Abstract
Background
The American College of Surgeons Commission on Cancer (CoC) revised operative quality standards recommending resection of lymph nodes from at least one hilar station and three different mediastinal stations in all curative-intent pulmonary resections. This study evaluated the prognostic value and factors associated with adherence to this new CoC standard in patients with resected clinical stage IA non-small cell lung cancer (NSCLC).
Methods
Retrospective review of 654 patients who underwent pulmonary resection for clinical IA NSCLC. The study population was divided into patients that met and did not meet the CoC standard.
Results
The CoC standard was met in only 254 (38.8%) patients. Factors associated with meeting the CoC standard included left-sided resections, open technique, and type of pulmonary resection. CoC standard was met in 51.6% of lobectomies, 29.9% of segmentectomies, and 17.1% of wedge resections (P<0.001). Nodal upstaging was more frequent in patients meeting the CoC standard (21.3% vs. 12.5% when standard not met; P=0.004). Time to recurrence [adjusted hazard ratio (aHR): 0.86, 95% confidence interval (CI): 0.63–1.17, P=0.33] and overall survival (aHR: 0.78, 95% CI: 0.58–1.05, P=0.10) were not different between CoC standard groups. However, patients not meeting the CoC standard and classified as pN0 exhibited an overall survival that resembled that of patients with pN1 disease.
Conclusions
Left-sided resections, open technique and lobectomy were associated with meeting the CoC standard. However, this standard did not have a significant impact on long-term outcomes. Larger studies with longer follow-up are needed to clarify the role of the CoC standard in patients with resected stage IA NSCLC.
Keywords: Non-small cell lung carcinoma, lung neoplasms, lymph nodes
Highlight box.
Key findings
• Resecting lymph nodes from at least 3 mediastinal and 1 hilar station in clinical stage IA non-small cell lung cancer (NSCLC) is affected by approach, type and laterality of lung resection.
• Resecting lymph nodes from at least 3 mediastinal and 1 hilar station is associated with nodal upstaging but not with recurrence or overall survival.
• However, patients not having at least 3 mediastinal and 1 hilar station lymph nodes collected during surgery and subsequently classified as pN0 seem to have worse overall survival.
What is known and what is new?
• The Commission on Cancer (CoC) revised operative quality standards recommending resection of lymph nodes from at least one hilar station and three different mediastinal stations in all curative-intent pulmonary resections.
• This manuscript investigates the prognostic value of meeting this standard in patients with clinical stage IA lung cancer and the clinical factors associated with standard compliance.
What is the implication, and what should change now?
• Meeting the CoC standard for lymph node harvest results in higher nodal upstaging. Surgeons should strive to maintain this standard when clinically appropriate in every operation.
Introduction
The importance of routine intraoperative lymph node evaluation as part of pulmonary resections for the treatment of lung cancer is a well-established concept. Nevertheless, the extent of node removal has been an ongoing debate. Guidelines have been proposed to help surgeons in the intraoperative node harvesting process (1), but standardization has been elusive. The ACOSOG Z0030 trial standardized the definition of a complete mediastinal lymphadenectomy during pulmonary resections (2), describing removal of one or more lymph nodes from stations 2, 4, 7, 8, and 9 on the right side and 5, 6, 7, 8, and 9 on the left side, in addition to hilar and intrapulmonary lymph nodes (3). The ACOSOG Z0030 trial failed to prove an overall survival advantage of radical mediastinal lymphadenectomy versus sampling but reinforced the value of lymph node evaluation for correct intraoperative lymph node staging (4). However, retrospective data from the SEER Database showed that only 62% of lung cancer resections were combined with mediastinal lymph node examination and that this impacted long term survival (5). Therefore, the extent of lymph node harvesting during pulmonary resection has been studied as a quality metric.
In an effort to establish quality standards in the surgical treatment of non-small cell lung cancer (NSCLC), the American College of Surgeons Commission on Cancer (CoC) initially required the examination of at least 10 lymph nodes during pulmonary resections (6). Although proven in some retrospective studies (7), the prognostic value of this metric was controversial. Recently, the CoC has revised the operative quality standards for the surgical treatment of NSCLC (standard 5.8) (8). This new CoC standard requires that all curative-intent pulmonary resections for NSCLC, small cell carcinoma, and carcinoid tumors resect lymph nodes from at least one numbered hilar station (N1) and at least three differently numbered mediastinal stations (N2). This new standard aligns with recommendations from the National Comprehensive Cancer Network (NCCN) (9), which has been associated with improvements in long-term survival (10,11). The CoC, a quality program of the American College of Surgeons, has included this in its Optimal Resources for Cancer Care 2020 standards, that have been updated in February of 2024 (12). Adherence to this standard will have implications in the CoC accreditation process.
Prior studies on the evaluation of lymph nodes during pulmonary resections have failed to present granular data on specific stations or patterns of lymph node harvesting. Moreover, most studies have included all resectable NSCLC stages in their analyses. With an increase in detection of clinical stage IA NSCLC (<3 cm) secondary to adoption of lung cancer screening (13), it is unclear whether lymph node evaluation standards have the same impact in this population. The present study evaluated the prognostic value of adherence to the new CoC standard in patients who underwent resection of clinical stage IA NSCLC. Secondarily, we looked at factors associated with meeting the CoC standard by analyzing detailed data on intraoperative lymph node evaluation. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-971/rc).
