Abstract
Respiratory syncytial virus (RSV) infection is the major cause of severe bronchiolitis in infants. Pathology of this infection is partly due to excessive proinflammatory leukocyte influx mediated by chemokines. Although direct infection of the respiratory epithelium by RSV may induce chemokine secretion, little is known about the role of cytokine networks. We investigated the effects of conditioned medium (CM) from RSV-infected monocytes (RSV-CM) on respiratory epithelial (A549) cell chemokine release. RSV-CM, but not control CM (both at a 1:5 dilution), stimulated interleukin-8 (IL-8) secretion from A549 cells within 2 h, and secretion increased over 72 h to 11,360 ± 1,090 pg/ml without affecting cell viability. In contrast, RSV-CM had only a small effect on RANTES secretion. RSV-CM interacted with direct RSV infection to synergistically amplify IL-8 secretion from respiratory epithelial cells (levels of secretion at 48 h were as follows: RSV-CM alone, 8,140 ± 2,160 pg/ml; RSV alone, 12,170 ± 300 pg/ml; RSV-CM plus RSV, 27,040 ± 5,260 pg/ml; P < 0.05). RSV-CM induced degradation of IκBα within 5 min but did not affect IκBβ. RSV-CM activated transient nuclear binding of NF-κB within 1 h, while activation of NF-IL6 was delayed until 8 h and was still detectable at 24 h. Promoter-reporter analysis demonstrated that NF-κB binding was essential and that NF-IL6 was important for IL-8 promoter activity in RSV-CM-activated cells. Blocking experiments revealed that the effects of RSV-CM depended on monocyte-derived IL-1 but that tumor necrosis factor alpha was not involved in this network. In summary, RSV infection of monocytes results in and amplifies direct RSV-mediated IL-8 secretion from respiratory epithelial cells by an NF-κB-dependent, NF-IL6-requiring mechanism.
Respiratory syncytial virus (RSV) is a major respiratory pathogen. RSV-induced bronchiolitis and pneumonia in infants result in at least 91,000 hospital admissions in the United States each year and 20,000 admissions annually in the United Kingdom (2, 18). RSV is also recognized as a contributor to illness among adult populations including the immunosuppressed and the elderly (24, 28). Further, RSV is a precipitant of asthma attacks and infection may predispose susceptible children to recurrent wheezing later in life (55, 66). Tissue damage in RSV-associated respiratory infection is due to the adverse consequences of inflammatory cell influx as well as to direct effects of viral infection. Severe infection is characterized by extensive inflammatory cell recruitment into the lower airways initially by neutrophils and then by T lymphocytes, monocytes, and eosinophils (27, 40, 49). At later stages of infection, T lymphocytes and monocytes predominate in the cellular infiltrate (1).
Recruitment of leukocytes to the site of RSV infection is regulated by chemokines secreted by epithelial cells and monocytes. Interleukin-8 (IL-8), a C-X-C chemokine, is a chemoattractant and activator for neutrophils and T cells (7, 8, 44) and more recently has been shown to have a role in monocyte recruitment (33). RANTES, a C-C chemokine, is an attractant and activator of monocytes, T lymphocytes, basophils, and eosinophils (13, 60, 62). Both these chemokines, but not others such as monocyte chemotactic protein 3 and macrophage inflammatory protein-1β (MIP-1β), are secreted from bronchial epithelial cells directly infected with RSV in vitro (3, 11, 50). Elevated IL-8 and RANTES concentrations have been found in nasal lavage samples from children with RSV infection (51), and concentrations of these chemokines correlated with white blood cell counts in nasal washes from children with RSV disease (63). Recently IL-8, along with MIP-1α and MIP-1β was also shown to be released from RSV-infected neutrophils (38). We have demonstrated elevated systemic IL-8 concentrations in infants with severe RSV bronchiolitis compared to healthy controls (14), and recent work suggests that plasma IL-8 may be a marker of disease severity in infants (15).
In vitro models of RSV infection of human respiratory epithelial cells and cell lines have provided useful tools to elucidate mechanisms of molecular control of chemokine secretion. However, studies have mainly focused on the direct effects of RSV infection of either epithelial cells or macrophages. For example, RSV-induced IL-8 secretion from pulmonary epithelial cells was dependent on activation of the transcription factors NF-κB and NF-IL6 (29, 37, 46). We have shown, using adenoviral vectors, that NF-κB is critical in regulating RANTES transcription and protein secretion, processes that are dependent on viral replication within epithelial cells (71). In addition, human alveolar macrophages and mononuclear cells infected with RSV secrete IL-8 (4, 10) and neutrophils exposed to RSV-antibody complexes also secrete high IL-8 concentrations (5). Little is known about whether mediators from RSV-infected leukocytes influence chemokine secretion from pulmonary epithelial cells, although, in bacterial infections, networks in which lipopolysaccharide-induced macrophage-derived mediators (but not direct stimulation) caused IL-8 secretion from pulmonary epithelial cells have been shown to exist (64).
