Abstract
Background
The rare incidence of small cell carcinoma of the esophagus (SCCE) makes prospective studies difficult to conduct, the efficacy of existing standard treatment regimens for SCCE is therefore highly controversial. This study aimed to explore differences in the efficacy of three different treatment regimens [upfront surgery, neoadjuvant chemotherapy (NCT), and chemoradiotherapy (CRT)] in patients with limited-stage SCCE (LS-SCCE).
Methods
In total, 483 patients with LS-SCCE were screened from five centers from June 2001 to June 2020, and 128 patients with LS-SCCE were screened from the Surveillance, Epidemiology, and End Results (SEER) database. A survival analysis of the patients who underwent upfront surgery, NCT, and CRT was performed. The primary endpoint was overall survival (OS).
Results
Treatment approaches for LS-SCCE differ between China and America. The data from the SEER database showed that aggressive treatment resulted in a significant survival benefit for patients [median OS (mOS), 16.0 vs. 1.0 months]. However, no significant survival difference was observed between the surgical and non-surgical treatments [China: hazard ratio (HR), 0.820; 95% confidence interval (CI): 0.618–1.088, P=0.17; SEER: HR, 0.717; 95% CI: 0.440–1.169, P=0.18]. CRT significantly improved the survival time of the patients aged >60 years (mOS, 20.9 vs. 36.0 months, P=0.007). NCT significantly prolonged the survival time of the patients who underwent esophagectomy (HR, 0.753; 95% CI: 0.569–0.995, P=0.046).
Conclusions
This study suggests that NCT provided a better survival benefit for patients with LS-SCCE than upfront surgery, LS-SCCE patients aged >60 years receiving CRT had survival benefit compared to those undergoing surgery.
Keywords: Small cell carcinoma of the esophagus (SCCE), limited-stage (LS), surgery, neoadjuvant chemotherapy (NCT), chemoradiotherapy (CRT)
Highlight box.
Key findings
• Neoadjuvant chemotherapy (NCT) is recommended rather than upfront surgery for patients aged ≤60 years, while chemoradiotherapy (CRT) is recommended for patients aged >60 years.
What is known, and what is new?
• The combination of surgery, chemotherapy, and radiotherapy for patients with limited-stage small cell carcinoma of the esophagus (LS-SCCE) has been recognized for improving patients’ outcomes, but there is a lack of large cohort studies comparing the efficacy of CRT, NCT, and upfront surgery.
• Aggressive treatments led to a significant improvement in the prognosis of patients with LS-SCCE. For patients aged ≤60 years, NCT significantly prolonged the median overall survival (mOS) compared with upfront surgery. For patients aged >60 years, CRT significantly prolonged the mOS compared with surgery.
What is the implication, and what should change now?
• Upfront surgery is not recommended for LS-SCCE patients aged ≤60 years, while CRT is recommended for LS-SCCE patients aged >60 years.
Introduction
Small cell carcinoma of the esophagus (SCCE) is a rare type of esophageal cancer that accounts for only 0.5–2.8% of all esophageal cancers (1-4). SCCE was first reported by McKeown in 1952 (5). Due to its low incidence, it is difficult to conduct prospective studies on SCCE. Therefore, most of the available literature reports are case reports or retrospective studies with small sample sizes.
As the most common extrapulmonary small cell carcinoma, SCCE has biological characteristics similar to those of small cell lung cancer, which is highly invasive and for which local lymph node and distant organ metastases develop early (6-10). Patients with SCCE have an extremely poor prognosis, with a median overall survival (mOS) of 8–13 months and a 5-year overall survival (OS) rate of approximately 6.7–18% (3,8,11-14).
The standard treatment regimen for SCCE remains controversial, and most available treatment regimens have been developed with reference to clinical guidelines for small cell lung cancer (4,15-17). The VALSG system classifies SCCE into limited-stage (LS) disease and extensive-stage (ES) disease. LS is defined as tumor confined to the esophagus and surrounding tissues with or without regional lymph node involvement (4). Most existing studies recommend surgery as the preferred treatment option for limited-stage small cell carcinoma of the esophagus (LS-SCCE). Most patients with LS-SCCE in the United States receive radiotherapy (RT), unlike in China, where surgery is the main treatment option (18). Gu et al. concluded that surgery alone in LS disease is sufficient to control disease progression (19). However, Meng et al. found that definitive chemoradiotherapy (CRT) in patients with LS-SCCE improved patient survival compared to surgery combined with RT, suggesting that CRT should be the preferred treatment option (20). Conversely, Zhu et al. suggested that radical surgery and RT were equally effective in treating patients with LS-SCCE (21).