Methods
Study population
This study included all patients with clinical stage IA pulmonary adenocarcinoma and squamous cell carcinoma who underwent curative-intent surgery at Mayo Clinic in Rochester, MN from January 1, 2005 until December 31, 2014. Patients with a prior history of lung cancer, histologies other than adenocarcinoma or squamous cell carcinoma, patients who received neoadjuvant therapy, resections larger than lobectomy, and non-R0 resections were excluded.
Pre-operative evaluation of lymph nodes was primarily based on imaging and left to the discretion of the individual surgeon. Operative evaluation and extent of lymph node removal was also at the discretion of the individual surgeon. To note, our institution participated of ACOSOG Z0030, and some patients included in this retrospective analysis were subjected to randomization between lymph node sampling versus dissection as part of that trial.
The database was reviewed for demographics, comorbidities, clinical characteristics, operative procedures, pathologic characteristics, and follow-up data. Data on the number of resected lymph nodes and detailed data on individual mediastinal (stations 2–9) and hilar (stations 10–14) lymph node stations sampled were collected. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Mayo Clinic Institutional Review Board (protocol #17-010802) and individual consent for this retrospective analysis was waived due to the retrospective nature.
Statistical analysis and study endpoints
REDCap (Research Electronic Data Capture) tools hosted at Mayo Clinic were used to store data obtained from electronic medical records. REDCap represents a secure means by which data capture can be performed for research studies. Stata/SE 13 (StataCorp LP, College Station, TX, USA) was used to perform the statistical analysis after data were exported. Variables were analyzed as proportions, means or medians according to their nature.
The study population was divided into two groups according to the CoC Standard 5.8. Patients who had ≥1 numbered hilar lymph node stations (stations 10–14) and ≥3 numbered mediastinal lymph node stations (stations 2–9) were classified as meeting the CoC standard, while patients with <1 hilar station or <3 mediastinal stations were classified as not meeting the standard. Involvement of carcinoma in any lymph nodes on final pathology defined nodal upstaging (i.e., pN1-3). Time to recurrence was defined as the months from the date of surgery to the first documented evidence of tumor recurrence or last known follow-up. Overall survival was defined as the time in months from the date of surgery to death by any cause or last known follow-up.
Categorical variables were compared using Fisher’s exact test. Continuous variables were compared using Student’s t-test or the Mann-Whitney U test. Factors associated with meeting the CoC standard were analyzed using logistic regression. The impact of the CoC standard on recurrence and overall survival was analyzed using the Kaplan-Meier method with log-rank tests and the Cox proportional hazards method. For all comparisons, statistical significance was considered with a two-sided P value ≤0.05.
Results
Baseline characteristics and surgical treatment
Of 654 patients that underwent pulmonary resection for the treatment of clinical stage IA NSCLC, 254 (38.8%) patients met the CoC standard and 400 (61.2%) patients did not. Baseline characteristics of the entire cohort and comparisons between groups are shown in Table 1. During the study period, patients meeting the CoC standard more frequently had a preoperative tissue diagnosis and a positron emission tomography (PET) scan as part of preoperative staging. There were no differences in the rates of preoperative invasive mediastinal staging between groups. Regarding tumor characteristics, there were no differences in tumor size or histology between groups. However, left-sided tumors were observed more frequently in patients meeting the CoC standard (Table 1).
Table 1. Baseline characteristics and surgical treatment according to the Commission on Cancer standard.
