Abstract
Background
The morbidity and mortality of chronic pulmonary aspergillosis (CPA) are very high. We aimed to investigate the prognostic factors of patients with CPA, especially focusing on the underlying pulmonary disease and the probable co-infection of bacterial.
Methods
We retrospectively analyzed 106 CPA patients from November 2019 to August 2023. We collected the patient’s clinical medical records. Kaplan-Meier survival curves were used to analyze patient survival; log-rank tests were utilized to compare survival among groups. Univariate and multivariate Cox proportional hazards regression analyses were applied for identification of potential prognostic factors.
Results
The mean age at the time of diagnosis was 60.3±14.8 years; 74 (69.8%) patients were male. There was significant difference between patients with and without lung cancer (P<0.001), and with and without emphysema (P=0.02). Other prognosis factors associated with mortality were as follows: smoking (P=0.04), cough (P=0.01), simultaneous discovery with Gram-negative bacteria (P=0.02), and hypoalbuminemia (P=0.001) in log-rank tests. Multivariate Cox regression analyses showed that emphysema [hazard ratio (HR), 4.107; 95% confidence interval (CI): 1.414–11.933; P=0.009] and lung cancer (HR, 8.511; 95% CI: 2.494–29.047; P<0.001) were identified as independent predictors of mortality. The 1- and 3-year survival rates with emphysema were 75.2% and 64.9%, respectively, whereas those for patients without emphysema were 92.6% and 85.9%, respectively.
Conclusions
In the current study, emphysema and lung cancer were independent predictors of mortality. Therefore, we should pay attention to the patients with these underlying lung diseases in order to improve the prognosis.
Keywords: Chronic pulmonary aspergillosis (CPA), underlying lung diseases, lung cancer, emphysema, prognosis
Highlight box.
Key findings
• A retrospective study of 106 chronic pulmonary aspergillosis (CPA) patients showed that emphysema and lung cancer were the predictors of mortality.
What is known and what is new?
• Systemic glucocorticoids, diabetes, lung cancer, interstitial lung disease, and diabetes significantly affect the prognosis of CPA patients.
• This paper subtly found that smoking, simultaneous discovery with Gram-negative bacteria, hypoalbuminemia, emphysema, and lung cancer were associated with mortality of CPA.
What is the implication, and what should change now?
• Clinicians should pay attention to the patients with this underlying lung diseases and probable co-infections, in order to improve the prognosis of pulmonary aspergillus.
Introduction
Aspergillus is a saprophytic conidial mold isolated from soil; aspergillus spores commonly exist in the air, and the immunocompromised hosts may develop pulmonary aspergillosis disease following inhalation of airborne conidia (1). Depending on the underlying immunologic status of the host and the previous lung disease, aspergillus diseases can be classified into three groups: invasive pulmonary aspergillosis (IPA); chronic pulmonary aspergillosis (CPA), and allergic bronchopulmonary aspergillosis (ABPA) (2). IPA often occurs emergently in severely immunocompromised hosts. The occurrence of CPA is related to previous pulmonary diseases [such as bronchiectasis, chronic obstructive pulmonary disease (COPD), and interstitial lung disease (ILD)] and the status of mild immune impairment (3-6). The morbidity and mortality of CPA are still high. The annual incidence of CPA is 1,837,272 and the mortality is 18.5% (7).
In the course of fungal infection, immune status is very important (8). Patients with hematological disease, a medication history of glucocorticosteroid or immune suppressive drugs, bronchiectasis, COPD, and pulmonary cavity diseases may have a high risk of aspergillosis infection due to the damaged immune barrier or the declined immune cells (9). When the patient’s immunity is mildly to moderately impaired and complicated with underlying lung diseases [COPD, bronchiectasis, and tuberculosis (TB), etc.], the symptoms of the pulmonary underlying diseases will overlap with those of pulmonary aspergillosis (10). As a result, if aspergillus cannot be detected in time, a poor prognosis may be caused.
There are many studies reporting on the prognostic factors of CPA. The most significant and important prognostic factor is pulmonary TB. Some 40% of patients after TB treatment have residual lesions, including fibrosis, cavity, bronchiectasis, and calcification (11). The destruction of lung structure leads to susceptibility to aspergillus, especially aspergilloma, a common CPA. In Vietnam, Africa, and India, the incidence of CPA in patients with prior TB exceeds 50% (12-14). Lung cancer history and COPD, nontuberculous mycobacteria (NTM), sarcoidosis, and ILD can also significantly affect the mortality rate of aspergillosis patients (15,16). A study (17) showed that among the patients with acute exacerbation of COPD, 9.8% had a prevalence of CPA. NTM, especially fibrous cavity NTM, is a high-risk factor for CPA, on account of the prolonged use of antibiotics and impaired immunity. In countries where NTM is endemic, the incidence of CPA after NTM ranges from 3.9% to 16.7% (18).
In this study, we aimed to investigate the prognostic factors of pulmonary aspergillosis in patients, especially focusing on the probable co-infection of bacterial and common underlying pulmonary disease. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-831/rc).