The purpose of this study was to investigate whether RSV infection of monocytes could stimulate and interact with direct infection of respiratory epithelial cells to cause chemokine secretion. Monocytes were chosen for study, as they are particularly important in the immune response to RSV since they are specifically recruited to the site of infection in large numbers. Further, monocytes are the leukocytes most susceptible to RSV infection (23). We have investigated the specific mechanism by which monocyte-derived mediators cause respiratory epithelial IL-8 secretion in a cellular model of RSV infection since there are data which suggest that they have the potential to be involved in chemokine networks (9). We demonstrate that conditioned medium (CM) from purified, RSV-infected monocytes (RSV-CM) stimulate IL-8 secretion from both the epithelial A549 cell line and primary epithelial cells. In contrast, RSV-CM only minimally upregulated RANTES secretion from pulmonary epithelial cells. The combined effects of RSV-CM and direct RSV infection on epithelial cell IL-8 secretion were synergistic. RSV-CM-stimulated IL-8 secretion from epithelial cells was critically dependent on NF-κB and was associated with rapid degradation of IκBα, but not IκBβ. Promoter-reporter analysis demonstrated that RSV-CM-induced IL-8 secretion required NF-IL6 activation in addition to NF-κB binding, although gel shift assays revealed marked differences in the time course of activation of each of these transcription factors. Monocyte-derived IL-1 was found to be a critical (although not necessarily sufficient) mediator of the effects of RSV-CM. However, in contrast to what was found for models of bacterial infection, tumor necrosis factor alpha (TNF-α) played little role. In summary, we have identified a novel mechanism of causing a synergistic increase in IL-8 secretion in a model of human RSV infection. Such an amplification network may be a significant factor in the development of excessive inflammatory cell influx to the site of RSV infection in clinically susceptible individuals.
MATERIALS AND METHODS
RSV growth and titration.
RSV (strain A2) was propagated in HEp-2 cells, cultured in Eagle's minimal essential medium (MEM) supplemented with 10% fetal calf serum, 2 mM glutamine, and nonessential amino acids. Cells were inoculated with RSV at a multiplicity of infection (MOI) of 0.1 and harvested after 4 to 5 days. The cell suspension was spun at 13,000 × g, and the virus-containing pellet was rapidly resuspended in fresh media, aliquoted, and snap frozen. The virus titer was quantitated using the microplaque immunoperoxidase method (20). Briefly, RSV stocks were serially diluted onto HEp-2 monolayers in 96-well plates. After 24 h, cells were fixed and RSV plaques were identified and counted by colorimetric staining using goat anti-human anti-RSV antibody as the primary antibody (Biogenesis, Oxon, United Kingdom).
Preparation of CM.
Human monocytes were prepared from buffy coat residues (from the North London Blood Transfusion Center, Collindale, United Kingdom). Briefly, mononuclear cells were isolated by density gradient centrifugation over Ficoll-Paque (Amersham-Pharmacia, Bucks, United Kingdom) and monocytes were adhesion-purified on tissue culture plastic for 2 h. The monocytes were washed well to remove nonadherent lymphocytes and cultured in serum-free Dulbeccos's MEM in the presence or absence of RSV (MOI = 1). After 24 h, the CM were removed, filtered centrifugally through a 100-kDa membrane to remove cells plus RSV, and then aliquoted and stored at −80°C. RSV-CM was harvested from RSV-infected monocytes and control CM (C-CM) was derived from noninfected cells.
Epithelial cell culture.
The type II alveolar epithelial (A549) cell line (45) was cultured in Dulbecco's MEM plus 10% fetal calf serum, 2 mM glutamine, and 10 μg of ampicillin/ml. Primary normal human bronchial epithelial (NHBE) cells were purchased from BioWhittaker (Wokingham, United Kingdom) and were maintained in supplemented basal epithelial growth medium (BEGM). Epithelial cells were exposed to either C-CM or RSV-CM at a 1:5 dilution in serum-free media for various times. Cell viability was assessed by trypan blue exclusion.
RNA extraction and Northern blotting.
Epithelial cell monolayers were washed in ice-cold phosphate-buffered saline (PBS) and then lysed in 4 M guanidine isothiocyanate–1 mM dithiothreitol (DTT) and stored at −80°C. RNA was extracted by a modified version of the guanidinium thiocyanate-phenol-chloroform extraction protocol (21). Then RNA was washed in 70% ethanol, and approximately 10-μg aliquots were run out on denaturing formaldehyde–1% agarose gels. RNA was transferred by capillary blotting to Hybond-N (Amersham International, Amersham, United Kingdom) and cross-linked by UV irradiation.
Oligonucleotide probing.
Northern blots were prehybridized for 1 h at 56.5°C in 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) containing 1× Denhardt's solution, 0.05% sodium pyrophosphate, 0.5% sodium dodecyl sulfate (SDS), 50 μg of tRNA/ml, and 50 μg of polyadenylic acid/ml. Hybridization was then performed overnight with a γ-32P-end-labeled oligonucleotide probe for either IL-8 or β-actin mRNA (67). Blots were then washed three times in 2× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7])–0.1% SDS solution and autoradiographed with intensifying screens at −80°C. Autoradiographs were scanned (Umax, Power Look II) and analyzed with National Institutes of Health (NIH). Image, version 1.52 (NIH Research Services Branch, Bethesda, Md.). IL-8 mRNA densitometry values were normalized for total RNA using β-actin mRNA densitometry. Blots were stripped between probings by washing them at 65°C for 1 h in a solution of 5 mM Tris-HCl (pH 8.0), 0.1× Denhardt's solution, and 2 mM disodium EDTA.
Electrophoretic mobility gel shift analysis (EMSA).