| Variable | All (n=654) | No CoC standard (n=400) | CoC standard met (n=254) | P value |
|---|---|---|---|---|
| Age (years) | 69.4±9.6 | 69.6±9.8 | 69.2±9.3 | 0.63 |
| Gender | 0.63 | |||
| Male | 305 (46.6) | 190 (47.5) | 115 (45.3) | |
| Female | 349 (53.4) | 210 (52.5) | 139 (54.7) | |
| BMI (kg/m2) | 27.7±8.9 | 27.9±8.9 | 27.3±9.0 | 0.39 |
| Smoking history | 0.56 | |||
| Never | 112 (17.1) | 69 (17.3) | 43 (16.9) | |
| Former | 418 (63.9) | 253 (63.3) | 165 (65.0) | |
| Current | 120 (18.4) | 74 (18.5) | 46 (18.1) | |
| Missing | 4 (0.6) | 4 (1.0) | 0 (0.0) | |
| Zubrod score | 0.77 | |||
| 0 | 490 (74.9) | 301 (75.3) | 189 (74.4) | |
| 1 | 157 (24.0) | 94 (23.5) | 63 (24.8) | |
| 2 | 2 (0.3) | 2 (0.5) | 0 (0.0) | |
| 3 | 1 (0.2) | 1 (0.3) | 0 (0.0) | |
| 4 | 1 (0.2) | 0 (0.0) | 1 (0.4) | |
| Missing | 3 (0.5) | 2 (0.5) | 1 (0.4) | |
| Comorbidities | ||||
| Hypertension | 292 (44.7) | 181 (45.3) | 111 (43.7) | 0.75 |
| COPD | 142 (21.7) | 93 (23.3) | 49 (19.3) | 0.24 |
| Diabetes | 87 (13.3) | 57 (14.3) | 30 (11.8) | 0.41 |
| Coronary artery disease | 145 (22.2) | 97 (24.3) | 48 (18.9) | 0.12 |
| Myocardial infarction | 46 (7.0) | 29 (7.3) | 17 (6.7) | 0.88 |
| Congestive heart failure | 17 (2.6) | 10 (2.5) | 7 (2.8) | 0.81 |
| Peripheral vascular disease | 44 (6.7) | 28 (7.0) | 16 (6.3) | 0.87 |
| Cerebrovascular disease | 19 (2.9) | 10 (2.5) | 9 (3.5) | 0.48 |
| Prior chest surgery | 114 (17.4) | 70 (17.5) | 44 (17.3) | 1.00 |
| Preoperative needle biopsy | 207 (31.7) | 114 (28.5) | 93 (36.6) | 0.03 |
| Preoperative staging methods | ||||
| PET/PET-CT | 544 (83.2) | 321 (80.3) | 223 (87.8) | 0.01 |
| Invasive mediastinal staging | 231 (35.3) | 135 (33.8) | 96 (37.8) | 0.31 |
| Brain MRI | 80 (12.2) | 47 (11.8) | 33 (13.0) | 0.63 |
| Tumor size (cm) | 0.10 | |||
| <2 | 448 (68.5) | 284 (71.0) | 164 (64.6) | |
| 2–3 | 206 (31.5) | 116 (29.0) | 90 (35.4) | |
| Tumor laterality | 0.002 | |||
| Right | 387 (59.2) | 256 (64.0) | 131 (51.6) | |
| Left | 267 (40.8) | 144 (36.0) | 123 (48.4) | |
| Tumor location | 0.03 | |||
| RUL | 237 (36.2) | 160 (40.0) | 77 (30.3) | |
| RML | 32 (4.9) | 20 (5.0) | 12 (4.7) | |
| RLL | 118 (18.0) | 76 (19.0) | 42 (16.5) | |
| LUL | 166 (25.4) | 89 (22.3) | 77 (30.3) | |
| LLL | 101 (15.4) | 55 (13.8) | 46 (18.1) | |
| Histology | 0.64 | |||
| Adenocarcinoma | 492 (75.2) | 298 (74.5) | 194 (76.4) | |
| Squamous cell carcinoma | 162 (24.8) | 102 (25.5) | 60 (23.6) | |
| Surgical approach | <0.001 | |||
| Minimally invasive | 371 (56.7) | 257 (64.3) | 114 (44.9) | |
| Open | 283 (43.3) | 143 (35.8) | 140 (55.1) | |
| Type of lung resection | <0.001 | |||
| Wedge | 199 (30.4) | 165 (41.3) | 34 (13.4) | |
| Segmentectomy | 67 (10.2) | 47 (11.8) | 20 (7.9) | |
| Lobectomy | 388 (59.3) | 188 (47.0) | 200 (78.7) | |
| Postoperative complications | ||||
| Pneumothorax requiring intervention | 5 (0.8) | 3 (0.8) | 2 (0.8) | >0.99 |
| Bleeding | 1 (0.2) | 0 (0) | 1 (0.4) | 0.39 |
| Pneumonia | 15 (2.3) | 10 (2.5) | 5 (2.0) | 0.79 |
| Chylothorax | 10 (1.5) | 3 (0.8) | 7 (2.8) | 0.052 |
| Recurrent laryngeal nerve injury | 2 (0.3) | 2 (0.5) | 0 (0.0) | 0.52 |
| Atrial arrhythmia | 45 (6.9) | 26 (6.5) | 19 (7.5) | 0.64 |
| Pulmonary embolism | 2 (0.3) | 1 (0.3) | 1 (0.4) | >0.99 |
| Prolonged air leak | 60 (9.2) | 29 (7.3) | 31 (12.2) | 0.04 |
| Chest tube drainage (days) | 3 [2–5] | 3 [2–5] | 4 [3–6] | <0.001 |
| Length of stay (days) | 5 [3–7] | 4 [3–6] | 5 [4–7] | <0.001 |
Data are presented as mean ± SD, n (%) or median [IQR]. CoC standard, Commission on Cancer standard 5.8; BMI, body mass index; COPD, chronic obstructive pulmonary disease; PET, positron emission tomography; CT, computed tomography; MRI, magnetic resonance imaging; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe; SD, standard deviation; IQR, interquartile range.