An increasing number of studies have found that combined modality therapy, such as surgery combined with chemotherapy (CT) or RT, further improves the prognosis of patients with SCCE. Studies have also found that combined modality therapy improves the survival outcomes of patients with LS-SCCE (22,23). However, the small number of patients included in these clinical studies may be one of the reasons for the different conclusions reached by these studies. Thus, more clinical studies with large samples need to be conducted to explore the standard treatment options for LS-SCCE.
The present study investigated the efficacy of different treatment regimens for LS-SCCE by retrospectively examining patients with LS-SCCE who underwent upfront surgery, neoadjuvant chemotherapy (NCT), and CRT from five Chinese cancer centers. The results were compared to the American Surveillance, Epidemiology, and End Results (SEER) database (http://surveillance.cancer.gov/index.html). We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1394/rc).
Methods
Patients selection
This was a multicenter comparative clinical study (RENMIN201). Eligible patients were selected from June 2001 to June 2020 from the Renmin Hospital of Wuhan University, the West China Hospital, the Fourth Hospital of Hebei Medical University, the Tianjin Medical University Cancer Hospital, and the First Affiliated Hospital of Zhengzhou University. To be eligible for inclusion in this study, patients had to meet the following inclusion criteria: (I) have histopathologically proven pure primary SCCE; (II) have LS disease; and (III) have complete medical records. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had other types of esophageal cancer (e.g., adenocarcinoma, squamous cell carcinoma, or other types of neuroendocrine carcinoma); (II) had non-confined stage disease; and/or (III) had incomplete medical records. Detailed demographic and clinicopathological information of the eligible patients were retrospectively retrieved from their medical records. The specific process is shown in Figure S1.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Renmin Hospital of Wuhan University (approval number: WDRY2019–K093), and individual consent for this retrospective analysis was waived. This study was approved by all participating hospitals/institutes.
Treatment
We categorized the study population into the upfront surgery, NCT, and CRT groups based on the treatment modality. Patients in the upfront surgery group underwent surgical resection of the esophagus, which included open transthoracic esophagectomy and minimally invasive esophagectomy. After surgery, clinicians decide to conduct adjuvant chemotherapy if patients had an R1 or R2 resection. Patients in the NCT group received two cycles of NCT prior to surgery, and surgery was scheduled 2 to 4 weeks after the completion of CT. After surgery, the attending physician decided whether each patient would receive adjuvant therapy according to the same criteria as above. The chemotherapy regimens used in China: etoposide, 120 mg/m2 by intravenous (IV) bolus on days 1–3, and cisplatin, 75 mg/m2 by IV on day 1, every 3 weeks for 2–4 cycles; or irinotecan, 130 mg/m2, and cisplatin, 75 mg/m2, by IV on day 1, every 3 weeks for 2–4 cycles. Patients in the CRT group did not receive surgical treatment but received RT and CT. The CT regimen consisted of two cycles of etoposide + cisplatin, and synchronized radiation therapy of 50 Gy/25 f. This was followed by two cycles of consolidation CT with the same regimen.
Surveillance, Epidemiology, and End Results (SEER) database
SEER*Stat (version8.4.2) software was used to search the SEER database (8 registries, 12 registries, and 17 registries) for patients with SCCE (ICD-0–3: codes 8041/8045) from 2000 to 2020. Clinicopathological features, such as age at diagnosis, race, sex, primary tumor site, pathological grading, TNM staging, tumor size, and treatment modalities, were extracted. The survival outcomes and OS times of the patients were determined. OS was defined as the time interval from diagnosis to death or last follow up.
Statistical analyses
We used the Chi-squared test or Fisher’s exact test to compare the categorical variables between the groups. Propensity score matching (PSM) for upfront surgery vs. CRT, and NCT vs. CRT was performed using the MatchIt R package. To provide more accurate treatment recommendations for patients with LS-SCCE, we analyzed the survival data after stratification. The Kaplan-Meier method was used to estimate the OS rates. The log-rank test was used to compare differences between groups. A prognostic analysis of OS was performed using univariate and multivariate Cox proportional hazards regression models. All the factors associated with OS were included in the multivariate Cox regression analyses to test their association with potential predictors, regardless of their level of significance in the univariate analysis. The results are reported as hazard ratios (HRs) and 95% confidence intervals (CIs). Statistical significance was set at P<0.05. All the statistical analyses were performed using SPSS software (version 25.0, IBM, USA) and R version 4.3.2 (R Project for Statistical Computing).