A549 cells were grown in 50-mm-diameter petri dishes and stimulated with CM. At specific time points, nuclear extracts were prepared using a modified version of the protocol of Durand et al. (25). Protein levels were quantified spectrophotometrically at 590 nm using the Bradford assay (16). Ten micrograms of nuclear extract was added to 0.3 pmol of 32P-end-labeled double-stranded DNA probe in a binding buffer consisting of 10 mM Tris-HCl (pH 7.9), 50 mM NaCl, 0.25 mM EDTA, 1 mM DTT, 0.25 mg of bovine serum albumin/ml, 5 μg of poly(dI-dC)/ml, and 25% glycerol. The probes used were for the IL-8 NF-κB site (5′-GTGGAATTTCC-3′) and for NF-IL6 (5′-TGCAGATTGTGCAATGTACG-3′) (Oswel Labs, Southampton, United Kingdom). Bound and free probes were separated on SDS–5% polyacrylamide gel electrophoresis (PAGE) gels and visualized by autoradiography. Competition assays were carried out by adding a 10-fold excess of either specific unlabeled probe or irrelevant probe.
Western blotting.
For detection of IκBα and IκBβ, cells were lysed with ice-cold PBS containing 0.1% SDS, 0.1% NP-40, 0.5% deoxycholate, 10 mM NaF, 1 mM VaS04, 170 μg of phenylmethylsulfonyl fluoride/ml, and a cocktail of protease inhibitors (leupeptin, E64, chymostatin, pepstatin, antipain, bestatin, and Pefabloc; all at 1 μg/ml). Lysates were harvested and centrifuged at 800 × g for 5 min at 4°C to pellet debris. Supernatant was recovered, and an equal volume of loading buffer (containing 50 mM HEPES [pH 6.8], 10% glycerol, 5% DTT, 2% SDS, and bromophenol blue) was added. The samples were boiled for 5 min and then immediately frozen at −80°C. Proteins were resolved by SDS-PAGE and transferred by electroblotting to a nitrocellulose membrane (Amersham). Western blots were blocked with PBS containing 0.05% Tween 20 and 5% milk protein before being incubated at 4°C overnight with either 0.5 μg of rabbit anti-human IκBα or 0.8 μg of rabbit anti-human IκBβ (both from Santa Cruz Biotechnology, Santa Cruz, Calif.). After being washed with PBS containing 0.1% Tween 20, the blot was incubated with peroxidase-conjugated goat anti-rabbit immunoglobulin G (Sigma, Poole, United Kingdom), and bands were detected via chemiluminescence.
Cell transfection and luciferase assays.
Promoter-reporter constructs containing the 5′ flanking region of the IL-8 gene (bases −1370 to +82) inserted into the firefly luciferase gene expression plasmid pGL2-basic were a kind gift from W. Reed (University of North Carolina). Three variants containing either the wild-type promoter or a promoter with mutations (lowercase) in either the NF-κB binding region (cTaCgAgT) or in the NF-IL6 binding site (GaatAATTTCC) were used. Plasmid DNA was purified from the Escherichia coli host using the QIAGEN plasmid purification system according to manufacturer's instructions (Qiagen, West Sussex, United Kingdom). A549 cells were grown to 50% confluency in six-well dishes and cotransfected overnight using FuGENE6 (Boehringer Mannheim, Sussex, United Kingdom) with 5 μg of experimental DNA plus 0.25 μg of control vector pRL-TK, which constitutively expresses Renilla luciferase. Transfected cells were stimulated for 2 h with RSV-CM, lysed into passive lysis buffer (Promega, Southampton, United Kingdom), and subjected to one freeze-thaw cycle. Firefly and Renilla luciferase activities were then assayed in lysates using the Dual-Luciferase Reporter system (Promega). Luciferase activity was normalized to Renilla activity to control for transfection efficiency. Data were expressed as percentages of maximal activity, which was obtained by stimulation of the wild-type promoter by RSV-CM.
Cytokine-neutralizing experiments.
In order to investigate the potential effects of proinflammatory mediators TNF and IL-1 (which are known to be released from RSV-infected monocytes) within RSV-CM, blocking agents were used to antagonize their effects. IL-1 activity was inhibited by preincubating epithelial cell monolayers for 2 h with 2, 20, or 200 μg of IL-1 receptor antagonist (IL-1Ra) (Peprotech, London, United Kingdom)/ml. In other experiments, RSV-CM was preincubated with rabbit anti-human neutralizing antibody against TNF-α at concentrations of 5, 20, and 50 μg/ml for 1 h at 37°C according to the manufacturer's instructions (Peprotech). Since we have found that IL-6 does not induce IL-8 secretion from epithelial cells (data not shown), a rabbit anti-human neutralizing antibody against IL-6 was preincubated with RSV-CM as a negative control. Antibody-treated RSV-CM was then applied to epithelial cells as described above. IL-1Ra was shown to act specifically by its ability to inhibit the effect of recombinant IL-1β (20 ng/ml), but not of recombinant human TNF-α (10 ng/ml), on epithelial cell IL-8 secretion. Similarly, the specificity of anti-TNF-α was confirmed by the inhibition of TNF-α-induced, but not IL-1β-induced, IL-8 secretion.
ELISA and presentation and analysis of data.
IL-8, RANTES, TNF-α, IL-1β, and IL-6 concentrations in supernatants were measured by specific enzyme-linked immunosorbent assay (ELISA) using matched-pair antibodies and recombinant standards from R&D Systems Europe Ltd. (Oxon, United Kingdom). The lower limit of sensitivity of the assays was 16 to 31 pg/ml. All cytokine data presented are mean ± standard errors of the mean (SEM) of at least three independent experiments. Comparison between groups was done by paired t tests.
RESULTS
RSV-CM-induced chemokine secretion from A549 cells.