Methods
Patients and diagnostic criteria
We searched the medical records system of the Respiratory Department of Anhui Chest Hospital from November 2019 to August 2023, then collected medical records of patients who had been diagnosed with CPA. Those who met the inclusion criteria were included in our study. The diagnostic criteria for CPA were as follows: (I) clinical symptoms for more than 1 month, such as fever, cough, sputum, hemoptysis, dyspnea, and so on (19); (II) imaging abnormalities: the most significant imaging feature was a fungus ball (aspergilloma) in a pre-existing lung cavity, new cavity, or cavity progression, infiltration around the cavity, parenchymal destruction, fibrosis developing around a pre-existing cavity, and pleural thickening or effusion; (III) serum aspergillosis immunoglobulin G (IgG) concentration >120 AU/mL; (IV) microbial evidence of CPA: hypha detected upon direct microscopic examination; positive of Gomori’s methenamine silver staining in histopathologic section; positive fungal fluorescence staining in sputum or tracheoscopy lavage fluid, smear microscopy, and culture; galactomannan detection (GM test) in blood or lavage was high [the threshold for GM test in blood and bronchoalveolar lavage fluid (BALF) is 0.8 µg/L in one single sample] (20). Next-generation sequencing (NGS) in BALF can be used to detect aspergillosis. All patients had clinical presentation, imaging abnormalities, and at least one positive microbiological report. We collected the patient’s general information, disease history and medication history, the concurrent presence of other systemic diseases, underlying lung disease, biochemical tests, and the patient’s lung computed tomography (CT) images. In order to confirm whether the patient had aspergillus infection, we used sputum, blood, or BALF for fluorescence staining or culture of aspergillus, and further NGS examination could be performed if the diagnosis was still not confirmed. We performed (1,3)-beta-D-glucan test (G test), GM test (from blood or BALF), and specific antibodies related to aspergillus in some cases. If we could obtain lesion tissue samples, special pathological staining was performed. If it was determined that the patient had aspergillus infection, we initiated anti-aspergillus treatment, usually voriconazole, itraconazole, isavuconazole, amphotericin B, and so on. We collected medical records of these patients and then followed up by telephone; for patients who died, the end point of follow-up was defined as the date of death, and for surviving patients, the end point of follow-up was defined as the time of last telephone contact. Finally, we ruled out missing follow-ups and untreated patients. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Anhui Chest Hospital (No. KJ2024-051) and the requirement for individual consent for this retrospective analysis was waived.
Statistical analyses
Qualitative variables were shown as percentages and quantitative variables as mean ± standard deviation (SD). We used Kaplan-Meier curves to analyze patient survival. We analyzed the 1- and 3-year survival of emphysema in all patients; because the time of follow-up in this study was not very long, the data of 5-year survivals were deficient. Log-rank tests were utilized to compare survival among two groups. Univariate and multivariate Cox proportional hazards regression analyses were applied for identification of potential prognostic factors. All the relevant clinical potential prognostic factors were combined in the univariable analysis in sequence, with the method ‘Enter’. In addition, all the potential prognostic factors were combined in the multivariable analysis when P<0.05 in univariable analysis, also with the method ‘Enter’; the hazard ratio (HR) of a variable and 95% confidence interval (95% CI) were calculated. A P value <0.05 was considered statistically significant. Statistical analyses were performed using the software SPSS 27.0 (IBM Corp., Armonk, NY, USA) and Prism 10 (GraphPad Software, San Diego, CA, USA).
Results
Baseline characteristics
Finally, we collected 106 patients with CPA. Basic information is given in Table 1. The mean age at the time of diagnosis was 60.3±14.8 years. There were 48 (45.3%) patients older than 65 years; 74 (69.8%) patients were male. There were 24 (22.6%) patients with hypoalbuminemia, and 25 (23.6%) patients had a history of smoking. As for clinical manifestation, 52 (49.1%) patients had a productive cough, 19 (17.9%) had cough, 26 (24.5%) had fever, 40 (37.7%) had hemoptysis, and 37 (34.9%) had dyspnea. As for comorbidities, 19 (17.9%) had hypertension, 16 (15.1%) had diabetes, 10 (9.4%) had tumor, 6 (5.7%) had cerebral infarction, 7 (6.6%) had heart disease, 5 (4.7%) had autoimmune disease, and 4 (3.8%) had immunosuppressive diseases. As for the history of medications, 14 (13.2%) patients used inhaled corticosteroid, 5 (4.7%) used systemic corticosteroid because of lung transplantation, COPD, ILD, or hypersensitivity pneumonitis, and 1 (0.9%) patient used chemotherapeutics because of lung cancer. Bronchiectasis comprised 70.8% (n=75) of the underlying pulmonary diseases, followed by emphysema (37, 34.9%), lung TB scarring (25, 23.6%), COPD (23, 21.7%), lung cancer (8, 7.5%), active lung TB (7, 6.6%) and non-tuberculous mycobacterium (6, 5.7%), respectively.