Results
We analyzed the data of 483 patients from China and 157 patients from the SEER database. The median survival time of the patients in China was 25.3 months (95% CI: 21.2–29.4), while that of the patients in the SEER database was 16.0 months (95% CI: 12.3–19.7). In the entire cohort, the vast majority of patients were male (347, 71.8%), and had tumors located in the upper middle third of the esophagus (329, 68.1%) and positive lymph node metastases (309, 64%). Overall, 212 patients (43.9%) were aged ≤60 years, and 202 patients (41.8%) had an early T stage. The baseline clinical characteristics of the patients are summarized in Table 1.
Table 1. Differences in treatment approaches for limited-stage small cell esophageal cancer between China and the United States.
| Characteristics | China | SEER | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All (n=483) | S (n=141) | NCT (n=171) | CRT (n=171) | P | All (n=128) | S (n=17) | NAT (n=12) | CRT (n=67) | CT (n=26) | RT (n=6) | P | ||
| Sex | 0.80 | 0.12 | |||||||||||
| Female | 136 | 41 (30.1) | 50 (36.8) | 45 (33.1) | 42 | 4 (9.5) | 2 (4.8) | 20 (47.6) | 14 (33.3) | 2 (4.8) | |||
| Male | 347 | 100 (28.8) | 121 (34.9) | 126 (36.3) | 86 | 13 (15.1) | 10 (11.6) | 47 (54.7) | 12 (14.0) | 4 (4.7) | |||
| Age, years | 0.01 | 0.03 | |||||||||||
| ≤60 | 212 | 61 (28.8) | 89 (42.0) | 62 (29.2) | 36 | 5 (13.9) | 8 (22.2) | 17 (47.2) | 6 (16.7) | 0 (0.0) | |||
| >60 | 271 | 80 (29.5) | 82 (30.3) | 109 (40.2) | 92 | 12 (13.0) | 4 (4.3) | 50 (54.3) | 20 (21.7) | 6 (6.5) | |||
| Tumor location | 0.45 | 0.01 | |||||||||||
| Proximal/middle third | 329 | 101 (30.7) | 111 (33.7) | 117 (35.6) | 52 | 8 (15.4) | 2 (3.8) | 27 (51.9) | 15 (28.8) | 0 (0.0) | |||
| Distal third | 154 | 40 (26.0) | 60 (39.0) | 54 (35.1) | 61 | 8 (13.1) | 9 (14.8) | 33 (54.1) | 9 (14.8) | 2 (3.3) | |||
| Unknown | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 15 | 1 (6.7) | 1 (6.7) | 7 (46.7) | 2 (13.3) | 4 (26.7) | |||
| T stage | <0.001 | 0.15 | |||||||||||
| T1/2 | 202 | 74 (36.6) | 78 (38.6) | 50 (24.8) | 29 | 5 (17.2) | 2 (6.9) | 13 (44.8) | 7 (24.1) | 2 (6.9) | |||
| T3/4 | 281 | 67 (23.8) | 93 (33.1) | 121 (43.1) | 32 | 4 (12.5) | 4 (12.5) | 22 (68.8) | 1 (3.1) | 1 (3.1) | |||
| Unknown | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 67 | 8 (11.9) | 6 (9.0) | 32 (47.8) | 18 (26.9) | 3 (4.5) | |||
| N stage | <0.001 | 0.64 | |||||||||||
| N0 | 174 | 57 (32.8) | 76 (43.7) | 41 (23.6) | 20 | 3 (15.0) | 2 (10.0) | 13 (65.0) | 1 (5.0) | 1 (5.0) | |||
| N+ | 309 | 84 (27.2) | 95 (30.7) | 130 (42.1) | 11 | 1 (9.1) | 2 (18.2) | 6 (54.5) | 2 (18.2) | 0 (0.0) | |||
| Unknown | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 97 | 13 (13.4) | 8 (8.2) | 48 (49.5) | 23 (23.7) | 5 (5.2) | |||
Data are presented as n (%). S, upfront surgery; SEER, Surveillance, Epidemiology, and End Results; S, upfront surgery; NCT, neoadjuvant chemotherapy; CRT, chemoradiotherapy; NAT, neoadjuvant therapy; CT, chemotherapy; RT, radiotherapy.