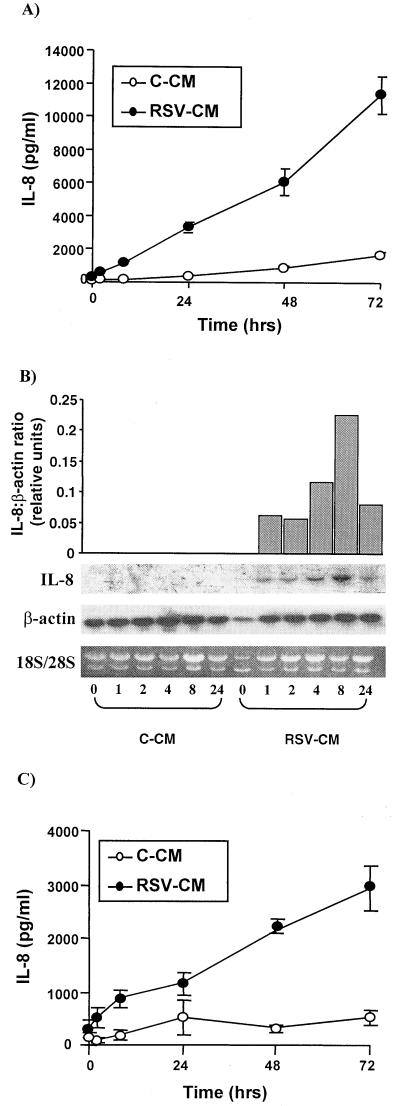
A549 cells were exposed to C-CM or RSV-CM at a 1:5 dilution and incubated for up to 72 h at 37°C, tissue culture supernatants were harvested, and cellular mRNA was extracted at specific time points. RSV-CM-treated cells secreted IL-8 protein within 2 h, and concentrations continued to increase over 72 h (Fig. 1A). Northern analysis demonstrated that IL-8 mRNA was present at 1 h, peaked at 8 h, and was still detectable at 24 h (Fig. 1B). In contrast C-CM caused minimal effect on IL-8 secretion or mRNA expression. The viability of pulmonary epithelial cells, as assessed by trypan blue exclusion, was not affected by either RSV-CM or C-CM. Percentages of viable cells following exposure to RSV-CM were 94.2% ± 1.4% and 90.5% ± 1.2% at 48 and 72 h, respectively. Corresponding percentages for C-CM were 94.5% ± 0.6% at 48 h and 92.4% ± 1.3% at 72 h (all viability counts were done in triplicate in two separate experiments).
FIG. 1.
Kinetics of RSV-CM-induced IL-8 from human respiratory epithelial cells. (A) A549 cells were stimulated with C-CM or RSV-CM at a 1:5 dilution. Cell culture supernatants were harvested at 0, 2, 8, 24, 48, and 72 h poststimulation, and IL-8 protein was measured by ELISA. (B) A549 cells were stimulated with C-CM or RSV-CM, and RNA was extracted from lysates taken at 0, 1, 2, 4, 8, and 34 h poststimulation. RNA was analyzed by ethidium bromide staining for 18S and 28S rRNA bands and by Northern blotting for IL-8 and β-actin mRNA. The graph depicts densitometrical analysis of IL-8 mRNA normalized for total mRNA using β-actin. (C) NHBE cells were stimulated with C-CM or RSV-CM as described for panel A. Results are the means ± SEM of three independent experiments (each using CM prepared on a separate occasion).
The A549 cell line is a well-established epithelial cell line which has been widely used as a model, particularly for RSV infection (3, 30, 37). However, it was important to confirm the physiological relevance of the present finding in primary human cells. Thus, NHBE cells were treated with RSV-CM or C-CM as described above, and supernatants were harvested at time points up to 72 h. Figure 1C shows that NHBE cells demonstrate a pattern of activation identical to that of A549 cells, with IL-8 protein levels similar to those for A549 detectable at 2 h; these levels increased up to 72 h in response to RSV-CM but not C-CM. This confirms that A549 cells are a good model in which to study responses to RSV-CM.
Since RANTES is secreted in response to direct RSV infection of pulmonary epithelial cells (11, 61), RANTES concentrations were also measured following exposure of A549 cells to RSV-CM. RSV-infected monocytes secreted appreciable levels of RANTES (levels of approximately 2 ng/ml were measured in undiluted RSV-CM), which is reflected at the 0-h time point of Fig. 2. However, RSV-CM only stimulated a small upregulation of RANTES secretion from epithelial cells (less than 500 pg/ml above levels at the zero time point), which principally occurred after 48 h (Fig. 2). Thus, RSV-CM has a much greater effect on IL-8 secretion than on RANTES release from respiratory epithelial cells.
FIG. 2.
Kinetics of RSV-CM-induced RANTES secretion from A549 cells. Cells were stimulated with C-CM or RSV-CM, and culture supernatants were harvested at specific time points up to 72 h. RANTES protein was measured in the supernatants by ELISA. Results are means ± SEM of three independent experiments (each using CM prepared on a separate occasion).
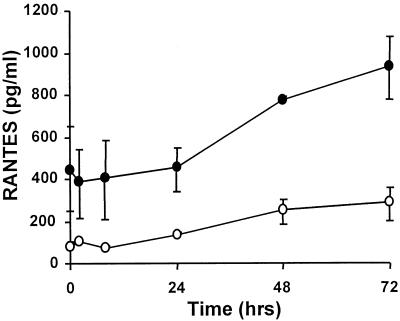
Combined effect of direct RSV infection and RSV-CM.