Table 1. Demographical characteristics and clinical data of chronic pulmonary aspergillosis.
| Variables | Value (n=106) |
|---|---|
| Age (years) | 60.3±14.8 |
| ≥65 | 48 (45.3) |
| <65 | 58 (54.7) |
| Gender | |
| Male | 74 (69.8) |
| Female | 32 (30.2) |
| Smoking | 25 (23.6) |
| Hypoalbuminemia | 24 (22.6) |
| Clinical manifestation | |
| Fever | 26 (24.5) |
| Productive cough | 52 (49.1) |
| Cough | 19 (17.9) |
| Hemoptysis | 40 (37.7) |
| Dyspnea | 37 (34.9) |
| Comorbidities | |
| Heart disease | 7 (6.6) |
| Diabetes | 16 (15.1) |
| Presence of tumor | 10 (9.4) |
| Autoimmune disease | 5 (4.7) |
| Hypertension | 19 (17.9) |
| Cerebral infarction | 6 (5.7) |
| Immunosuppressive diseases | 4 (3.8) |
| History of medications | |
| Chemotherapeutics | 1 (0.9) |
| Inhaled corticosteroid | 14 (13.2) |
| Systemic corticosteroid | 5 (4.7) |
| Underlying pulmonary disease | 82 (77.4) |
| Chronic obstructive pulmonary disease | 23 (21.7) |
| Asthma | 2 (1.9) |
| Lung tuberculosis (activity) | 7 (6.6) |
| Emphysema | 37 (34.9) |
| Pneumothorax | 2 (1.9) |
| Non-tuberculous mycobacterium | 6 (5.7) |
| Bronchiectasis | 75 (70.8) |
| Lung cancer | 8 (7.5) |
| Pneumoconiosis | 2 (1.9) |
| Interstitial lung disease | 1 (0.9) |
| Lung tuberculosis scarring | 25 (23.6) |
Data are presented as mean ± standard deviation or n (%).
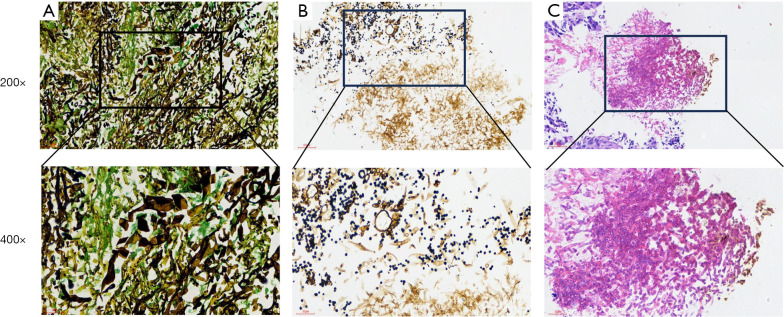
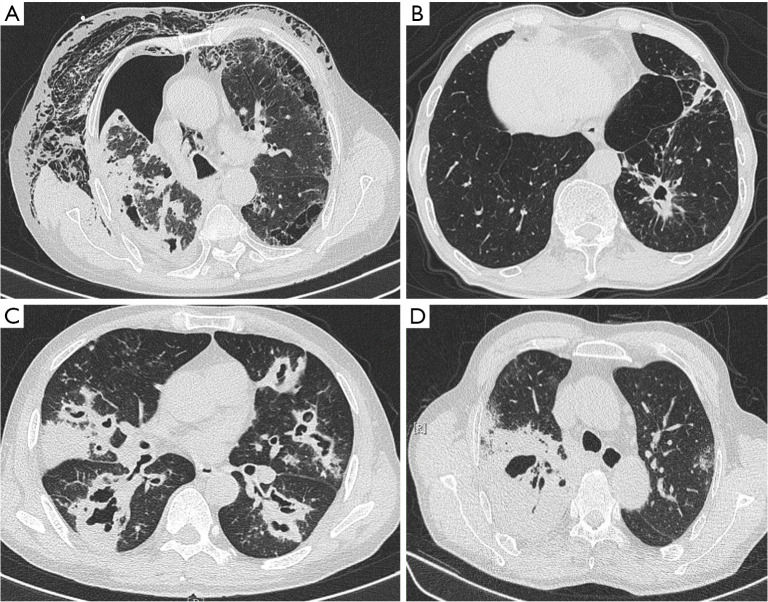
There were 5 (4.7%) patients diagnosed by histopathology (Figure 1), which showed the characteristic pathological pictures of pulmonary aspergillus infection. There were 82 (77.4%) patients who had positive microbiological tests of aspergillus, including fluorescence staining in sputum or BALF, smear microscopy and culture GM test in blood or BALF. Besides, 6 (5.7%) patients were detected using NGS. The most common aspergillus species found in our patients were Aspergillus flavus (A. flavus) (45, 42.5%), followed by Aspergillus fumigatus (A. fumigatus) (41, 38.7%), and Aspergillus niger (A. niger) (7, 6.6%). Some patients were coinfected with other microorganisms, 45 (42.5%) patients were simultaneously detected with Gram-negative bacteria, 34 (32.1%) with Gram-positive bacteria, 7 (6.6%) co-infected with TB, 2 (1.9%) co-infected with NTM, and 2 (1.9%) patients had mucor combined with aspergillosis. Characteristic CT images are shown in Figure 2.