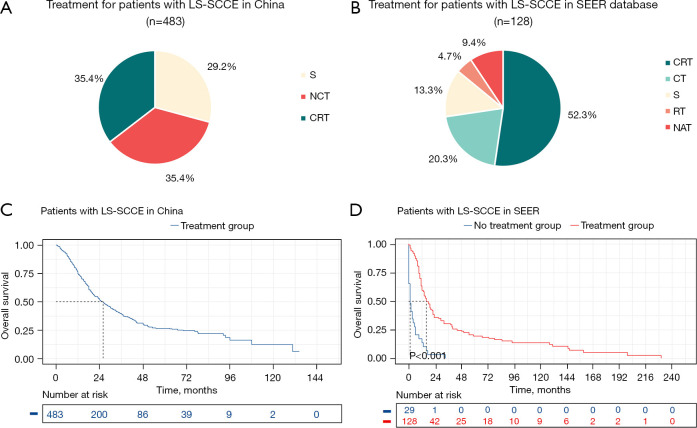
As Table 1 and Figure 1 show, in China, surgery (312, 64.6%) was the main treatment option; 141 patients (29.2%) received upfront surgery, 171 patients (35.4%) received NCT, and 171 patients (35.4%) received CRT. Furthermore, Chinese doctors more often choose surgical treatment for younger patients than older patients. The data from the SEER database showed that non-surgical treatment (99,77.3%), including CRT (67,52.3%), CT (26,20.3%), and RT (6, 4.7%), was predominant in the United States. Regardless of the clinical characteristics of the patients, physicians in the United States were more likely to choose non-surgical treatment before surgery. The treatment approaches for LS-SCCE still differ significantly in China and the United States. Meanwhile, according to the SEER database, the LS-SCCE patients who received aggressive treatment had a significant survival benefit compared to those who did not receive treatment (mOS, 16.0 vs. 1.0 months, P<0.001).
Figure 1.
Differences in treatment approaches for LS-SCCE between China and the United States. Percentages of different treatments for LS-SCCE in China (A) and the United States (B); survival curves for LS-SCCE patients in China (C) and the United States (D). LS-SCCE, limited-stage small cell carcinoma of the esophagus; SEER, Surveillance, Epidemiology, and End Results; S, upfront surgery; NCT, neoadjuvant chemotherapy; CRT, chemoradiotherapy; CT, chemotherapy; RT, radiotherapy; NAT, neoadjuvant therapy.
The univariate and multivariate Cox regression analyses of the Chinese patients after PSM showed that there was no statistically significant difference in prognosis between the surgical and non-surgical patients (HR, 0.820; 95% CI: 0.618–1.088, P=0.17) (Table 2, Figure S2). The results for the SEER patients database were similar (HR, 0.717; 95% CI: 0.440–1.169, P=0.18) (Table S1, Figure S2).
Table 2. Clinical and tumor characteristics of patients with LS-SCCE receiving surgical and non-surgical treatment.
| Characteristic | Before matching | After matching | |||||
|---|---|---|---|---|---|---|---|
| S + NCT (n=312) | CRT (n=171) | P | S + NCT (n=156) | CRT (n=156) | P | ||
| Sex | 0.58 | 0.90 | |||||
| Female | 91 (29.2) | 45 (26.3) | 39 (25.0) | 37 (23.7) | |||
| Male | 221 (70.8) | 126 (73.7) | 117 (75.0) | 119 (76.3) | |||
| Age, years | 0.02 | >0.99 | |||||
| ≤60 | 150 (48.1) | 62 (36.3) | 62 (39.7) | 62 (39.7) | |||
| >60 | 162 (51.9) | 109 (63.7) | 94 (60.3) | 94 (60.3) | |||
| Tumor location | >0.99 | 0.63 | |||||
| Proximal/middle third | 212 (67.9) | 117 (68.4) | 110 (70.5) | 105 (67.3) | |||
| Distal third | 100 (32.1) | 54 (31.6) | 46 (29.5) | 51 (32.7) | |||
| T | <0.001 | >0.99 | |||||
| T1/2 | 152 (48.7) | 50 (29.2) | 50 (32.1) | 50 (32.1) | |||
| T3/4 | 160 (51.3) | 121 (70.8) | 106 (67.9) | 106 (60.9) | |||
| N | <0.001 | >0.99 | |||||
| N0 | 133 (42.6) | 41 (24.0) | 41 (26.3) | 41 (26.3) | |||
| N+ | 179 (57.4) | 130 (76.0) | 115 (73.7) | 115 (73.7) | |||
Data are presented as n (%). LS-SCCE, limited-stage small cell carcinoma of the esophagus; S, upfront surgery; NCT, neoadjuvant chemotherapy; CRT, chemoradiotherapy.