In clinical RSV infection, pulmonary epithelial cells are likely be exposed to the virus itself as well as to cytokine networks, and so we extended the cellular model to take account of this. A549 cells were incubated with RSV-CM as before and concurrently exposed to a low-grade RSV infection (MOI, 0.05). The levels of IL-8 secreted following direct infection alone were similar at 24 h to those obtained using 1:5-diluted RSV-CM and were only slightly higher at 48 h (Fig. 3). This suggests that direct and indirect effects of RSV infection both contribute to total IL-8 secretion. Exposure of the epithelial cells to both RSV and RSV-CM induced greater IL-8 secretion than was elicited following either stimulus alone. IL-8 secretion was 2,688 ± 1,602 pg/ml for RSV alone, 2,998 ± 1,087 pg/ml for RSV-CM, and 9,698 ± 3,167 pg/ml for RSV plus RSV-CM at 24 h; it was 12,168 ± 2,958 pg/ml for RSV alone, 8,142 ± 2,158 pg/ml for RSV-CM, and 27,043 ± 5,261 pg/ml for RSV plus RSV-CM at 48 h (P < 0.05 for comparisons of the sum of the two single stimuli with the effect of dual stimulation by paired t test). Thus RSV-CM synergizes with direct RSV infection to amplify IL-8 secretion from pulmonary epithelial cells, further emphasizing the role of monocyte-derived mediators in RSV-driven respiratory epithelial cell IL-8 secretion.
FIG. 3.
Synergism between RSV-CM and RSV infection of A549 cells. A549 cells were incubated with media alone (control), C-CM, or RSV-CM or were exposed to direct RSV infection (MOI = 0.05) with (RSV + RSV-CM) or without (RSV) RSV-CM. Cell culture supernatants were harvested after 24 or 48 h of culture, and IL-8 was measured by ELISA. Results are means ± SEM of three independent experiments. ∗, P < 0.05 for a comparison of the sum of the two single stimuli with the effect of dual stimulation (paired t test).
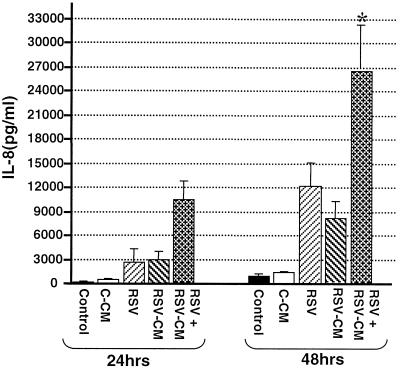
RSV-CM induced IκB degradation and NF-κB activation.
After it had been established that RSV-CM induced significant IL-8 secretion from respiratory epithelial cells both directly and synergistically with direct RSV infection, the mechanisms underlying RSV-CM-induced transcriptional activation of IL-8 were investigated. NF-κB is an important family of Rel-related transcription factors necessary for the transcription of many chemokine genes (34). NF-κB is normally held within the cytoplasm by the inhibitory protein IκBα. Following cellular stimulation, phosphorylation, ubiquitination, and subsequent proteolysis of IκBα liberate NF-κB (19, 35), allowing nuclear translocation and binding to specific κB sites within promoters (34). IκBβ is a close homolog of IκBα which may be involved in the control of more-prolonged cellular responses than those regulated by IκBα (72). To investigate the degradation of IκBα and IκBβ, cell lysates were obtained from C-CM- or RSV-CM-treated A549 cells, and equal amounts of protein were run in Western blots. IκBα levels rapidly diminished within 5 min of RSV-CM treatment, indicating that degradation had occurred (Fig. 4A). This effect was transient, with some IκBα reaccumulation at 15 min poststimulation and with levels returning completely to normal after 45 min. No changes in cytoplasmic IκBα levels were observed in C-CM-treated cells. In contrast, IκBβ was not degraded in response to CM (Fig. 4B).
FIG. 4.
Kinetics of IκB degradation and resynthesis in CM-stimulated A549 cells. A549 cells were stimulated by C-CM or RSV-CM, and cytoplasmic lysates were prepared at specific time points after stimulation. Lysates were resolved by SDS-PAGE, and Western blotting was performed for IκBα (A) and IκBβ (B).
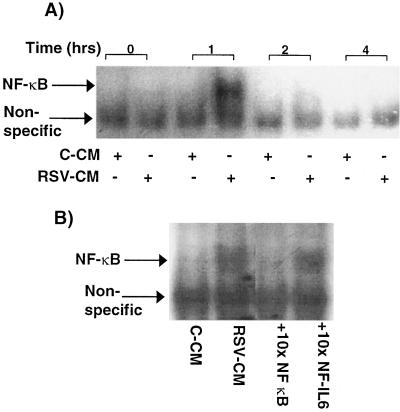
In order to determine whether RSV-CM-induced IκBα degradation was associated with nuclear translocation of NF-κB, gel shift assays were performed. Nuclear proteins extracted from CM-treated A549 cells were run out by PAGE with an oligonucleotide containing the consensus NF-κB binding site. Extracts from RSV-CM-exposed, but not C-CM-exposed, A549 cells demonstrated clear NF-κB binding activity at 1 h poststimulation, which was no longer detectable at 2 h (Fig. 5A). These kinetics of NF-κB activation were consistent with the observed degradation and resynthesis of IκBα. The addition of 10× excess cold NF-κB probe blocked the binding to the labeled probe, whereas excess cold oligonucleotide that was specific for the NF-IL6 binding site had no effect, thus confirming the specificity of the binding reaction (Fig. 5B).
FIG. 5.