Figure 1.
Characteristic pathological pictures of pulmonary aspergillus infection. (A) Silver hexamine staining shows mucor; (B) silver hexamine staining shows aspergillus; (C) HE staining of pathological sections showed aspergillus. HE, hematoxylin-eosin staining.
Figure 2.
Computed tomography shows some typical features of CPA. (A) A case of CPA complicated with pneumothorax and subcutaneous emphysema, and eventually died; (B) a case of emphysema with CPA; (C) a case of CPA due to bronchogenic spread; (D) a case co-infected by Aspergillus fumigatus, Aspergillus flavus and mucor. He died of massive hemoptysis. CPA, chronic pulmonary aspergillosis.
Prognostic factors
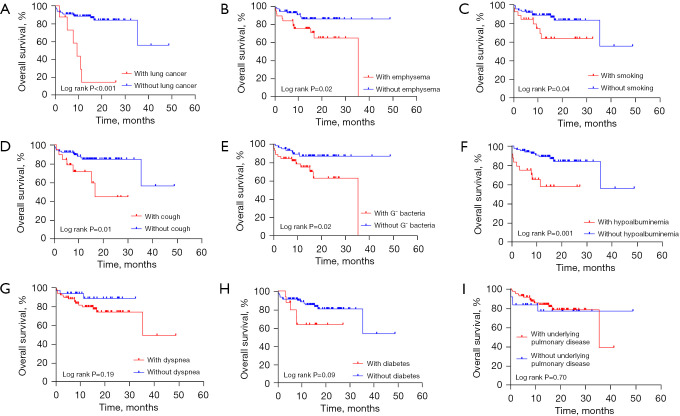
At the final follow-up time, 22 (20.8%) patients had died. Notably, 3 patients had died of a sudden hemoptysis. The 1- and 3-year survival rates with emphysema were 75.2% and 64.9%, respectively, and those for patients without emphysema were 92.6% and 85.9%, respectively. The survival curves according to different factors are shown in Figure 3. There was significant a difference between patients with and without lung cancer (P<0.001) (Figure 3A). Significant differences were also found in patients with and without emphysema (P=0.02) (Figure 3B). Other factors associated with mortality were as follows: smoking (P=0.04) (Figure 3C), cough (P=0.01) (Figure 3D), simultaneous discovery with Gram-negative bacteria (P=0.02) (Figure 3E), and hypoalbuminemia (P=0.001) (Figure 3F). In contrast, dyspnea (P=0.19) (Figure 3G), diabetes (P=0.09) (Figure 3H), underlying pulmonary disease (P=0.70) (Figure 3I), active lung TB, NTM, bronchiectasis, and lung TB scarring were not related to mortality (Table 2).
Figure 3.
Kaplan-Meier curves for survival probability in pulmonary aspergillosis according to lung cancer (A), emphysema (B), smoking (C), cough (D), Gram-negative bacteria (G− bacteria) (E), hypoalbuminemia (F), dyspnea (G), diabetes (H), and underlying pulmonary disease (I).
Table 2. Underlying pulmonary conditions and their effect on 106 chronic pulmonary aspergillosis.
| Underlying pulmonary diseases | Patients | Kaplan-Meier analysis | |||||
|---|---|---|---|---|---|---|---|
| 1-year survival (%) | 3-year survival (%) | P value (log-rank test) |
|||||
| With | Without | With | Without | ||||
| COPD | 23 (21.7) | 73±9.5 | 92.7±2.9 | – | 84.5±4.4 | 0.21 | |
| Lung tuberculosis | 7 (7.1) | 85.7±13.2 | 90.8±2.9 | – | 86.2±3.6 | 0.94 | |
| Emphysema | 37 (34.9) | 75.2±7.2 | 92.6±3.2 | 64.9±9.3 | 85.9±4.8 | 0.02 | |
| NTM | 6 (5.7) | 83.3±15.2 | 91±2.9 | – | 86.4±3.5 | 0.89 | |
| Bronchiectasis | 75 (70.8) | 91.9±3.2 | 76.2±7.9 | 86.6±4.2 | 33.4±24.1 | 0.052 | |
| Lung cancer | 8 (7.5) | 14.6±13.5 | 90.8±2.9 | – | 88.3±3.3 | <0.001 | |
| Lung tuberculosis scarring | 25 (23.6) | 77.9±8.8 | 91.3±3.1 | 62.3±15.6 | 83.6±4.4 | 0.28 | |
Data are presented as mean ± standard deviation or n (%). COPD, chronic obstructive pulmonary disease; NTM, non-tuberculous mycobacterium.
