in patients with diabetes mellitus. Clin Nutr 2016;35:308-16. 10.1016/j.clnu.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 37.Zheng J, Cheng J, Wang T, et al. Does HbA1c Level Have Clinical Implications in Diabetic Patients Undergoing Coronary Artery Bypass Grafting? A Systematic Review and Meta-Analysis. Int J Endocrinol 2017;2017:1537213. 10.1155/2017/1537213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halkos ME, Lattouf OM, Puskas JD, et al. Elevated preoperative hemoglobin A1c level is associated with reduced long-term survival after coronary artery bypass surgery. Ann Thorac Surg 2008;86:1431-7. 10.1016/j.athoracsur.2008.06.078 [DOI] [PubMed] [Google Scholar]
- 39.Lee GA, Wyatt S, Topliss D, et al. A study of a pre-operative intervention in patients with diabetes undergoing cardiac surgery. Collegian 2014;21:287-93. 10.1016/j.colegn.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 40.Sato H, Carvalho G, Sato T, et al. The association of preoperative glycemic control, intraoperative insulin sensitivity, and outcomes after cardiac surgery. J Clin Endocrinol Metab 2010;95:4338-44. 10.1210/jc.2010-0135 [DOI] [PubMed] [Google Scholar]
- 41.Gustafsson UO, Thorell A, Soop M, et al. Haemoglobin A1c as a predictor of postoperative hyperglycaemia and complications after major colorectal surgery. Br J Surg 2009;96:1358-64. 10.1002/bjs.6724 [DOI] [PubMed] [Google Scholar]
- 42.Goodenough CJ, Liang MK, Nguyen MT, et al. Preoperative Glycosylated Hemoglobin and Postoperative Glucose Together Predict Major Complications after Abdominal Surgery. J Am Coll Surg 2015;221:854-61.e1. 10.1016/j.jamcollsurg.2015.07.013 [DOI] [PubMed] [Google Scholar]
- 43.Sreedharan R, Khanna S, Shaw A. Perioperative glycemic management in adults presenting for elective cardiac and non-cardiac surgery. Perioper Med (Lond) 2023;12:13. 10.1186/s13741-023-00302-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim HJ, Shim JK, Youn YN, et al. Influence of preoperative hemoglobin A1c on early outcomes in patients with diabetes mellitus undergoing off-pump coronary artery bypass surgery. J Thorac Cardiovasc Surg 2020;159:568-76. 10.1016/j.jtcvs.2019.01.086 [DOI] [PubMed] [Google Scholar]
- 45.Kotfis K, Szylińska A, Listewnik M, et al. Diabetes and elevated preoperative HbA1c level as risk factors for postoperative delirium after cardiac surgery: an observational cohort study. Neuropsychiatr Dis Treat 2019;15:511-21. 10.2147/NDT.S196973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holt RIG, Barnard-Kelly K, Dritsakis G, et al. Developing an intervention to optimise the outcome of cardiac surgery in people with diabetes: the OCTOPuS pilot study. Pilot Feasibility Stud 2021;7:157. 10.1186/s40814-021-00887-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Navaratnarajah M, Rea R, Evans R, et al. Effect of glycaemic control on complications following cardiac surgery: literature review. J Cardiothorac Surg 2018;13:10. 10.1186/s13019-018-0700-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levy N, Dhatariya K. Pre-operative optimisation of the surgical patient with diagnosed and undiagnosed diabetes: a practical review. Anaesthesia 2019;74 Suppl 1:58-66. 10.1111/anae.14510 [DOI] [PubMed] [Google Scholar]
- 49.Marx N, Davies MJ, Grant PJ, et al. Guideline recommendations and the positioning of newer drugs in type 2 diabetes care. Lancet Diabetes Endocrinol 2021;9:46-52. 10.1016/S2213-8587(20)30343-0 [DOI] [PubMed] [Google Scholar]
- 50.Florentin M, Kostapanos MS, Papazafiropoulou AK. Role of dipeptidyl peptidase 4 inhibitors in the new era of antidiabetic treatment. World J Diabetes 2022;13:85-96. 10.4239/wjd.v13.i2.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sattar N, Petrie MC, Zinman B, et al. Novel Diabetes Drugs and the Cardiovascular Specialist. J Am Coll Cardiol 2017;69:2646-56. 10.1016/j.jacc.2017.04.014 [DOI] [PubMed] [Google Scholar]
- 52.Padda IS, Mahtani AU, Parmar M. Sodium-Glucose Transport Protein 2 (SGLT2) Inhibitors. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 [cited 2024 Sep 1]. Available online: http://www.ncbi.nlm.nih.gov/books/NBK576405/ [PubMed] [Google Scholar]
- 53.Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N Engl J Med 2022;387:1089-98. 10.1016/j.jchf.2021.11.006 [DOI] [PubMed] [Google Scholar]
- 54.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019;393:31-9. 10.1016/S0140-6736(18)32590-X [DOI] [PubMed] [Google Scholar]
- 55.Monzo L, Ferrari I, Cicogna F, et al. Sodium-glucose co-transporter-2 inhibitors eligibility in patients with heart failure with reduced ejection fraction. Int J Cardiol 2021;341:56-9. 10.1016/j.ijcard.2021.08.035 [DOI] [PubMed] [Google Scholar]