Activation of NF-κB in RSV-CM-stimulated A549 cells. (A) Nuclear extracts were prepared from A549 cells at 0, 1, 2, and 4 h poststimulation with either C-CM or RSV-CM. Equal quantities of nuclear protein were mixed with 32P-labeled, double-stranded oligonucleotides specific for the NF-κB binding sequence and were resolved by PAGE. Bound complexes were visualized by autoradiography. (B) Competition experiments were performed on extracts prepared 1 h poststimulation with RSV-CM. NF-κB binding to labeled probe was competed out with a 10-fold excess of unlabeled NF-κB probe but not with a 10-fold excess of unlabeled, irrelevant (NF-IL6-binding) probe.
RSV-CM stimulated nuclear binding of NF-IL6.
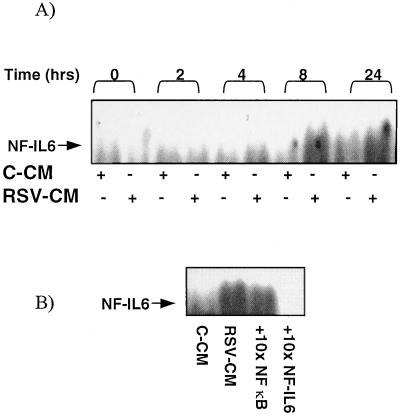
Transcriptional activation of the IL-8 gene involves the binding of other transcription factors in addition to NF-κB. RSV-induced IL-8 secretion from the epithelium has been shown to involve NF-IL6 as well as NF-κB (46). NF-IL6 binds to a region adjacent to the NF-κB site in the IL-8 promoter region. We therefore determined whether RSV-CM induced NF-IL6 translocation in A549 cells. Nuclear extracts were prepared as before and analyzed by EMSA using a probe containing the consensus NF-IL6 binding sequence. RSV-CM, but not C-CM, induced weak but apparent NF-IL6 binding activity. However, the time course of NF-IL6 binding was markedly delayed compared to that seen for NF-κB (Fig. 6A). Binding activity was first apparent at 8 h and was still present after 24 h. The specificity of the binding was confirmed by the ability of a 10-fold excess of the NF-IL6 probe to compete out the signal and the failure of excess NF-κB to affect binding (Fig. 6B).
FIG. 6.
Activation of NF-IL6 in RSV-CM-stimulated A549 cells. (A) Nuclear extracts were prepared from A549 cells at 0, 2, 4, 8, and 24 h poststimulation with either C-CM or RSV-CM. Equal quantities of nuclear protein were mixed with 32P-labeled, double-stranded oligonucleotides specific for the NF-IL6 binding sequence and were resolved by PAGE. Bound complexes were visualized by autoradiography. (B) Competition experiments were performed on extracts prepared 24 h poststimulation with RSV-CM. NF-IL-6 binding to labeled probe was not competed out with a 10-fold excess of unlabeled NF-κB probe but was completely blocked with a 10-fold excess of unlabeled NF-IL6 probe.
RSV-CM induced IL-8 promoter activity.
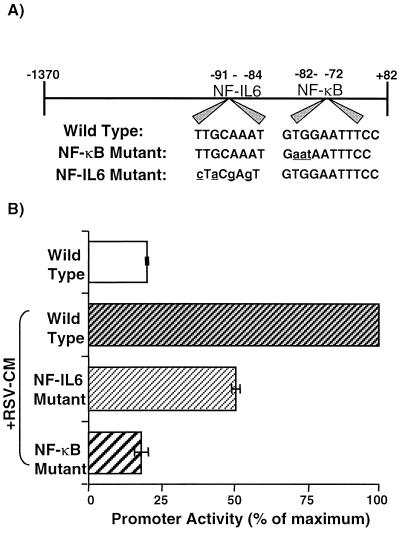
After it was established that RSV-CM activated NF-κB and NF-IL6 binding in respiratory epithelial cells, the functional importance of these observations for IL-8 gene activation was investigated. Cells were cotransfected with plasmids containing variants of the IL-8 promoter upstream of the luciferase gene (Fig. 7A) and a control vector expressing Renilla luciferase. RSV-CM caused a fourfold increase in promoter activity in cells containing the wild-type promoter, compared with unstimulated, transfected cells (Fig. 7B). Reporter activity was decreased by approximately 50% by mutation of the NF-IL6-binding site in the IL-8 promoter and was completely abolished by disruption of the NF-κB-binding site. Thus, RSV-CM-induced activation of the IL-8 gene is dependent on NF-κB activity but also requires NF-IL6 binding for full activity.
FIG. 7.
RSV-CM-induced IL-8 promoter activity: effect of NF-κB- or NF-IL6-binding region mutations on activation. (A) Schematic representation of the wild-type IL-8 promoter (bases −1370 to +82) inserted upstream of the luciferase gene in pGL2-basic. Lowercase letters, construct variants containing mutations in either the NF-κB or NF-IL6 sequences. (B) A549 cells were grown to 50% confluency and then were cotransfected with the wild-type, NF-κB-mutated, or NF-IL6-mutated IL-8 promoter constructs plus a control plasmid, pRL-TK, which expresses low, constitutive levels of Renilla luciferase. Cells were stimulated the following day with RSV-CM, and lysates were prepared and assayed for luciferase activity. Data are percentages of maximal promoter activity (obtained from RSV-CM-stimulated cells containing the wild-type promoter construct) after normalization of experimental plasmid values with control plasmid luciferase activity. Results are the means ± SEM of three independent experiments (each using CM prepared on a separate occasion).