The results of the univariate and multivariate Cox analyses are shown in Table 3. There were five significant prognostic variables associated with mortality identified in univariate Cox regression analysis: smoking (HR, 2.538; 95% CI: 1.020–6.314; P=0.045), emphysema (HR, 2.849; 95% CI: 1.164–6.972; P=0.02), lung cancer (HR, 7.054; 95% CI: 2.649–18.787; P<0.001), with Gram-negative bacteria (HR, 2.966; 95% CI: 1.180–7.455; P=0.02), and hypoalbuminemia (HR, 3.976; 95% CI: 1.608–9.834; P=0.003). NTM (HR, 1.151; 95% CI: 0.153–8.637; P=0.89), bronchiectasis (HR, 0.425; 95% CI: 0.175–1.032; P=0.059), and COPD (HR, 1.810; 95% CI: 0.687–4.770; P=0.23) were not significantly associated with mortality. Multivariate Cox regression analysis showed that emphysema (HR, 4.107; 95% CI: 1.414–11.933; P=0.009) and lung cancer (HR, 8.511; 95% CI: 2.494–29.047; P<0.001) were independent predictors of mortality (Table 3).
Table 3. Analysis of prognostic factors related to mortality in 106 patients with chronic pulmonary aspergillosis.
| Characteristics | Univariate | Final multivariate | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Age | 1.518 (0.628–3.671) | 0.35 | – | – | |
| Male sex | 0.023 (0.000–1.057) | 0.053 | – | – | |
| Dyspnea | 2.261 (0.929–5.500) | 0.07 | – | – | |
| Fever | 1.219 (0.438–3.390) | 0.70 | – | – | |
| Hypoalbuminemia | 3.976 (1.608–9.834) | 0.003 | 1.746 (0.617–4.940) | 0.29 | |
| Underlying pulmonary condition | 0.818 (0.296–2.258) | 0.70 | – | – | |
| Smoking | 2.538 (1.020–6.314) | 0.045 | 1.061 (0.354–3.185) | 0.91 | |
| COPD | 1.810 (0.687–4.770) | 0.23 | – | – | |
| Lung tuberculosis (activity) | 0.045 (0.000–185.665) | 0.47 | – | – | |
| Emphysema | 2.849 (1.164–6.972) | 0.02 | 4.107 (1.414–11.933) | 0.009 | |
| Non-tuberculous mycobacterium | 1.151 (0.153–8.637) | 0.89 | – | – | |
| Bronchiectasis | 0.425 (0.175–1.032) | 0.059 | – | – | |
| Lung cancer | 7.054 (2.649–18.787) | <0.001 | 8.511 (2.494–29.047) | <0.001 | |
| Lung tuberculosis scarring | 1.701 (0.643–4.497) | 0.28 | – | – | |
| Diabetes | 2.324 (0.831–6.495) | 0.11 | – | – | |
| Use of systemic glucocorticoids | 1.516 (0.201–11.441) | 0.69 | – | – | |
| Gram-positive bacteria | 1.882 (0.778–4.551) | 0.16 | – | – | |
| Gram-negative bacteria | 2.966 (1.180–7.455) | 0.02 | 1.988 (0.748–5.287) | 0.17 | |
CI, confidence interval; COPD, chronic obstructive pulmonary disease.
Discussion
In the present study, we retrospectively analyzed the prognostic factors of 106 patients with CPA. We found that some prognostic factors were significant with the mortality of CPA. It has important significance for predicting the prognosis of patients.
From the basic patient information, we found that the most common symptoms were productive cough, hemoptysis, dyspnea, then fever, respectively. Similarly, Yu et al. (21) found that the main symptoms were cough, fever, dyspnea, and hemoptysis in CPA patients without granulosis. Bronchiectasis was the most common underlying pulmonary disease in our study, followed by emphysema, lung TB scarring, and COPD. The proportion varies among different study populations. Salzer et al. (22) found that the most common underlying pulmonary diseases in CPA patients were emphysema, COPD, and bronchiectasis, which is similar to our findings. Most studies have found that TB infection, COPD, and bronchiectasis are the most common underlying diseases in CPA (23). Bronchiectasis, as a significant imaging feature, can be found in a variety of diseases, such as TB or NTM, ABPA, congenital bronchiectasis, and so on. Bronchiectasis increases the chance of aspergillus infection. Yu et al. (21) found that the incidence of aspergillosis in patients complicated with bronchiectasis was higher than that in those without bronchiectasis. Besides, patients often have multiple underlying lung diseases; bronchiectasis comorbid with COPD, previous pulmonary TB, or NTM pulmonary disease will increase the risk of aspergillosis infection because of the abnormalities in mucociliary clearance and established lung structure in these comorbid diseases.