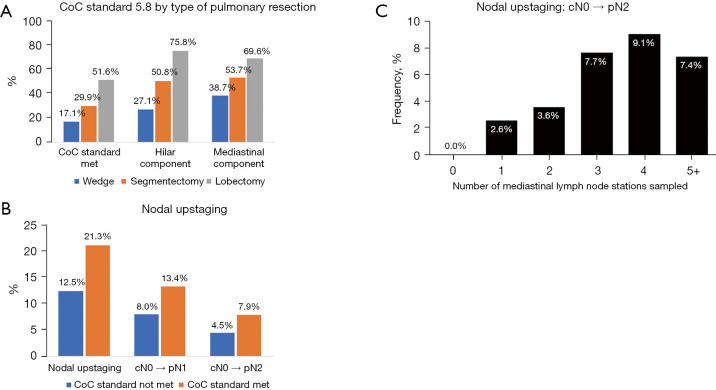
Minimally invasive approaches were selected in 371 (56.7%) patients (Table 1). However, only 114 (30.7%) of these patients met the CoC standard. In contrast, 140 of 283 (49.5%) patients having an open approach met the standard. Type of pulmonary resection was significantly associated with meeting the CoC standard with increasing compliance observed with larger resections (Table 1 and Figure 1A). Overall, wedge resections showed low rates of both the hilar and mediastinal lymph node harvest.
Figure 1.
Evaluation of meeting the CoC standard and rates of nodal upstaging. (A) Rates of CoC standard compliance according to type of pulmonary resection. (B) Lymph node upstaging according to CoC standard compliance and (C) number of N2 stations harvested. CoC, Commission on Cancer.
Postoperative complications are shown in Table 1. We found a higher rate of prolonged air leaks in patients meeting the CoC standard, and a non-statistically significant higher rate of chylothorax. Other complications that could be attributed to a more extensive lymph node dissection such as bleeding, recurrent laryngeal nerve injury, and atrial arrhythmia were not different between groups. The duration of chest drainage and length of stay were longer for patients meeting the CoC standard.
Lymph node evaluation and factors associated with meeting the CoC standard
The total number of resected lymph nodes and the number of lymph node stations sampled were significantly higher in patients meeting the CoC (Table 2). In patients who met the CoC standard, 159 (62.6%) had 3 mediastinal stations sampled, 76 (29.9%) had 4, and 19 (7.5%) had 5 or more. For hilar lymph node stations, 144 (56.7%) patients had 1 station, 102 (40.2%) had 2, and 8 (3.2%) had 3. Among patients not meeting the CoC standard, direct reasons included the harvest of <1 N1 station in 272 (68.0%) patients or harvest of <3 N2 stations in 271 (67.8%) patients. There were 128 patients (32.0%) that met the hilar component but failed to meet the mediastinal component. Of these, 101 (78.9%) patients had 2 N2 stations sampled, 23 (18.0%) had 1, and 4 (3.1%) had none.
Table 2. Lymph node evaluation and pathological characteristics according to the Commission on Cancer standard.
| Variable | All (n=654) | No CoC standard (n=400) | CoC standard met (n=254) | P value |
|---|---|---|---|---|
| Pathologic T stage | 0.48 | |||
| pT1 | 414 (63.3) | 259 (64.8) | 155 (61.0) | |
| pT2 | 183 (28.0) | 110 (27.5) | 73 (28.7) | |
| pT3 | 28 (4.3) | 17 (4.3) | 11 (4.3) | |
| pT4 | 29 (4.4) | 14 (3.5) | 15 (5.9) | |
| Pathologic N stage | 0.01 | |||
| pN0 | 550 (84.1) | 350 (87.5) | 200 (78.7) | |
| pN1 | 66 (10.1) | 32 (8.0) | 34 (13.4) | |
| pN2 | 38 (5.8) | 18 (4.5) | 20 (7.9) | |
| Pathologic M stage | 0.65 | |||
| pM0 | 649 (99.2) | 396 (99.0) | 253 (99.6) | |
| pM1 | 5 (0.8) | 4 (1.0) | 1 (0.4) | |
| High-risk features | ||||
| Visceral pleural invasion | 149 (22.8) | 91 (22.8) | 58 (22.8) | >0.99 |
| Lymphovascular invasion | 41 (6.3) | 24 (6.0) | 17 (6.7) | 0.74 |
| Lymph node evaluation | ||||
| Number of resected lymph nodes | 5 [3–7] | 3 [2–5] | 6 [5–8] | <0.001 |
| Number of lymph node stations sampled | 4 [3–5] | 3 [2–4] | 5 [4–6] | <0.001 |
| Number of N1 stations sampled | 1 [0–1] | 0 [0–1] | 1 [1–2] | <0.001 |
| Number of N2 stations sampled | 3 [2–3] | 2 [1–3] | 3 [3–4] | <0.001 |
| One or more N1 stations | 382 (58.4) | 128 (32.0) | 254 (100.0) | <0.001 |
| Three or more N2 stations | 383 (58.6) | 129 (32.3) | 254 (100.0) | <0.001 |
| Nodal upstaging | 104 (15.9) | 50 (12.5) | 54 (21.3) | 0.004 |
Data are presented as n (%) or median [IQR]. CoC, Commission on Cancer; IQR, interquartile range.