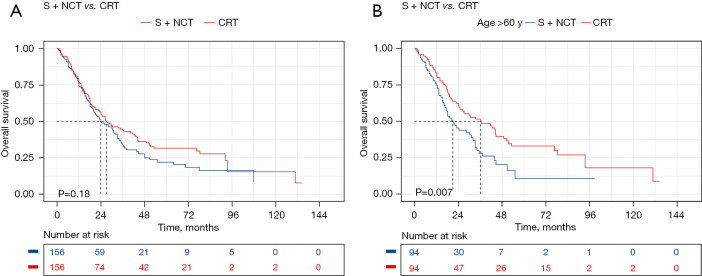
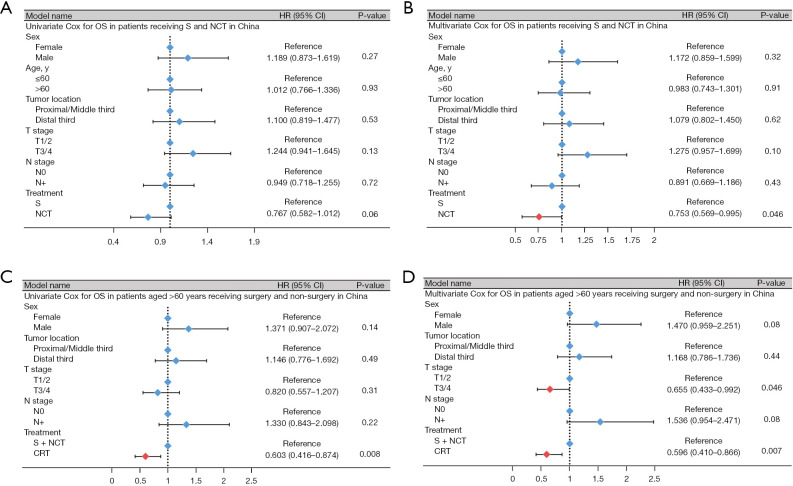
Because the SEER database had more missing patient data and fewer surgical patients, we attempted to determine the optimal treatment strategy for patients with LS-SCCE by performing a stratified survival analysis on the data of 483 patients in China. The stratified analysis showed CRT significantly improved the mOS of patients aged >60 years (upfront surgery + NCT vs. CRT: mOS, 20.9 vs. 36.0 months, P=0.007), and similar results were also found in the multivariate Cox regression analysis (HR, 0.596, 95% CI: 0.410–0.866, P=0.007) (Figures 2,3, Table S2, Figures S3,S4).
Figure 2.
Survival analysis of surgical vs. non-surgical groups after PSM. (A) Surgical vs. non-surgical group Kaplan-Meier curves; (B) Kaplan-Meier curves for patients aged >60 years in the surgical vs. non-surgical groups. PSM, propensity score matching; S, upfront surgery; NCT, neoadjuvant chemotherapy; CRT, chemoradiotherapy.
Figure 3.
Forest plot for univariate and multivariate Cox regression analyses. (A,B) Univariate and multivariate Cox regression analyses of S vs. NCT in China. (C,D) Univariate and multivariate Cox regression analyses of S + NCT vs. CRT in a subgroup of patients aged older than 60 years in China. S, upfront surgery; NCT, neoadjuvant chemotherapy; CRT, chemoradiotherapy; OS, overall survival; HR, hazard ratio; CI, confidence interval.
The number of patients undergoing NCT is increasing annually as treatment approaches evolve. Therefore, we also evaluated the effects of different surgical approaches in patients with LS-SCCE. The univariate and multivariate Cox regression analyses showed that NCT was a strong protective factor in patients with LS-SCCE (HR, 0.753; 95% CI: 0.569–0.995, P=0.046) (Figure 3, Table S3). Moreover, in patients aged ≤60 years, NCT significantly prolonged survival compared to upfront surgery (upfront surgery vs. NCT: mOS, 19.6 vs. 27.0 months, P=0.046). Similarly, in male patients, NCT showed an advantage (upfront surgery vs. NCT: mOS, 19.5 vs. 26.0 months, P=0.03) (Table S4, Figure S5).
Finally, to explore the differences between NCT and CRT, we performed a survival analysis after PSM. No statistically significant difference was observed between NCT and CRT in either the univariate or multivariate Cox analyses (HR, 0.931; 95% CI: 0.661–1.313, P=0.69) (Table S5, Figure S2); however, CRT still resulted in a longer survival time for patients aged >60 years (NCT vs. CRT: mOS, 23.0 vs. 44.5 months, P=0.03) (Table S6, Figure S5).