- 56.Pabel S, Hamdani N, Singh J, et al. Potential Mechanisms of SGLT2 Inhibitors for the Treatment of Heart Failure With Preserved Ejection Fraction. Front Physiol 2021;12:752370. 10.3389/fphys.2021.752370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bergenstal RM, Mullen DM, Strock E, et al. Randomized comparison of self-monitored blood glucose (BGM) versus continuous glucose monitoring (CGM) data to optimize glucose control in type 2 diabetes. J Diabetes Complications 2022;36:108106. 10.1016/j.jdiacomp.2021.108106 [DOI] [PubMed] [Google Scholar]
- 58.Scrimgeour LA, Potz BA, Sellke FW, et al. Continuous Glucose Monitoring in the Cardiac ICU: Current Use and Future Directions. Clin Med Res (N Y) 2017;6:173-6. [PMC free article] [PubMed] [Google Scholar]
- 59.Underwood P, Seiden J, Carbone K, et al. Early Identification of Individuals with Poorly Controlled Diabetes Undergoing Elective Surgery: Improving A1C Testing in the Preoperative Period. Endocr Pract 2015;21:231-6. 10.4158/EP14228.OR [DOI] [PubMed] [Google Scholar]
- 60.Garg R, Schuman B, Bader A, et al. Effect of Preoperative Diabetes Management on Glycemic Control and Clinical Outcomes After Elective Surgery. Ann Surg 2018;267:858-62. 10.1097/SLA.0000000000002323 [DOI] [PubMed] [Google Scholar]
- 61.Mendez CE, Wainaina N, Walker RJ, et al. Preoperative Diabetes Optimization Program. Clin Diabetes 2018;36:68-71. 10.2337/cd17-0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knight JB, Subramanian H, Sultan I, et al. Prehabilitation of Cardiac Surgical Patients, Part 1: Anemia, Diabetes Mellitus, Obesity, Sleep Apnea, and Cardiac Rehabilitation. Semin Cardiothorac Vasc Anesth 2022;26:282-94. 10.1177/10892532221121118 [DOI] [PubMed] [Google Scholar]
- 63.ElSayed NA, Aleppo G, Aroda VR, et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023;46:S19-40. 10.2337/dc23-S002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Engelman DT, Ben Ali W, Williams JB, et al. Guidelines for Perioperative Care in Cardiac Surgery: Enhanced Recovery After Surgery Society Recommendations. JAMA Surg 2019;154:755-66. 10.1001/jamasurg.2019.1153 [DOI] [PubMed] [Google Scholar]
- 65.Thongsuk Y, Hwang NC. Perioperative Glycemic Management in Cardiac Surgery: A Narrative Review. J Cardiothorac Vasc Anesth 2024;38:248-67. 10.1053/j.jvca.2023.08.149 [DOI] [PubMed] [Google Scholar]
- 66.Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg 2003;125:1007-21. 10.1067/mtc.2003.181 [DOI] [PubMed] [Google Scholar]
- 67.Golden SH, Peart-Vigilance C, Kao WH, et al. Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes. Diabetes Care 1999;22:1408-14. 10.2337/diacare.22.9.1408 [DOI] [PubMed] [Google Scholar]
- 68.Smiley DD, Umpierrez GE. Perioperative glucose control in the diabetic or nondiabetic patient. South Med J 2006;99:580-9; quiz 590–1. 10.1097/01.smj.0000209366.91803.99 [DOI] [PubMed] [Google Scholar]
- 69.Galindo RJ, Fayfman M, Umpierrez GE. Perioperative Management of Hyperglycemia and Diabetes in Cardiac Surgery Patients. Endocrinol Metab Clin North Am 2018;47:203-22. 10.1016/j.ecl.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Draznin B, Gilden J, Golden SH, et al. Pathways to quality inpatient management of hyperglycemia and diabetes: a call to action. Diabetes Care 2013;36:1807-14. 10.2337/dc12-2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lazar HL, Chipkin SR, Fitzgerald CA, et al. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation 2004;109:1497-502. 10.1161/01.CIR.0000121747.71054.79 [DOI] [PubMed] [Google Scholar]
- 72.Friedberg SJ, Lam YW, Blum JJ, et al. Insulin absorption: a major factor in apparent insulin resistance and the control of type 2 diabetes mellitus. Metabolism 2006;55:614-9. 10.1016/j.metabol.2005.12.004 [DOI] [PubMed] [Google Scholar]
- 73.Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009;32:1119-31. 10.2337/dc09-9029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grant B, Chowdhury TA. New guidance on the perioperative management of diabetes. Clin Med (Lond) 2022;22:41-4. 10.7861/clinmed.2021-0355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC); European Association for the Study of Diabetes (EASD); Rydén L, et al. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD - summary. Diab Vasc Dis Res 2014;11:133-73. [DOI] [PubMed]
- 76.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001;345:1359-67. 10.1056/NEJMoa011300 [DOI] [PubMed] [Google Scholar]
- 77.Hruska LA, Smith JM, Hendy MP, et al. Continuous insulin infusion reduces infectious complications in diabetics following coronary surgery. J Card Surg 2005;20:403-7. 10.1111/j.1540-8191.2005.200472.x [DOI] [PubMed] [Google Scholar]