Overall, node upstaging was significantly more frequent in patients meeting the CoC standard (21.3% vs. 12.5% when not meeting the standard; P=0.004), with higher rates of both pN1 and pN2 disease (Figure 1B). Overall, there was a direct correlation between the number of mediastinal stations sampled and upstaging to pN2 disease (Figure 1C). The same was not true for hilar lymph nodes and upstaging to pN1 disease (Figure S1). Nodal upstaging was observed in 22/129 (17.1%) patients meeting the mediastinal component but not the hilar component of the standard, 21/128 (16.4%) patients meeting the hilar component but not the mediastinal component, and in 7/143 (4.9%) patients no meeting either of the components of the standard. Among 128 patients that met the hilar component of the standard but failed to meet the mediastinal component, nodal upstaging and pN2 disease was observed in 16/101 (15.8%) and 6/101 (5.9%) patients with two N2 stations sampled, 5/23 (21.7%) and 0/23 (0%) with one, and 0/4 and 0/4 in patients with no N2 nodes sampled, respectively.
Factors associated with a higher likelihood of meeting the CoC standard included a preoperative tissue diagnosis, left-sided resections, open approach, and anatomical pulmonary resections (Table 3). However, only laterality, open approach, and lobectomy remained associated with the CoC standard after multivariable analysis. The rates of meeting the CoC standard and the types of pulmonary resections performed over the study period are shown in Figures S2,S3.
Table 3. Risk factor analysis for meeting the Commission on Cancer standard.
| Variable | Univariate | Multivariable | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Preoperative needle biopsy (yes vs. no) | 1.45 (1.04–2.03) | 0.030 | 1.37 (0.96–1.97) | 0.08 | |
| Laterality (left vs. right) | 1.67 (1.21–2.30) | 0.002 | 1.70 (1.21–2.40) | 0.002 | |
| Technique (open vs. minimally invasive) | 2.21 (1.60–3.04) | <0.001 | 1.57 (1.11–2.23) | 0.01 | |
| Type of resection | |||||
| Wedge | 1.00 | 1.00 | |||
| Segmentectomy | 2.07 (1.09–3.92) | 0.026 | 1.68 (0.87–3.24) | 0.12 | |
| Lobectomy | 5.16 (3.39–7.85) | <0.001 | 4.55 (2.95–7.04) | <0.001 | |
OR, odds ratio; CI, confidence interval.
The effect of pulmonary resection laterality is further shown in Table S1. There was a higher number of lymph node stations sampled in left-sided resections by virtue of a higher number of N2 stations sampled. The rates of sampling of each nodal station according to laterality of resection are shown in Figure S4. When looking at mediastinal stations potentially accessible from both sides, harvest of station 4 was significantly more frequent in right-sided resections (right 317/387, 81.9% vs. left 93/267, 34.8%; P<0.001), there were similar rates for station 7 (right 320/387, 82.7% vs. left 207/267, 77.5%; P=0.11) and station 8 (right 50/387, 12.9% vs. left 23/267, 8.6%; P=0.10), while station 9 was more frequently harvested in left-sided resections (right 179/387, 46.3% vs. left 182/267, 68.2%; P<0.001).
The CoC standard was met in 123 (46.1%) patients who underwent a left-sided resection and in 131 (33.9%) patients that had a right-sided resection (P=0.002). Meeting the hilar component was not significantly impacted by laterality, however, the mediastinal component of the CoC standard was more frequently achieved in left-sided resections (65.5% vs. 53.8% in right-sided resections; P=0.003). Among patients meeting the CoC standard, the most frequent combinations of resected N2 lymph node stations in right-sided resections were stations 4+7+9 in 65 (49.6%) patients, 2+4+7 in 17 (13.0%), 4+7+8+9 in 15 (11.5%), and 2+4+7+9 in 13 (9.9%). For left-sided resections, the most frequent combinations were stations 5+7+9 in 42 (34.2%) patients, 5+6+7+9 in 20 (16.3%), 4+7+9 in 19 (15.5%), and 4+5+7+9 in 14 (11.4%) patients.