Discussion
Due to the extremely low incidence of SCCE, large prospective clinical studies are difficult to conduct, and the existing National Comprehensive Cancer Network (NCCN) guidelines (https://www.nccn.org) do not yet include recommendations for the treatment of SCCE, and only refer to the treatment guidelines for small cell lung cancer. We summarized the large sample of domestic and international SCCE studies over the past 10 years (Table S7). Specifically, we compared the changes in the NCCN guidelines for the treatment of small cell lung cancer over the past 10 years and drew three conclusions based on the available findings. First, there are regional differences in the preferred treatment options for LS-SCCE. In China, patients with LS-SCCE tend to undergo esophagectomy, however, the rate of NCT among surgical patients is increasing, as is the rate of CRT due to changes in treatment concepts. Patients with LS-SCCE in the United States tend to undergo CRT as their primary treatment modality (18). Meanwhile, due to its efficacy and safety, concurrent CRT remains a central treatment modality for small cell lung cancer (16,17). Second, the use of surgery as the primary treatment option for patients with LS disease has been accepted by most researchers in China. Kukar et al. and Xu et al. found that the mOS of surgical patients was significantly longer than that of non-surgical patients (24,25). Third, as treatment approaches have evolved, combined modality therapy is now thought to further improve the survival time of patients with LS-SCCE (22,23).
The survival analysis of patients who did and who did not receive treatment for LS-SCCE from the SEER database showed that the mOS of the patients who were aggressively treated was significantly longer than that of the patients who were not actively treated (14.0 vs. 1.0 months, P<0.001). Consequently, aggressive therapeutic interventions are necessary for patients with LS-SCCE.
NCT combined with surgery is an emerging treatment option for LS-SCCE; however, few studies have explored its efficacy for LS-SCCE. Cai et al. compared the mOS of 171 patients with LS-SCCE who underwent NCT in combination with surgery and 109 patients who underwent surgery alone, and found that patients who underwent NCT in combination with surgery had a significantly prolonged mOS compared to those who underwent surgery alone (26). However, no study has been conducted to further compare the differences in efficacy between the three treatment modalities of upfront surgery, NCT, and CRT.
Our study combined data from 483 LS-SCCE patients who received upfront surgery, NCT, and CRT from June 2001 to June 2020 at five oncology centers in China to compare differences in efficacy between these three treatment modalities for LS-SCCE for the first time. Using PSM, we compared the differences in efficacy between surgery and non-surgery, between different surgical modalities, and between NCT and CRT. Data from the Chinese population and SEER database showed no prognostic differences between the surgical and non-surgical patients. The multivariate Cox analysis of the SEER data showed that later N stage was a protective factor for patients with LS-SCCE (HR, 0.266; 95% CI: 0.104–0.681, P=0.006); however, this might be associated with incompletely matched clinical characteristics. We also performed a stratified analysis and found that in patients aged >60 years, non-surgical treatments had better outcomes than surgical treatments. No difference was found between the surgical and non-surgical treatments in patients aged ≤60 years, but it is important to note that NCT had a greater survival benefit than upfront surgery in this population. Interestingly, when we performed a multivariate analysis of patients aged >60 years, we found that patients with higher T stages had a better prognosis, but no such statistical significance was observed in the univariate analysis. This might be related to the fact that the older patients with higher T stages received better nutritional support and care. Zhu et al. compared the OS and progression-free survival (PFS) of 458 patients with LS-SCCE from 2000 to 2020 in China who received three regimens of CT, CT plus radical surgery, and CT plus definitive RT (CT + RT) and found that there was no statistically significant difference in the OS and PFS of these patients before and after PSM for CT + RT compared with CT + radical surgery (21). However, it should be noted that patients in the CT + radical surgery group were not further stratified into those receiving neoadjuvant therapy and upfront surgery, which might have reduced the efficacy of the neoadjuvant treatment.
We also examined the treatment cycle and treatment benefit ratios of different treatment regimens. Taking into account the existing literature and the NCCN guidelines for the treatment of small cell lung cancer, the existing treatment regimens for patients with LS-SCCE are as follows: the NCT regimen usually consists of two cycles of NCT; the adjuvant regimen usually consists of four to six cycles of adjuvant CT (ACT), or RT with a total dose range of 28–70 Gy and a single RT dose range of 1.8–2.0 Gy; and CRT regimens usually consist of four to six cycles of CT, and RT with a total dose range of 45–70 Gy and a single RT dose range of 1.5–2 Gy (24,26-28). The NCT regimen has advantages in terms of the length of the treatment cycle and the simplicity of the treatment regimen over upfront surgery, and at the same time enables patients to achieve a longer survival time in a shorter treatment period.
