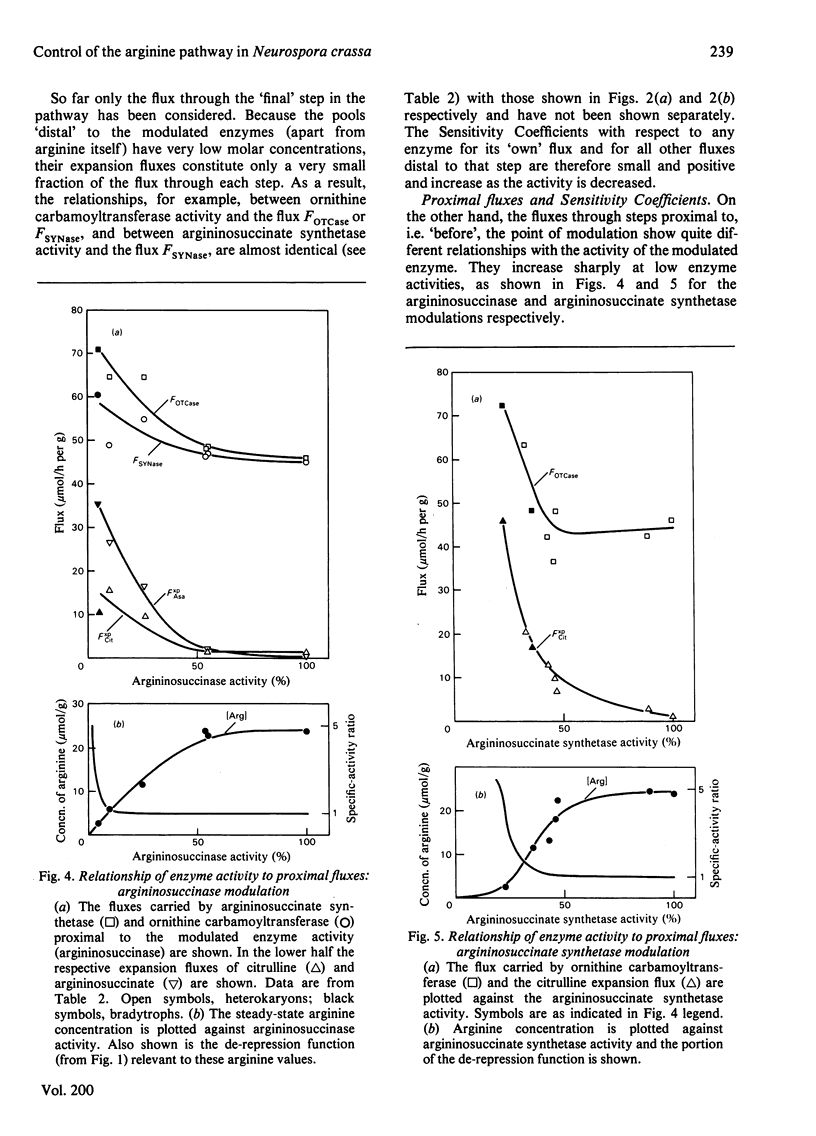
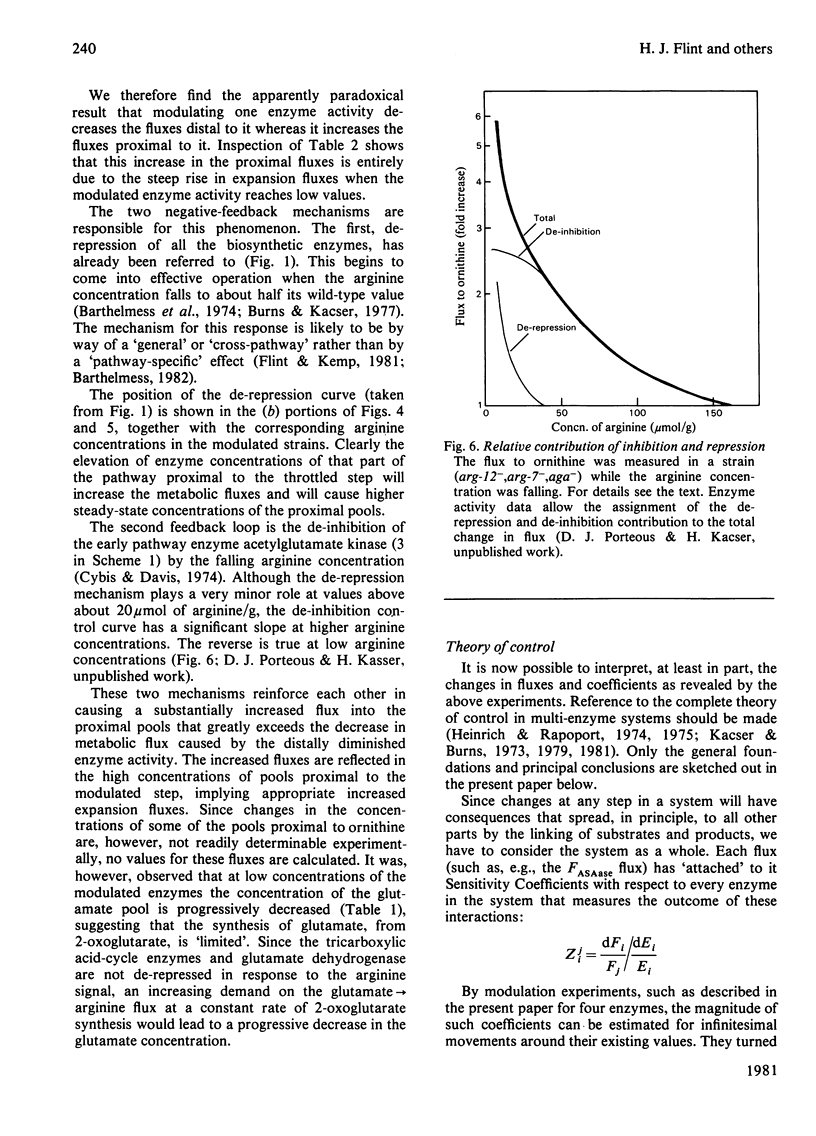
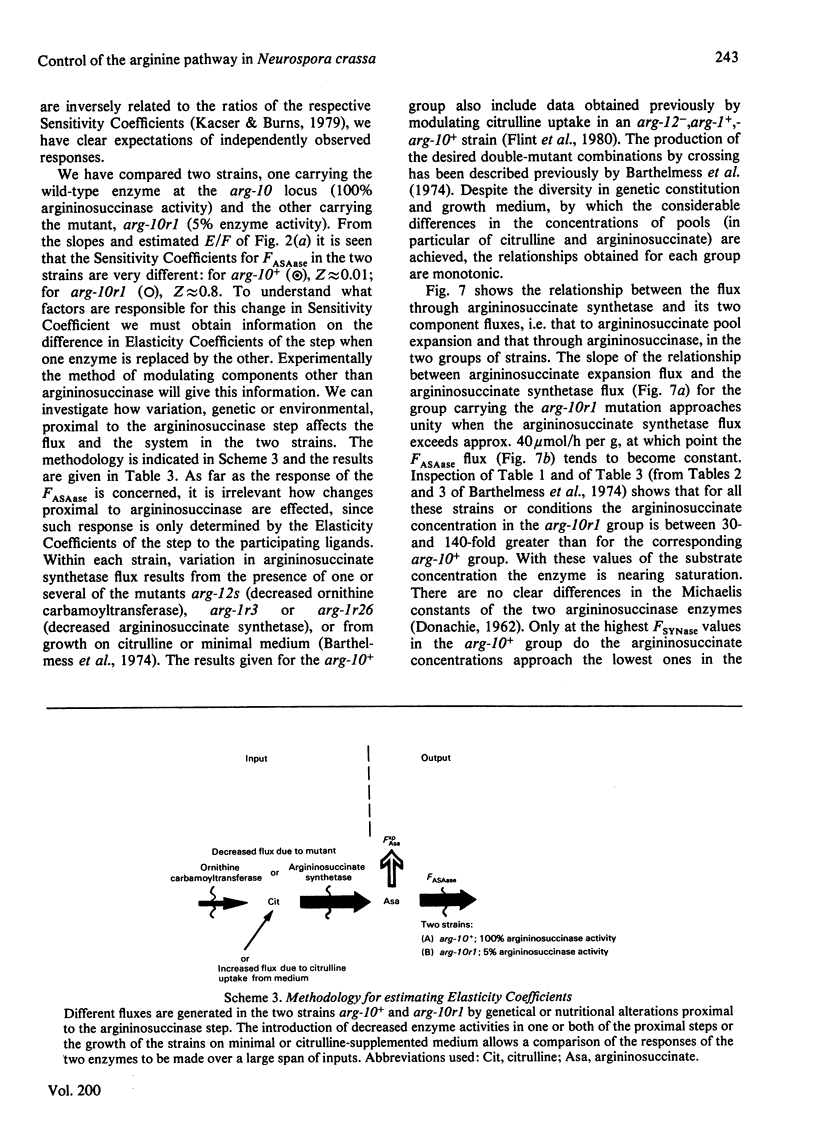
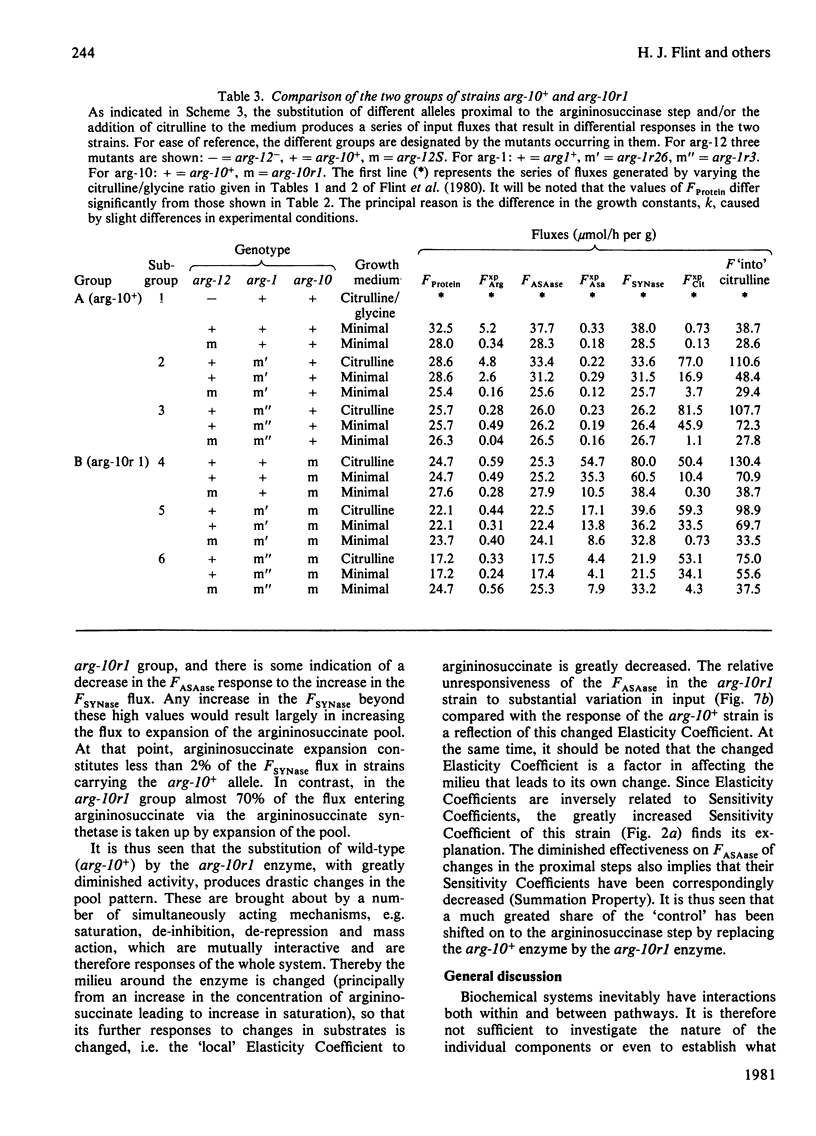
Abstract
The influence of particular enzyme activities on the flux of metabolites in a pathway can be estimated by 'modulating' enzymes (i.e. changing turnover or concentration) and measuring the response in various parts of the system. By controlling the nuclear ration of two genetically different nuclear types in heterokaryons, the enzyme concentrations at four different steps in the arginine pathway were decreased over a range. This range was extended by the use of bradytrophs, mutant strains specifying enzymes with greatly diminished enzyme activities. Strains altered simultaneously at more than one step were also constructed by genetic recombination. By measuring the outputs of the pathway and the steady-state concentrations of intermediate pools, the fluxes in different parts of the pathway were calculated. This allowed the construction of flux/enzyme relationships, the slope of which is a measure of the sensitivity of a flux to the change in enzyme activity at that step. All fluxes were found to be considerably buffered for quite substantial decreases in the activities of all enzymes. Mass action plays an important part in this phenomenon, as do inhibition and repression. Because of the existence of expansion fluxes in growing systems, we find quantitatively different fluxes in different parts of the single pathway. For the same reason some enzyme modulations given decreased fluxes in one part and increased fluxes in another. The understanding of control in the pathway thus involves consideration of many mechanisms operating simultaneously and the estimation of changes in the whole system. The concept of a 'rate-limiting step' is found to be inadequate and is replaced by a quantitative measure, the Sensitivity Coefficient, which takes account of all the interactions. It is shown that control of the flux is shared among all the enzymes of the pathway. The results are discussed in terms of the theory of flux control.