Compliance with the CoC standards and oncologic outcomes
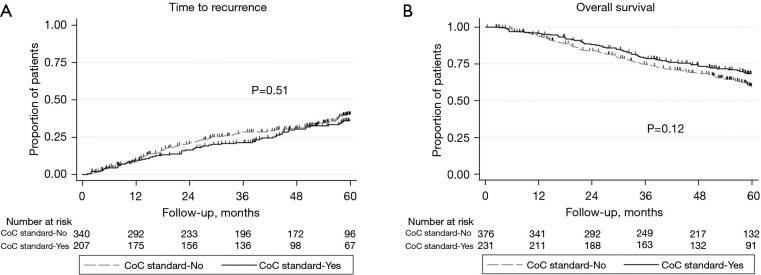
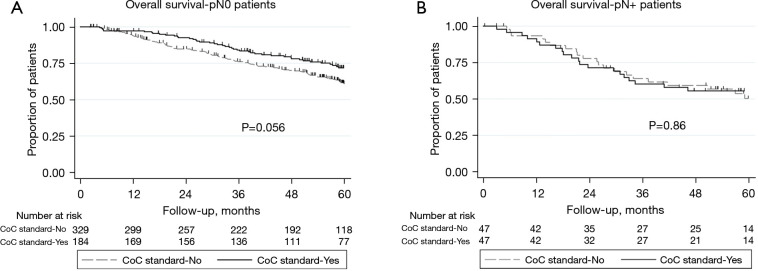
Median follow up for recurrence was 47.3 months (interquartile range, 19.4–60.5). Time to recurrence was not different between patients that met and did not meet the CoC standard (HR: 0.90, 95% CI: 0.67–1.22, P=0.51; Figure 2A). This remained true after adjusting for type of pulmonary resection, visceral pleural invasion, and pathological T and N stage [adjusted hazard ratio (HR): 0.86, 95% confidence interval (CI): 0.63–1.17, P=0.33]. Median follow up for overall survival was 53.8 months (interquartile range, 29.2–61.3). There were no significant differences in long-term overall survival between CoC standard groups (HR: 0.80, 95% CI: 0.60–1.06, P=0.12; Figure 2B), even after adjusting for age, gender, smoking history, histology, type of pulmonary resection, and pathological T and N stage (adjusted HR: 0.78, 95% CI: 0.58–1.05, P=0.10). The impact of meeting the CoC standard seemed more pronounced among patients classified as pN0 (Figure 3) as worse overall survival trended towards significance when the standard was not met (P=0.056). Patients not meeting the CoC standard and classified as pN0 exhibited an overall survival that resembled that of patients with pN1 disease (Figure 4).
Figure 2.
Recurrence and survival associated with meeting the CoC standard. (A) Time to cancer recurrence and (B) overall survival after resected clinical stage IA NSCLC according to CoC standard 5.8. CoC, Commission on Cancer; NSCLC, non-small cell lung cancer.
Figure 3.
Overall survival based on meeting CoC standard 5.8. (A) Patients without postoperative nodal upstaging and (B) patients with postoperative upstaging. CoC, Commission on Cancer.
Figure 4.
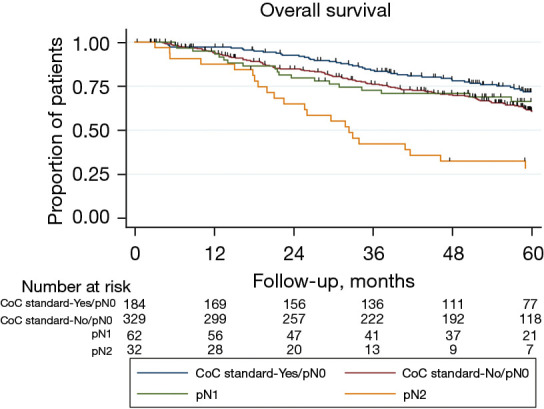
Overall survival by individual pathological N stage and CoC standard 5.8. CoC, Commission on Cancer.
Discussion
In a time when the transition to minimally invasive surgery is almost complete due to proven advantages over thoracotomy (14), and the value of sublobar lung resections has been demonstrated in patients with peripheral NSCLC <2 cm in size (15,16) an inadequate intraoperative lymph node evaluation may stage the type of tumor resection as R-undetermined (R-Un) due to incomplete mediastinal assessment (17). The CoC Accreditation Program of the American College of Surgeons aims at establishing quality standards while providing lung cancer care. Standard 5.8 of the Cancer Surgery Standards mandates that lymph nodes from at least one numbered hilar station (N1) and at least three numbered mediastinal station (N2) are collected intraoperatively in all pulmonary resections performed with curative intent in cases of NSCLC, small cell lung cancer, and carcinoid tumors. The present study shows that, in a historical cohort of clinical stage IA NSCLC patients who underwent pulmonary resection, only 38.8% met the new CoC standard for intraoperative lymph node evaluation. Although the study period predates the current standard, lymph node evaluation improved during the study period, mostly by virtue of increased hilar lymph node evaluation. Interestingly, 244 of 654 (37.3%) patients in the entire study missed meeting the current CoC standard by only 1 lymph node station, as 143 of 272 (52.3%) patients lacked 1 hilar station when they met the mediastinal component, and 101 of 271 (37.3%) meeting the hilar component lacked only 1 mediastinal station. This shows a potential robust improvement in CoC standard rates with a slightly more proactive role by surgeons.
We identified that an open approach, lobectomy, and left-sided resections were independently associated with achieving the CoC standard. Particularly, we found low rates of CoC standard compliance with wedge resections due to low rates of hilar lymph node evaluation. With the expected increased adoption of sublobar resections for the treatment of stage I NSCLC after the results of the JCOG0802 (15) and CALGB140503 trials (16), it is imperative that surgeons strive to maximize lymph node harvest in those patients.