We found that the traditional methods of microbiological examination were still very important; 77.4% of patients had positive results using microbiological tests (fluorescence staining or culture in sputum or BALF), but NGS and histopathology could be helpful when traditional tests were negative. Akram et al. (24) found that the positive rate of microbiological culture in BALF or sputum was 84.9% in all CPA patients. Among the more than 250 kinds of aspergillus species, A. fumigatus, A. flavus, Aspergillus terreus (A. terreus), and A. niger were more common (25). Through analysis of aspergillus species, the most common aspergillus species found in our patients was A. flavus (42.5%), followed by A. fumigatus (38.7%), and A. niger (7.7%), respectively. Akram et al. found that the most common aspergillus species was A. fumigatus, which accounted for 45.9%, then A. flavus 37.2%, A. niger 9.2%, and A. terreus 7.8% (24). In our study, we found that patients had other bacterial infections while simultaneous merging with aspergillus infection. There were 2 patients in our study who had mucor combined with aspergillosis; this incidence rate is high, which may because our tests are more comprehensive, enabling a higher detection rate. When aspergillus and mucor are co-infected at the same time, the diagnosis and treatment strategies are complex (26). Mucormycosis is known to have a high mortality rate (27). We can imagine when pulmonary aspergillus coinfects with mucor, the mortality could be increased. Our results showed that CPA simultaneously discovered with Gram-negative bacteria was common, 32.1% patients were also found to have Gram-positive bacteria, some of them probable co-infected with all, whereas 42.5% of patients were detected with Gram-negative bacteria; the rate of co-infection with Gram-negative bacteria was higher than that of Gram-positive bacteria. However, we did not analyze the specific bacterial species, because the testing method was mostly direct smear microscopy of respiratory specimens. Recent studies have discovered the interaction of Pseudomonas aeruginosa (PA) and aspergillus. They interact with each other in the process of infecting. Volatile organic compounds secreted by PA stimulate A. fumigatus growth, and when co-cultured with filamentous fungi, the elastase produced by PA is increased, which can destroy the immunity of the host (28-30).
CPA often occurs in a structural lung disease caused by TB, but it is not easy to identify the simultaneous coinfection of TB and pulmonary aspergillus because of the similarity of symptoms and imaging features (31). We found seven patients co-infected with TB and pulmonary aspergillus. We hypothesize that some CPA patients may be infected with aspergillus at the time of TB infection. However, the interaction between TB and aspergillus in the course of infection has been rarely reported. We found six cases of co-infection of NTM and pulmonary aspergillus; NTM often occurs on the structural lung diseases, and patients often have immune dysfunction, which is also a high-risk factor for pulmonary aspergillosis. The clinical characteristics and imaging manifestations of these two diseases are very similar. So, the coinfection of the two pathogenic bacteria can often be underestimated. Moreover, the combination of NTM and aspergillus can lead to high mortality (32).
Our study found that the prognosis of CPA with lung cancer was significantly worse than that of CPA without lung cancer. Similar to our findings, a large sample study found that the risk of mortality of CPA patients with a history of lung cancer was high (33). In addition to the Gram-negative bacteria, pulmonary fungi can also be present (34). Fungal infections involve T cell-mediated immunity. While chemotherapy kills tumor cells it also affects immune cells. Immunotherapy may activate certain T cells, resulting in dysregulated immunity (35). In addition, distinguishing pulmonary aspergillosis from cancer recurrence is challenging; the early diagnosis of CPA localized in a single lung after non-small cell lung cancer surgery is important to improve the survival in patients with CPA (36). Therefore, the prognosis of lung cancer patients with aspergillus infection is poor. Research shows that the infection of aspergillus after lung cancer surgery had a profound impact on restrictive lung function deterioration (37).
Another important finding in our research is that emphysema is a poor prognostic factor of pulmonary aspergillosis. Emphysema characterized by severe diffuse lung destruction or airway obstruction is a high-risk factor for aspergillus infection, due to the disturbed local clearance, and emphysema often combines with COPD which requires inhaled corticosteroid or systemic use of glucocorticoids so the host immunity is weakened. Emphysema was the only factor associated with higher mortality in CPA (38). Emphysema is the most frequent underlying chronic pulmonary disease. When CPA occurs, the 5-year mortality is higher (16), so emphysema could worsen the prognosis of CPA. In addition, among Mycobacterium avium complex pulmonary disease, emphysema can increase the incidence of CPA, then contribute to poor prognosis owing to severe pulmonary infection (39).
There are some limitations in our study. First, this is a retrospective study, which may have caused some selection bias. The selection of the study population was limited by time and place. Second, the number of CPA patients is relatively small. Since we only studied CPA, IPA was not included in our research. Third, our follow-up time was relatively short, 5-year survival was deficient and future studies could extend the follow-up time.
Conclusions
We found that underlying pulmonary diseases, lung cancer, and emphysema are poor prognostic factors for CPA. In clinical work, more attention should be paid to the patients with underlying lung diseases, as timely diagnosis and treatment may improve their prognosis.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by the Foundation of Major Scientific Research Project of Health Commission of Anhui Province in 2023 (No. AHWJ2023A10125).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Anhui Chest Hospital (No. KJ2024-051) and the requirement for individual consent for this retrospective analysis was waived.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-831/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-831/coif). The authors have no conflicts of interest to declare.