The study had a number of strengths. First, this was a large-scale, multicenter, clinical study that included 483 patients from five oncology centers in China. Second, this study was the first to compare the differences in the efficacy of the three treatment modalities (i.e., upfront surgery, NCT, and CRT). Third, this study further stratified the survival analysis of patients with different clinical characteristics to provide more detailed guidance on the optimal treatment regimen for patients with different clinical characteristics. Finally, we could compare our data to the American SEER database.
This study had several limitations. First, this was a retrospective cohort study with an information bias or confounding factors. Different oncology centers may differ in terms of their specific treatment processes, information collection, and other aspects, which might have introduced bias. These potential biases should be considered when interpreting our findings. Second, the number of clinical features included in the study was limited, and more clinical features need to be included in the future to achieve precise treatment. Third, limitations of the data resulted in some cohorts still having mismatched clinical characteristics after PSM, which may have influenced the study conclusions. Finally, we only compared the OS of the three treatment modality cohorts and were unable to obtain other end points of the patients, such as PFS, quality of life, side effects, etc. We hope that more prospective studies in the future will allow a more detailed comparison of the above parameters.
Conclusions
In this study, we found that NCT provided a better survival benefit for patients aged ≤60 years with LS-SCCE than upfront surgery, CRT should be the preferred treatment option for patients aged >60 years.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was supported by the Central Leading Local Science and Technology Development Special Foundation (No. ZYYD2020000169) and the Interdisciplinary Innovative Talents Foundation of Renmin Hospital of Wuhan University (No. JCRCWL-2022–004).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Renmin Hospital of Wuhan University (approval number: WDRY2019–K093), and individual consent for this retrospective analysis was waived. This study was approved by all participating hospitals/institutes.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1394/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1394/coif). The authors have no conflicts of interest to declare.
Data Sharing Statement
Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1394/dss
References
- 1.Ji A, Jin R, Zhang R, et al. Primary small cell carcinoma of the esophagus: progression in the last decade. Ann Transl Med 2020;8:502. 10.21037/atm.2020.03.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeene PM, Geijsen ED, Muijs CT, et al. Small Cell Carcinoma of the Esophagus: A Nationwide Analysis of Treatment and Outcome at Patient Level in Locoregional Disease. Am J Clin Oncol 2019;42:534-8. 10.1097/COC.0000000000000546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li R, Yang Z, Shao F, et al. Multi-omics profiling of primary small cell carcinoma of the esophagus reveals RB1 disruption and additional molecular subtypes. Nat Commun 2021;12:3785. 10.1038/s41467-021-24043-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu XJ, Luo JD, Ling Y, et al. Management of small cell carcinoma of esophagus in China. J Gastrointest Surg 2013;17:1181-7. 10.1007/s11605-013-2204-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mckeown F. Oat-cell carcinoma of the oesophagus. J Pathol Bacteriol 1952;64:889-91. 10.1002/path.1700640420 [DOI] [PubMed] [Google Scholar]
- 6.Li J, Ma J, Wang H, et al. Population-based analysis of small cell carcinoma of the esophagus using the SEER database. J Thorac Dis 2020;12:3529-38. 10.21037/jtd-20-1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai J, Zhao F, Pan S. Clinicopathological Characteristics and Survival of Small Cell Carcinoma of the Salivary Gland: a Population-Based Study. Cancer Manag Res 2019;11:10749-57. 10.2147/CMAR.S231446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang F, Liu DB, Zhao Q, et al. The genomic landscape of small cell carcinoma of the esophagus. Cell Res 2018;28:771-4. 10.1038/s41422-018-0039-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen B, Yang H, Ma H, et al. Radiotherapy for small cell carcinoma of the esophagus: outcomes and prognostic factors from a retrospective study. Radiat Oncol 2019;14:210. 10.1186/s13014-019-1415-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nayal B, Vasudevan G, Rao AC, et al. Primary Small Cell Carcinoma of The Esophagus - An Eight Year Retrospective Study. J Clin Diagn Res 2015;9:EC04-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen SB, Yang JS, Yang WP, et al. Treatment and prognosis of limited disease primary small cell carcinoma of esophagus. Dis Esophagus 2011;24:114-9. 10.1111/j.1442-2050.2010.01112.x [DOI] [PubMed] [Google Scholar]