We found significant differences in meeting the CoC standard according to laterality, with right-sided resection meeting the standard less frequently due to lower sampling of mediastinal stations. We found a lower rate of harvest of station 9 lymph nodes in right-sided resections. This is an interesting finding, since station 9 lymph nodes are equally accessible from both the right and left chest. Lower rates of resection of station 9 might explain a large part of the observed difference in achieving the CoC standard in right-sided resections, as they were harvested only in 30% of patients not achieving the CoC standard while they were harvested in 77% of patients achieving the standard. Not surprisingly, the most frequent lymph node harvest combination meeting the CoC standard in right resections included stations 4+7+9. This contrasts with left resections, where 47% of patients not achieving the CoC standard and 94% of those achieving it had station 9 resected. It merits consideration that the observed differences in station 9 resection might have an anatomical substrate. Autopsy studies have demonstrated important anatomical differences with station 9 lymph nodes being encountered more frequently in the left chest and in larger numbers (18). Also, we found very low rates of harvest of stations 2, 3 and 8. This is definitely an area for improvement, particularly when considering that identification of lymph node stations based on intraoperative photographs is not consistent among cardiothoracic surgeons (19). On the contrary, resection of station 3 lymph nodes have shown no prognostic impact after right-sided resections (20).
We found that meeting the CoC standard was associated with nodal upstaging which has downstream implications on prognosis and candidacy for adjuvant therapies. For instance, patients whose surgical pathology returns consistent with pN1 or greater disease should be evaluation for consideration of adjuvant therapy based on results of biomarker testing, as this can improve long-term outcomes (21-24). If nodal disease remains occult, therapies with proven benefit remain unavailable to patients. However, we did not find significant differences in recurrence or overall survival. Patients that experienced nodal upstaging (pN+), had similar overall survival regardless of meeting the CoC standard or not. However, we did observe a non-significant separation of the overall survival curves in patients that were classified as pN0 and did not meet the CoC standard, which suggests that occult nodal disease might have been missed by a less thorough lymph node evaluation. The lack of statistically significant long-term impact might be secondary to our sample size or length of follow-up. This also raises the question if the CoC standard has the same impact in clinical stage IA NSCLC as it does when it has been evaluated in broader lung cancer populations (7,10).
While it appears that meeting the CoC standard does impact identification of occult nodal disease, it should not be neglected to note that meeting the CoC standard trended towards higher rates of postoperative complications such as chyle leak, prolonged airleak, increased days with a chest tube and increased hospital length of stay. Surgeons may subconsciously avoid additional removal of lymph nodes in cases of perceived “lower risk” pulmonary nodules or in patients with significant comorbidities, in an effort to mitigate potential complications. This deserves further scrutiny.
There are limitations to our study. Its retrospective nature may reflect biases and discrepancies in data accuracy, particularly regarding the number of lymph nodes or stations sampled. This represents a historical cohort that reflects knowledge and practices at the time of surgery. Indeed, it is incontrovertible that the proficiency of lymph node evaluation must have improved after overcoming the learning curve of video-assisted thoracoscopic lung resections. It would be important for future studies to analyze intraoperative lymph node evaluation in a more contemporary cohort of patients, so that the impact of other factors such as the size of invasive components, histology subtypes, and genetic mutations can be assessed. Also, it represents the experience at a single large academic medical center that performs a high volume of lung resections. Furthermore, we cannot account for nuances in different surgeons techniques during intraoperative lymph node evaluation. The external validity of our results should be assessed for each population and center. Importantly, the routine use of intraoperative frozen section analysis of all resected specimens in our institution, including all resected lymph nodes, might have impacted the intraoperative sampling of mediastinal lymph nodes. Our study lacks data to determine if lymph nodes were present and not harvested from a specific lymph node station or to determine if a particular lymph node station was explored and no lymph nodes were identified, which could be related to anatomical variations. Finally, our study did not collect data on other factors that might have influenced the decision to harvest a particular lymph node station during surgery, such us technical difficulty secondary to adhesions or bleeding.
Conclusions
We identified that an open approach, lobectomy, and left-sided resections were associated with meeting the CoC standard in patients undergoing pulmonary resection for clinical stage IA NSCLC. We also found significant differences in harvest of individual mediastinal lymph node stations according to laterality of resection which impacted meeting the CoC standard. In this analysis, the CoC standard was associated with nodal upstaging, but not with cancer recurrence or overall survival in patients having surgery for clinical stage IA NSCLC. However, patients that did not meet the CoC standard and were classified as pN0 appear to experience lower overall survival; this finding deserves further investigation. Overall, this manuscript reiterates the importance of adequate intraoperative lymph node assessment, the factors that can influence this, and the possible prognostic implications that are correlated with it.
Supplementary
The article’s supplementary files as
Acknowledgments
We thank the Thoracic Surgery Research Office at Mayo Clinic in Rochester, MN for their work and continuous support.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Mayo Clinic Institutional Review Board (protocol #17-010802) and individual consent for this retrospective analysis was waived due to the retrospective nature.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-971/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-971/coif). J.S.R. reports patents pending with Imvaria, Phase and Medview, and consulting role with companies Elucent, Noah, Isola, and Vergent. The other authors have no conflicts of interest to declare.