Data Sharing Statement
Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-831/dss
References
- 1.Kwon-Chung KJ, Sugui JA. Aspergillus fumigatus--what makes the species a ubiquitous human fungal pathogen? PLoS Pathog 2013;9:e1003743. 10.1371/journal.ppat.1003743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax 2015;70:270-7. 10.1136/thoraxjnl-2014-206291 [DOI] [PubMed] [Google Scholar]
- 3.Cadena J, Thompson GR, 3rd, Patterson TF. Aspergillosis: Epidemiology, Diagnosis, and Treatment. Infect Dis Clin North Am 2021;35:415-34. 10.1016/j.idc.2021.03.008 [DOI] [PubMed] [Google Scholar]
- 4.Yamakawa H, Nishizawa T, Ohta H, et al. Patient background and prognosis of chronic pulmonary aspergillosis in fibrosing interstitial lung disease. Medicine (Baltimore) 2022;101:e29936. 10.1097/MD.0000000000029936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Soyza A, Aliberti S. Bronchiectasis and Aspergillus: How are they linked? Med Mycol 2017;55:69-81. 10.1093/mmy/myw109 [DOI] [PubMed] [Google Scholar]
- 6.Kurosaki F, Bando M, Nakayama M, et al. Clinical features of pulmonary aspergillosis associated with interstitial pneumonia. Intern Med 2014;53:1299-306. 10.2169/internalmedicine.53.1578 [DOI] [PubMed] [Google Scholar]
- 7.Ikuta KS, Meštrović T, Naghavi M. Global incidence and mortality of severe fungal disease. Lancet Infect Dis 2024;24:e268. 10.1016/S1473-3099(24)00102-6 [DOI] [PubMed] [Google Scholar]
- 8.Feys S, Gonçalves SM, Khan M, et al. Lung epithelial and myeloid innate immunity in influenza-associated or COVID-19-associated pulmonary aspergillosis: an observational study. Lancet Respir Med 2022;10:1147-59. 10.1016/S2213-2600(22)00259-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bongomin F, Harris C, Foden P, et al. Innate and Adaptive Immune Defects in Chronic Pulmonary Aspergillosis. J Fungi (Basel) 2017;3:26. 10.3390/jof3020026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barberan J, Sanz F, Hernandez JL, et al. Clinical features of invasive pulmonary aspergillosis vs. colonization in COPD patients distributed by gold stage. J Infect 2012;65:447-52. 10.1016/j.jinf.2012.07.006 [DOI] [PubMed] [Google Scholar]
- 11.Menon B, Nima G, Dogra V, et al. Evaluation of the radiological sequelae after treatment completion in new cases of pulmonary, pleural, and mediastinal tuberculosis. Lung India 2015;32:241-5. 10.4103/0970-2113.156233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen NTB, Le Ngoc H, Nguyen NV, et al. Chronic Pulmonary Aspergillosis Situation among Post Tuberculosis Patients in Vietnam: An Observational Study. J Fungi (Basel) 2021;7:532. 10.3390/jof7070532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ocansey BK, Otoo B, Adjei A, et al. Chronic pulmonary aspergillosis is common among patients with presumed tuberculosis relapse in Ghana. Med Mycol 2022;60:myac063. 10.1093/mmy/myac063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singla R, Singhal R, Rathore R, et al. Risk factors for chronic pulmonary aspergillosis in post-TB patients. Int J Tuberc Lung Dis 2021;25:324-6. 10.5588/ijtld.20.0735 [DOI] [PubMed] [Google Scholar]
- 15.Kimura Y, Sasaki Y, Suzuki J, et al. Prognostic factors of chronic pulmonary aspergillosis: A retrospective cohort of 264 patients from Japan. PLoS One 2021;16:e0249455. 10.1371/journal.pone.0249455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maitre T, Cottenet J, Godet C, et al. Chronic pulmonary aspergillosis: prevalence, favouring pulmonary diseases and prognosis. Eur Respir J 2021;58:2003345. 10.1183/13993003.03345-2020 [DOI] [PubMed] [Google Scholar]
- 17.Palanivel J, Mohapatra MM, Rajaram M, et al. Prevalence and risk factors for chronic pulmonary aspergillosis in chronic obstructive pulmonary disease patients with acute exacerbations. Monaldi Arch Chest Dis 2024. [Epub ahead of print]. doi: . 10.4081/monaldi.2024.2927 [DOI] [PubMed] [Google Scholar]
- 18.Phoompoung P, Chayakulkeeree M. Chronic Pulmonary Aspergillosis Following Nontuberculous Mycobacterial Infections: An Emerging Disease. J Fungi (Basel) 2020;6:346. 10.3390/jof6040346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ray A, Chowdhury M, Sachdev J, et al. Efficacy of LD Bio Aspergillus ICT Lateral Flow Assay for Serodiagnosis of Chronic Pulmonary Aspergillosis. J Fungi (Basel) 2022;8:400. 10.3390/jof8040400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Oliveira VF, Silva GD, Taborda M, et al. Systematic review and meta-analysis of galactomannan antigen testing in serum and bronchoalveolar lavage for the diagnosis of chronic pulmonary aspergillosis: defining a cutoff. Eur J Clin Microbiol Infect Dis 2023;42:1047-54. 10.1007/s10096-023-04639-0 [DOI] [PubMed] [Google Scholar]