Full text
PDF





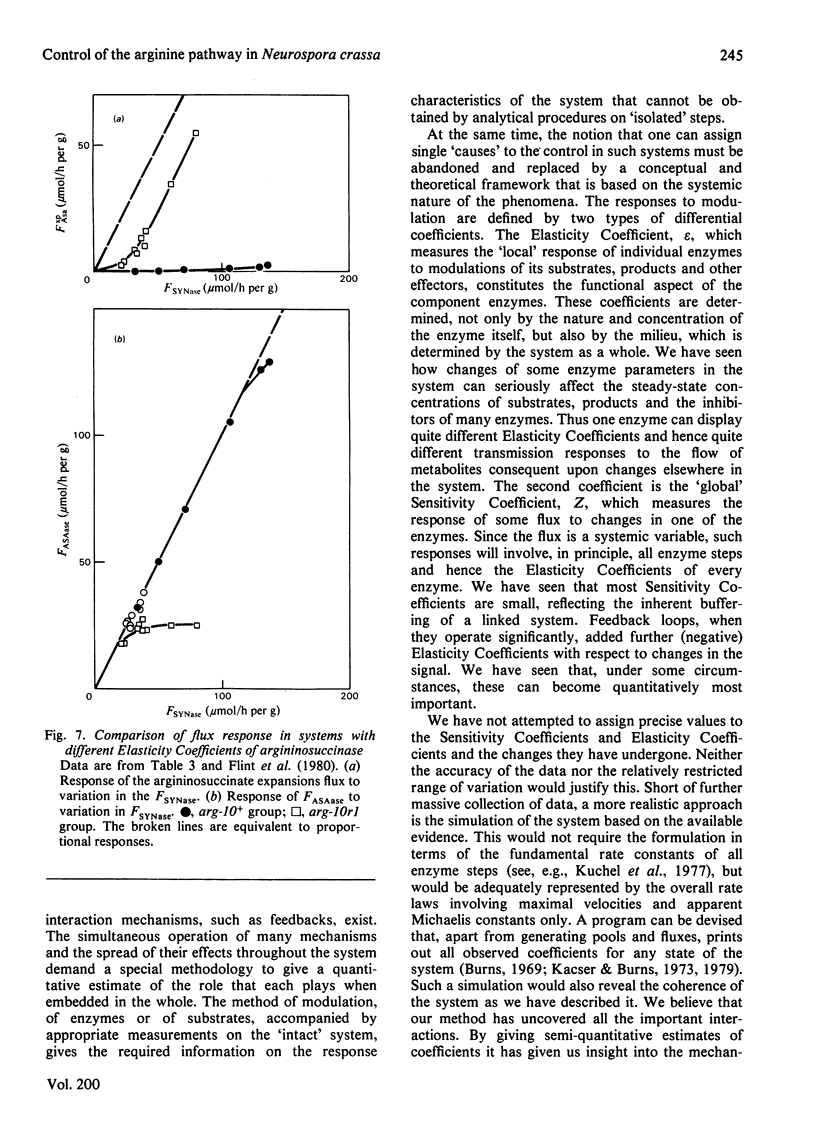

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
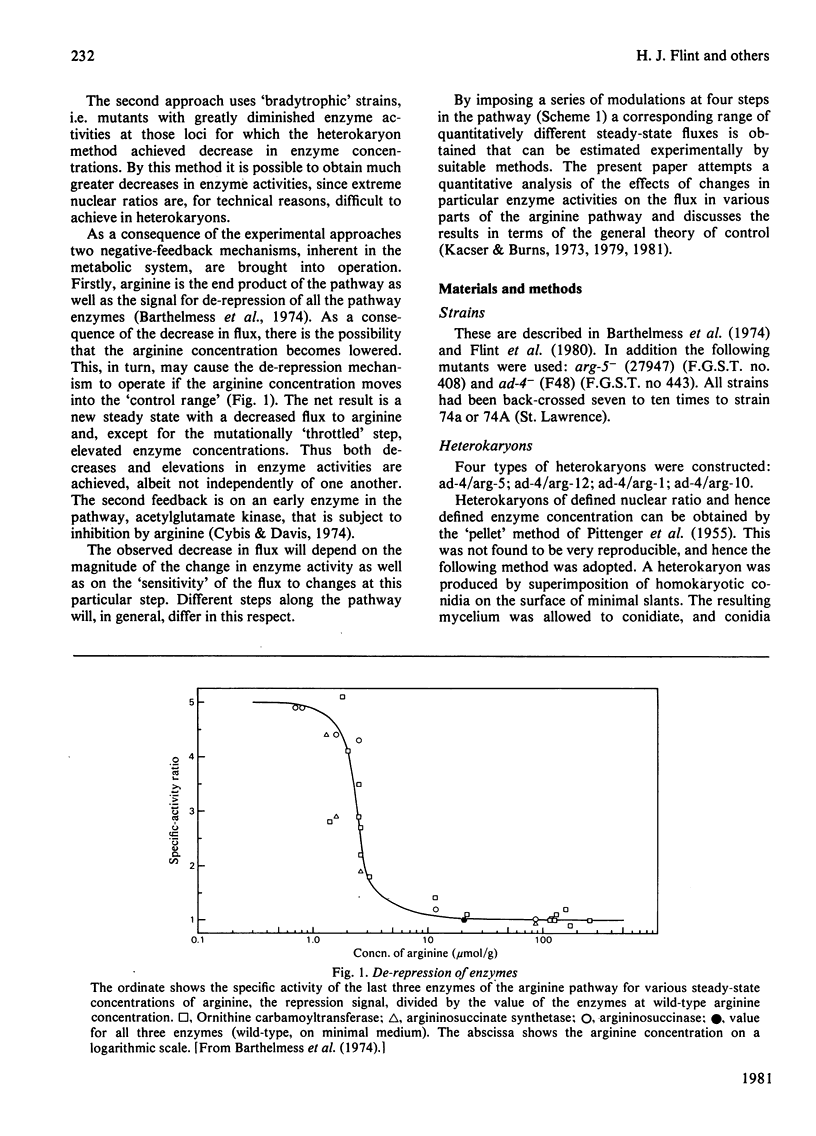
- Barthelmess I. B., Curtis C. F., Kacser H. Control of the flux to arginine in Neurospora crassa: de-repression of the last three enzymes of the arginine pathway. J Mol Biol. 1974 Aug 5;87(2):303–316. doi: 10.1016/0022-2836(74)90151-x. [DOI] [PubMed] [Google Scholar]
- Burns J. A., Kacser H. Allosteric repression: an analysis. J Theor Biol. 1977 Sep 21;68(2):199–213. doi: 10.1016/0022-5193(77)90159-x. [DOI] [PubMed] [Google Scholar]
- Burns J. A. Steady states of general multi-enzyme networks and their associated properties. Computational approaches. FEBS Lett. 1969 Mar;2 (Suppl 1)(NaN):S30–S33. doi: 10.1016/0014-5793(69)80073-6. [DOI] [PubMed] [Google Scholar]