- 12.Hou X, Wei JC, Wu JX, et al. Multidisciplinary modalities achieve encouraging long-term survival in resectable limited-disease esophageal small cell carcinoma. PLoS One 2013;8:e69259. 10.1371/journal.pone.0069259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang C, Zhang G, Xue L, et al. Patterns and prognostic values of programmed cell death-ligand 1 expression and CD8 + T-cell infiltration in small cell carcinoma of the esophagus: a retrospective analysis of 34 years of National Cancer Center data in China. Int J Surg 2024;110:4297-309. 10.1097/JS9.0000000000000064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hudson E, Powell J, Mukherjee S, et al. Small cell oesophageal carcinoma: an institutional experience and review of the literature. Br J Cancer 2007;96:708-11. 10.1038/sj.bjc.6603611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song Z, Liu Y, Cheng G, et al. Distinct mutational backgrounds and clonal architectures implicated prognostic discrepancies in small-cell carcinomas of the esophagus and lung. Cell Death Dis 2021;12:472. 10.1038/s41419-021-03754-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petty WJ, Paz-Ares L. Emerging Strategies for the Treatment of Small Cell Lung Cancer: A Review. JAMA Oncol 2023;9:419-29. 10.1001/jamaoncol.2022.5631 [DOI] [PubMed] [Google Scholar]
- 17.Deek MP, Haigentz M, Jabbour SK. Waiting for Big Changes in Limited-Stage Small-Cell Lung Cancer: For Now, More of the Same. J Clin Oncol 2023;41:2326-30. 10.1200/JCO.22.02316 [DOI] [PubMed] [Google Scholar]
- 18.Xiao Q, Xiao H, Ouyang S, et al. Primary small cell carcinoma of the esophagus: Comparison between a Chinese cohort and Surveillance, Epidemiology, and End Results (SEER) data. Cancer Med 2019;8:1074-85. 10.1002/cam4.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu YM, Yang YS, Shi GD, et al. Limited-stage small cell carcinoma of the esophagus treated with curative esophagectomy: A multicenter retrospective cohort study. J Surg Oncol 2022;126:1396-402. 10.1002/jso.27073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng MB, Zaorsky NG, Jiang C, et al. Radiotherapy and chemotherapy are associated with improved outcomes over surgery and chemotherapy in the management of limited-stage small cell esophageal carcinoma. Radiother Oncol 2013;106:317-22. 10.1016/j.radonc.2013.01.008 [DOI] [PubMed] [Google Scholar]
- 21.Zhu J, Wang Y, Sun H, et al. Surgery versus radiotherapy for limited-stage small cell esophageal carcinoma: a multicenter, retrospective, cohort study in China (ChiSCEC). Int J Surg 2024;110:956-64. 10.1097/JS9.0000000000000912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen S, Wang XM, Wu F, et al. Primary Small Cell Carcinoma of the Esophagus in a Large Multicenter Cohort: Prognostic Factors and Treatment Strategies in the Modern Era. Int J Radiat Oncol Biol Phys 2023;117:e286-7. [Google Scholar]
- 23.Sun H, Wang Q, Wang Y, et al. Treatment Strategies for Limited-Stage Primary Small Cell Carcinoma of the Esophagus: A Multicenter Retrospective Trial from China. Int J Radiat Oncol Biol Phys 2022;114:e161-2. [Google Scholar]
- 24.Xu L, Li Y, Liu X, et al. Treatment Strategies and Prognostic Factors of Limited-Stage Primary Small Cell Carcinoma of the Esophagus. J Thorac Oncol 2017;12:1834-44. 10.1016/j.jtho.2017.09.1966 [DOI] [PubMed] [Google Scholar]
- 25.Kukar M, Groman A, Malhotra U, et al. Small cell carcinoma of the esophagus: a SEER database analysis. Ann Surg Oncol 2013;20:4239-44. 10.1245/s10434-013-3167-3 [DOI] [PubMed] [Google Scholar]
- 26.Cai G, Wang J, Zou B, et al. Preoperative Chemotherapy for Limited-stage Small Cell Carcinoma of the Esophagus. Ann Thorac Surg 2022;114:1220-8. 10.1016/j.athoracsur.2021.08.059 [DOI] [PubMed] [Google Scholar]
- 27.Zhao K, Huang Z, Si Y, et al. Use of Chemoradiotherapy as a Treatment Option for Patients with Limited-Stage Primary Small Cell Carcinoma of the Esophagus. Cancer Manag Res 2021;13:613-23. 10.2147/CMAR.S278914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding J, Ji J, Zhu W, et al. A retrospective study of different treatments of limited-stage small-cell esophageal carcinoma and associated prognostic factor analysis. Dis Esophagus 2013;26:696-702. 10.1111/dote.12017 [DOI] [PubMed] [Google Scholar]