- 21.Yu Q, He J, Xing B, et al. Potential value of serum Aspergillus IgG antibody detection in the diagnosis of invasive and chronic pulmonary aspergillosis in non-agranulocytic patients. BMC Pulm Med 2020;20:89. 10.1186/s12890-020-1125-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salzer HJ, Heyckendorf J, Kalsdorf B, et al. Characterization of patients with chronic pulmonary aspergillosis according to the new ESCMID/ERS/ECMM and IDSA guidelines. Mycoses 2017;60:136-42. 10.1111/myc.12589 [DOI] [PubMed] [Google Scholar]
- 23.Camara B, Reymond E, Saint-Raymond C, et al. Characteristics and outcomes of chronic pulmonary aspergillosis: a retrospective analysis of a tertiary hospital registry. Clin Respir J 2015;9:65-73. 10.1111/crj.12105 [DOI] [PubMed] [Google Scholar]
- 24.Akram W, Ejaz MB, Mallhi TH, et al. Clinical manifestations, associated risk factors and treatment outcomes of Chronic Pulmonary Aspergillosis (CPA): Experiences from a tertiary care hospital in Lahore, Pakistan. PLoS One 2021;16:e0259766. 10.1371/journal.pone.0259766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugui JA, Kwon-Chung KJ, Juvvadi PR, et al. Aspergillus fumigatus and related species. Cold Spring Harb Perspect Med 2014;5:a019786. 10.1101/cshperspect.a019786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teng P, Han X, Zhang S, et al. Mixed invasive pulmonary Mucor and Aspergillus infection: a case report and literature review. Chin Med J (Engl) 2022;135:854-6. 10.1097/CM9.0000000000001839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis 2019;19:e405-21. 10.1016/S1473-3099(19)30312-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keown K, Reid A, Moore JE, et al. Coinfection with Pseudomonas aeruginosa and Aspergillus fumigatus in cystic fibrosis. Eur Respir Rev 2020;29:200011. 10.1183/16000617.0011-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao J, Yu W. Interaction between Pseudomonas aeruginosa and Aspergillus fumigatus in cystic fibrosis. PeerJ 2018;6:e5931. 10.7717/peerj.5931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan K, Yin H, Wang J, et al. Subtle relationships between Pseudomonas aeruginosa and fungi in patients with cystic fibrosis. Acta Clin Belg 2022;77:425-35. 10.1080/17843286.2020.1852850 [DOI] [PubMed] [Google Scholar]
- 31.Kim SH, Kim MY, Hong SI, et al. Invasive Pulmonary Aspergillosis-mimicking Tuberculosis. Clin Infect Dis 2015;61:9-17. 10.1093/cid/civ216 [DOI] [PubMed] [Google Scholar]
- 32.Fayos M, Silva JT, López-Medrano F, et al. Non-Tuberculous Mycobacteria and Aspergillus Lung Co-Infection: Systematic Review. J Clin Med 2022;11:5619. 10.3390/jcm11195619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin SH, Kim BG, Kang J, et al. Incidence and Risk Factors of Chronic Pulmonary Aspergillosis Development during Long-Term Follow-Up after Lung Cancer Surgery. J Fungi (Basel) 2020;6:271. 10.3390/jof6040271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Li J, Wu Q, et al. Pathogen distribution in pulmonary infection in chinese patients with lung cancer: a systematic review and meta-analysis. BMC Pulm Med 2023;23:402. 10.1186/s12890-023-02681-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morelli T, Fujita K, Redelman-Sidi G, et al. Infections due to dysregulated immunity: an emerging complication of cancer immunotherapy. Thorax 2022;77:304-11. 10.1136/thoraxjnl-2021-217260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugimoto S, Soh J, Suzawa K, et al. Pulmonary aspergillosis as a late complication after surgery for locally advanced non-small cell lung cancer treated with induction chemoradiotherapy. Surg Today 2020;50:863-71. 10.1007/s00595-020-01960-5 [DOI] [PubMed] [Google Scholar]
- 37.Kim BG, Choi YS, Shin SH, et al. Mortality and lung function decline in patients who develop chronic pulmonary aspergillosis after lung cancer surgery. BMC Pulm Med 2022;22:436. 10.1186/s12890-022-02253-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koyama K, Ohshima N, Suzuki J, et al. Evaluation of clinical characteristics and prognosis of chronic pulmonary aspergillosis depending on the underlying lung diseases: Emphysema vs prior tuberculosis. J Infect Chemother 2015;21:795-801. 10.1016/j.jiac.2015.08.006 [DOI] [PubMed] [Google Scholar]
- 39.Takasaka N, Hosaka Y, Fukuda T, et al. Impact of emphysema on the prognosis of Mycobacterium avium complex pulmonary disease. Respir Med 2022;192:106738. 10.1016/j.rmed.2022.106738 [DOI] [PubMed] [Google Scholar]