Role of cytokines in RSV-CM-induced IL-8 secretion.
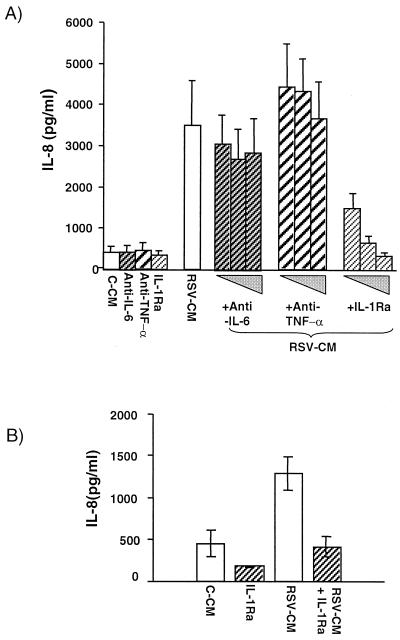
The identity of the active constituents within RSV-CM that were responsible for driving IL-8 secretion from pulmonary epithelial cells was next investigated. Monocytes are capable of producing large quantities of proinflammatory cytokines, and RSV infection of mononuclear cells or macrophages results in secretion of TNF-α, IL-1, and IL-6 (4, 10, 31, 70). Since both TNF-α and IL-1 have the potential to stimulate IL-8 secretion, the action of these cytokines was blocked in specific experiments. In specific experiments, either A549 cells were preincubated with the IL-1 receptor antagonist or RSV-CM itself was pretreated with specific anti-TNF-α (at 5, 20, or 50 μg/ml). Since we demonstrated that IL-6 and the neutralizing antibody to IL-6 do not induce IL-8 secretion from respiratory epithelial cells (data not shown), neutralizing anti-IL-6 was used as a negative control. The inhibitors alone had no effect on IL-8 secretion, and neither anti-TNF-α nor anti-IL-6 affected RSV-CM-induced IL-8 release. The amount of anti-TNF-α antibody used was sufficient to neutralize 8 ng of recombinant TNF-α/ml, i.e., an approximately 50-fold excess over that required to neutralize all the TNF-α measured in RSV-CM (data not shown). In contrast, IL-1Ra inhibited IL-8 secretion caused by RSV-CM in a dose-dependent manner, with 200 μg of IL-1Ra/ml totally blocking IL-8 secretion, indicating that IL-1 is a necessary component for this response (Fig. 8A). The response was specific since IL-1Ra did not affect IL-8 secretion from A549 cells stimulated with recombinant TNF-α (10 ng/ml), whereas the neutralizing antibody to TNF-α completely inhibited such secretion (data not shown). Similarly, experiments using IL-1Ra in primary cells demonstrated that IL-1 was absolutely required for the effects of RSV-CM on NHBE cells (Fig. 8B), which further confirms that IL-1 is a critical mediator in this network. We have other very detailed data which indicate that IL-1 alone may account for all the effects of RSV-CM; these data are from experiments in which unstimulated respiratory epithelial cells were spiked with cytokine, and the mechanisms by which this is regulated will be the subject of a separate paper.
FIG. 8.
Effect of IL-1Ra or neutralizing anti-TNF-α on RSV-CM-induced IL-8 secretion from human epithelial cells. (A) A549 cells were preincubated for 2 h in the absence or presence of human Il-1Ra (2, 20, or 200 ng/ml) prior to addition of RSV-CM. Alternatively, RSV-CM was incubated for 1 h in the absence or presence of rabbit anti-human neutralizing anti-TNF-α (5, 20, or 50 μg/ml) prior to its addition to A549 cell cultures. RSV-CM was also incubated with rabbit anti-human neutralizing anti-IL-6 at 5, 20, and 50 μg/ml as a negative control. Culture supernatants were harvested after 24 h and analyzed for IL-8 content by ELISA. (B) NHBE cells were incubated in the presence of IL-1Ra (200 ng/ml) as described for panel A. IL-8 in supernatants harvested after 24 h was measured by ELISA. All results are the means ± SEM of three independent experiments.
DISCUSSION
In this study, we have demonstrated that networks between human monocytes and epithelial cells during RSV infection are important in upregulating IL-8 secretion. We have further shown that direct and indirect effects of RSV infection on pulmonary epithelial cells interact synergistically to increase IL-8 secretion. RSV-CM acts at a molecular level to activate NF-κB and NF-IL6 in epithelial cells, resulting in transcription and IL-8 mRNA generation, which are followed by IL-8 protein secretion. Monocyte-derived IL-1, but not TNF-α, is absolutely required (although not necessarily sufficient) for the effects of RSV-CM. Our results therefore emphasize that the epithelium has an active proinflammatory role in RSV infection and is more than a simple reservoir for viral replication.