Data Sharing Statement
Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-971/dss
References
- 1.Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. 10.1016/j.ejcts.2006.08.008 [DOI] [PubMed] [Google Scholar]
- 2.Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. 10.1016/j.jtcvs.2010.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darling GE, Allen MS, Decker PA, et al. Number of lymph nodes harvested from a mediastinal lymphadenectomy: results of the randomized, prospective American College of Surgeons Oncology Group Z0030 trial. Chest 2011;139:1124-9. 10.1378/chest.10-0859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osarogiagbon RU, Decker PA, Ballman K, et al. Survival Implications of Variation in the Thoroughness of Pathologic Lymph Node Examination in American College of Surgeons Oncology Group Z0030 (Alliance). Ann Thorac Surg 2016;102:363-9. 10.1016/j.athoracsur.2016.03.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osarogiagbon RU, Yu X. Mediastinal lymph node examination and survival in resected early-stage non-small-cell lung cancer in the surveillance, epidemiology, and end results database. J Thorac Oncol 2012;7:1798-806. 10.1097/JTO.0b013e31827457db [DOI] [PubMed] [Google Scholar]
- 6.Osarogiagbon RU, D'Amico TA. Improving lung cancer outcomes by improving the quality of surgical care. Transl Lung Cancer Res 2015;4:424-31. 10.3978/j.issn.2218-6751.2015.08.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou H, Tapias LF, Gaissert HA, et al. Lymph Node Assessment and Impact on Survival in Video-Assisted Thoracoscopic Lobectomy or Segmentectomy. Ann Thorac Surg 2015;100:910-6. 10.1016/j.athoracsur.2015.04.034 [DOI] [PubMed] [Google Scholar]
- 8.Katz MHG, Francescatti AB, Hunt KK, et al. Technical Standards for Cancer Surgery: Commission on Cancer Standards 5.3-5.8. Ann Surg Oncol 2022;29:6549-58. 10.1245/s10434-022-11375-w [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 3.2022).
- 10.Osarogiagbon RU, Ray MA, Faris NR, et al. Prognostic Value of National Comprehensive Cancer Network Lung Cancer Resection Quality Criteria. Ann Thorac Surg 2017;103:1557-65. 10.1016/j.athoracsur.2017.01.098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smeltzer MP, Faris NR, Ray MA, et al. Association of Pathologic Nodal Staging Quality With Survival Among Patients With Non-Small Cell Lung Cancer After Resection With Curative Intent. JAMA Oncol 2018;4:80-7. 10.1001/jamaoncol.2017.2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American College of Surgeons C on C. Optimal Resources for Cancer Care : 2020. Standards. Comm Cancer, Am Coll Surg 2020:1-106. [Google Scholar]
- 13.National Lung Screening Trial Research Team ; Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim E, Batchelor TJP, Dunning J, et al. Video-Assisted Thoracoscopic or Open Lobectomy in Early-Stage Lung Cancer. NEJM Evid 2022;1:EVIDoa2100016. [DOI] [PubMed]
- 15.Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. 10.1016/S0140-6736(21)02333-3 [DOI] [PubMed] [Google Scholar]
- 16.Altorki N, Wang X, Kozono D, et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N Engl J Med 2023;388:489-98. 10.1056/NEJMoa2212083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards JG, Chansky K, Van Schil P, et al. The IASLC Lung Cancer Staging Project: Analysis of Resection Margin Status and Proposals for Residual Tumor Descriptors for Non-Small Cell Lung Cancer. J Thorac Oncol 2020;15:344-59. 10.1016/j.jtho.2019.10.019 [DOI] [PubMed] [Google Scholar]
- 18.Kiyono K, Sone S, Sakai F, et al. The number and size of normal mediastinal lymph nodes: a postmortem study. AJR Am J Roentgenol 1988;150:771-6. 10.2214/ajr.150.4.771 [DOI] [PubMed] [Google Scholar]
- 19.Yang CJ, Veeramachaneni N, Hurd J, et al. A National Survey of Surgeons Evaluating the Accuracy of Mediastinal Lymph Node Identification. Clin Lung Cancer 2023;24:445-52. 10.1016/j.cllc.2023.03.005 [DOI] [PubMed] [Google Scholar]
- 20.Y Yang MZ , Tan ZH, Li JB, et al. Station 3A lymph node dissection does not improve long-term survival in right-side operable non-small-cell lung cancer patients: A propensity score matching study. Thorac Cancer 2022;13:2106-16. 10.1111/1759-7714.14456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 2021;398:1344-57. 10.1016/S0140-6736(21)02098-5 [DOI] [PubMed] [Google Scholar]
- 22.Wu YL, Tsuboi M, He J, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:1711-23. 10.1056/NEJMoa2027071 [DOI] [PubMed] [Google Scholar]
- 23.O'Brien M, Paz-Ares L, Marreaud S, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol 2022;23:1274-86. 10.1016/S1470-2045(22)00518-6 [DOI] [PubMed] [Google Scholar]
- 24.Wu YL, Dziadziuszko R, Ahn JS, et al. Alectinib in Resected ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2024;390:1265-76. 10.1056/NEJMoa2310532 [DOI] [PubMed] [Google Scholar]