- Cybis J. J., Davis R. H. Acetylglutamate kinase: a feedback-sensitive enzyme of arginine biosynthesis in Neurospora. Biochem Biophys Res Commun. 1974 Sep 23;60(2):629–634. doi: 10.1016/0006-291x(74)90287-3. [DOI] [PubMed] [Google Scholar]
- Cybis J., Davis R. H. Organization and control in the arginine biosynthetic pathway of Neurospora. J Bacteriol. 1975 Jul;123(1):196–202. doi: 10.1128/jb.123.1.196-202.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONACHIE W. D. THE REGULATION OF PYRIMIDINE BIOSYNTHESIS IN NEUROSPORA CASSA. II. HETEROKARYONS AND THE ROLE OF THE "REGULATORY MECHANISMS". Biochim Biophys Acta. 1964 Feb 10;82:293–302. doi: 10.1016/0304-4165(64)90300-9. [DOI] [PubMed] [Google Scholar]
- Davis R. H. Sources of urea in Neurospora. Biochim Biophys Acta. 1970 Aug 14;215(2):412–414. doi: 10.1016/0304-4165(70)90042-5. [DOI] [PubMed] [Google Scholar]
- Flint H. J., Kemp B. F. General control of arginine biosynthetic enzymes in Neurospora crassa. J Gen Microbiol. 1981 May;124(1):129–140. doi: 10.1099/00221287-124-1-129. [DOI] [PubMed] [Google Scholar]
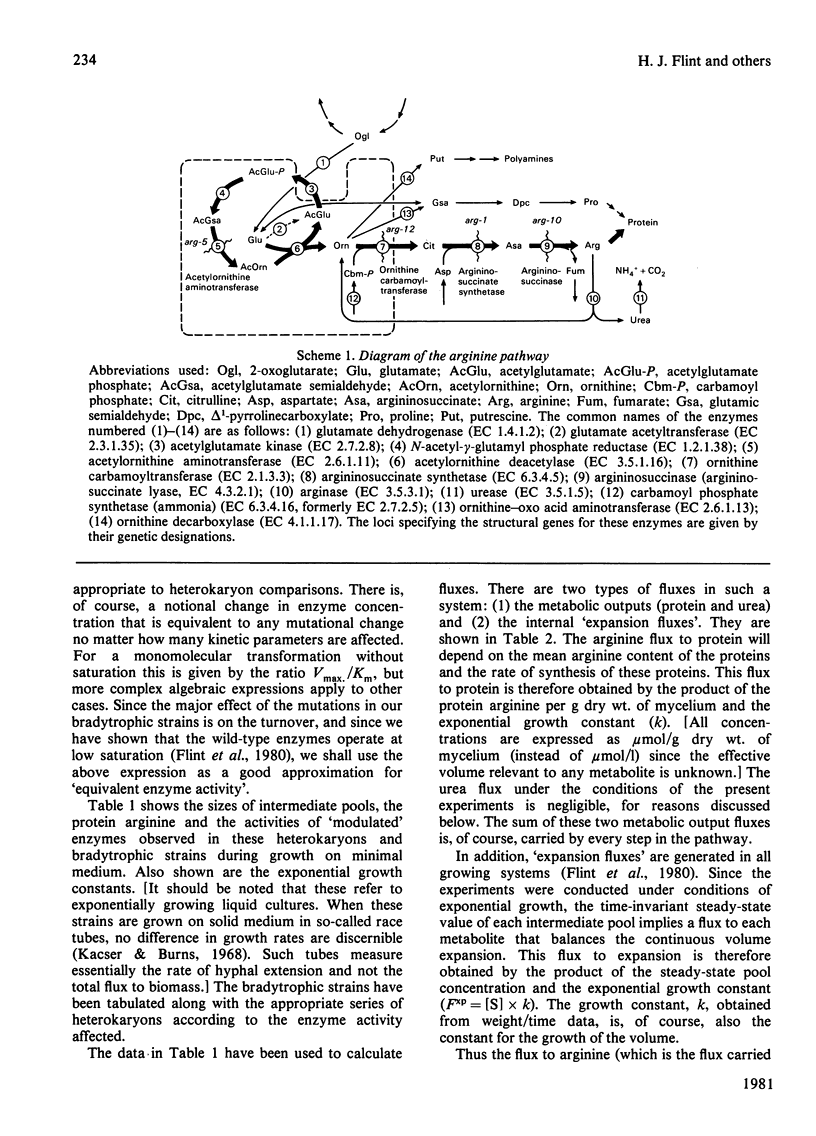
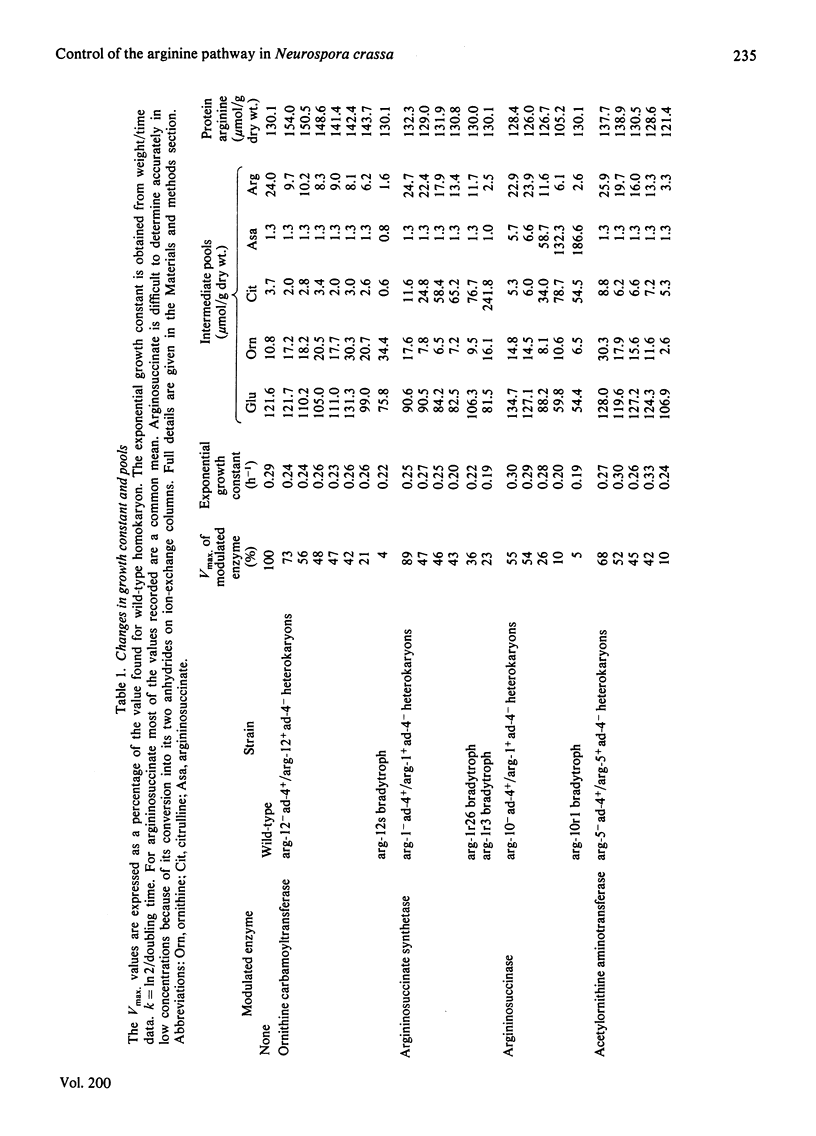
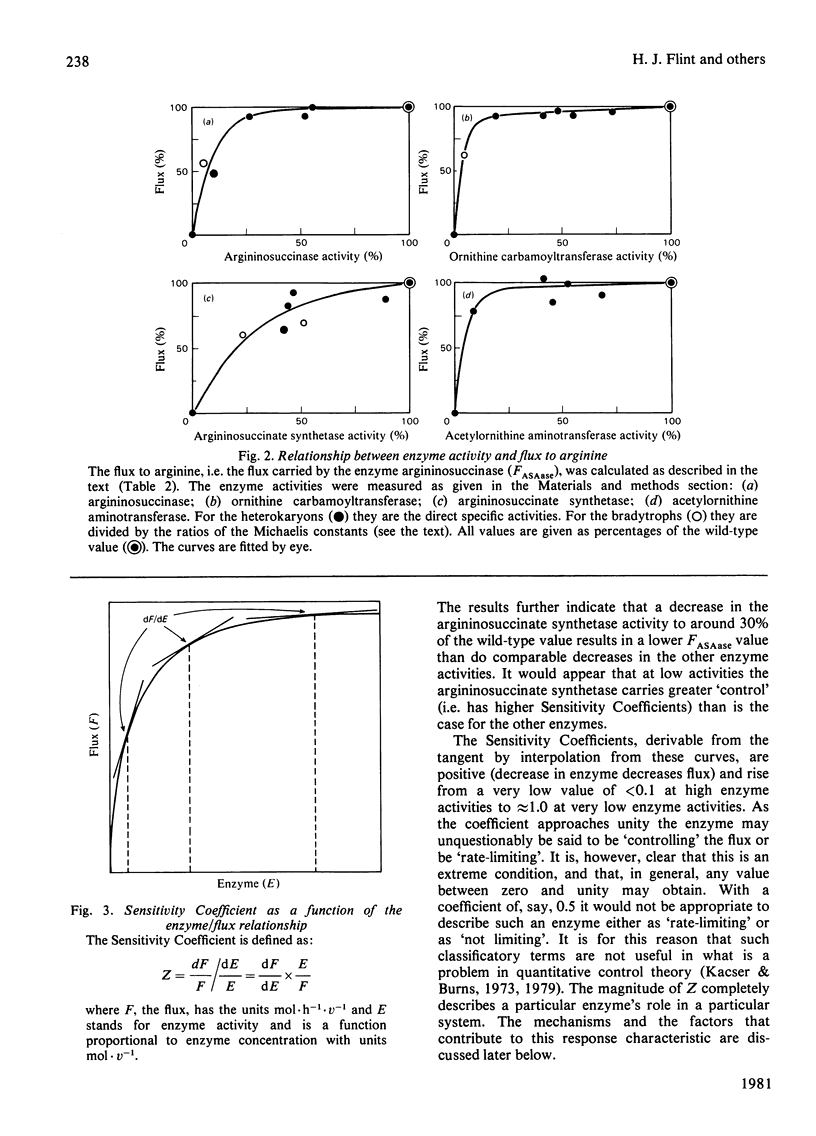
- Flint H. J., Porteous D. J., Kacser H. Control of the flux in the arginine pathway of Neurospora crassa. The flux from citrulline to arginine. Biochem J. 1980 Jul 15;190(1):1–15. doi: 10.1042/bj1900001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich R., Rapoport T. A. Mathematical analysis of multienzyme systems. II. Steady state and transient control. Biosystems. 1975 Jul;7(1):130–136. doi: 10.1016/0303-2647(75)90050-7. [DOI] [PubMed] [Google Scholar]
- Kacser H., Burns J. A. MOlecular democracy: who shares the controls? Biochem Soc Trans. 1979 Oct;7(5):1149–1160. doi: 10.1042/bst0071149. [DOI] [PubMed] [Google Scholar]
- Kacser H., Burns J. A. The control of flux. Symp Soc Exp Biol. 1973;27:65–104. [PubMed] [Google Scholar]
- Kacser H., Burns J. A. The molecular basis of dominance. Genetics. 1981 Mar-Apr;97(3-4):639–666. doi: 10.1093/genetics/97.3-4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchel P. W., Roberts D. V., Nichol L. W. The simulation of the urea cycle: correlation of effects due to inborn errors in the catalytic properties of the enzymes with clinical-biochemical observations. Aust J Exp Biol Med Sci. 1977 Jun;55(3):309–326. doi: 10.1038/icb.1977.26. [DOI] [PubMed] [Google Scholar]
- Rapoport T. A., Heinrich R., Jacobasch G., Rapoport S. A linear steady-state treatment of enzymatic chains. A mathematical model of glycolysis of human erythrocytes. Eur J Biochem. 1974 Feb 15;42(1):107–120. doi: 10.1111/j.1432-1033.1974.tb03320.x. [DOI] [PubMed] [Google Scholar]
- Subramanian K. N., Weiss R. L., Davis R. H. Use of external, biosynthetic, and organellar arginine by Neurospora. J Bacteriol. 1973 Jul;115(1):284–290. doi: 10.1128/jb.115.1.284-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