We have shown that substantial IL-8 concentrations are secreted by indirect, monocyte-dependent pathways, in addition to the previously described secretion as a consequence of direct infection of epithelial cells and macrophages by RSV (3, 10, 11, 31, 70). IL-8 secretion is important in the recruitment of neutrophils and T lymphocytes to the area of infection (7, 8, 33, 44). Such cells may also contribute to subsequent chemokine secretion. There is good evidence that RSV-infected neutrophils may be an important source of IL-8 (38, 41). T lymphocytes have the potential to modulate IL-8 secretion both positively and negatively (68), but their predominant effect in response to clinical RSV infection has not been determined. In contrast to these observations on IL-8 secretion and to the effects of direct RSV infection (11, 61), RSV-CM had only a small effect on RANTES secretion from epithelial cells. RSV-CM caused only a 2-fold increase in epithelial cell RANTES secretion, compared with a 35-fold increase in IL-8 secreted. This negative effect on RANTES is consistent with another recent study in which cellular networks were also shown to be important in the secretion of monocyte chemotactic protein 1 and MIP-1α (12). These data are also consistent with other known differences between the molecular regulation of IL-8 and RANTES secretion. For example, whereas TNF-α alone is able to activate epithelial cell IL-8 secretion, it is unable to cause RANTES secretion from either respiratory or colonic epithelial cells unless used in combination with gamma interferon (71, 73). In another example of the different regulation of these chemokines, TNF-α and IL-1β both cause release of IL-8 and RANTES from rheumatoid synovial cells but IL-4 downregulates the induced RANTES mRNA and augments the IL-8 message (56). At the level of transcriptional regulation, overexpression of the NF-κB regulator, IκB-related protein, in alveolar epithelial cells specifically upregulates RANTES, but not IL-8 or MIP-1α, gene expression due to specific sequestration of suppressive p50 homodimers (58).
A critical observation was that the interaction between RSV-CM and direct low-grade RSV infection of A549 cells resulted in synergistic increases in IL-8 secretion at 24 and 48 h. The mechanism of this synergy is being further investigated but may operate at the transcriptional level. Transcriptional control of IL-8 is cell and stimulus specific. For example, hydrogen peroxide selectively stimulates IL-8 secretion for epithelial cells but not endothelial cells (43), and, in respiratory epithelial cells, hydrogen peroxide-induced IL-8 activation requires selective binding of AP-1 to the promoter, whereas stimulation by TNF-α correlates with NF-κB binding (59). We found that the IL-8 secretion induced by RSV-CM alone was critically dependent on NF-κB nuclear translocation which was associated with degradation of IκBα but not of IκBβ. The resynthesis of IκBα had a somewhat unusual biphasic pattern. The transcription factor response was transient, as is characteristic for IκBα-driven acute NF-κB responses (34) in which NF-κB itself signals for resynthesis of IκBα (69). Such rapid degradation and resynthesis of NF-κB have been well documented when monocytes were exposed to a variety of inflammatory stimuli and pathogens (6). However, the kinetics of NF-κB activation contrasted with those following direct RSV infection of epithelial cells, which we and others have found to cause delayed (onset between 2 and 8 h) and prolonged activation of NF-κB, which is still detectable at 24 h (32, 46, 71); these findings are consistent with the fact that both IκBα and IκBβ are degraded in response to direct RSV infection (36). It is therefore possible to hypothesize that, in combination, RSV-CM may activate an early and direct RSV infection a delayed, sustained NF-κB-dependent response, with one consequence being a synergistic increase in IL-8 secretion, as observed. This response would be similar to the synergistic increase in IL-8 secretion from melanoma cells stimulated by both trans-retinoic acid and TNF-α, which involved enhanced NF-κB binding activity, possible via removal of repressive factors (48).
Although NF-κB is pivotal in RSV infection, the relationship between this and other transcription factors may be vital in gene activation. EMSAs showed that NF-IL6 was also activated by RSV-CM, and although the kinetics of nuclear binding of NF-IL6 were very different from those observed for NF-κB, reporter assays demonstrated that this was necessary for full activation of the IL-8 promoter by RSV-CM. Our data are consistent with a previous study which showed binding of NF-IL6 in an EMSA that was evident at 3 h and that persisted until 48 h (46). Cooperation between NF-IL6 and NF-κB also appears necessary for direct RSV-induced IL-8 gene expression in respiratory epithelial cells (30), although AP-1 may also have a key role (47). Similarly, IL-8 expression driven by IL-1 involves transcriptional activation of the promoter by both NF-κB p65 and NF-IL6 in Jurkat cells (42) and regulation depends on the ratio of NF-κB to NF-IL6 family members (65). In contrast, TNF-α-induced IL-8 secretion from A549 cells was dependent on the NF-κB subunits RelA, NF-κB1, and c-Rel but did not require NF-IL6 (17). Such differential activation and binding of inducible transcription factors may be an element contributing to synergistic responses.
Our experiments demonstrated that monocyte-derived IL-1 is a vital component responsible for the activity of RSV-CM. We have observed a similar role for IL-1 in a cellular model of pulmonary tuberculosis (74). In contrast, IL-6 and TNF-α, also secreted by monocytes infected with RSV (4, 10), did not have an important role although TNF has the potential to contribute to IL-8 secretion (9); IL-6 was a control. In this model RSV-infected monocytes may release insufficient bioactive TNF-α to activate epithelial cells. RSV-infected macrophages release approximately 1,500 pg of TNF-α/ml and around 2,000 pg of peripheral blood mononuclear cells/ml, which would translate to only 300 to 400 pg/ml after a 1:5 dilution such as we have used for RSV-CM (4, 10). In addition, Becker et al. and Panuska et al. noted a discrepancy between measured and bioactive TNF after RSV infection and suggested that there was concurrent induction of a TNF inhibitor (10, 53). Thus, pulmonary networks appear pathogen specific, and the findings for RSV infection contrast with the fact that both IL-1β and TNF-α were involved in IL-8 secretion from A549 cells exposed to CM from lipopolysaccharide-stimulated macrophages (64). This emphasizes the importance of investigating actual functional effects of RSV-CM rather than simply measuring cytokine content. In the present studies we did not discriminate between the effects of IL-1α and -1β, but IL-1β is the major secreted form of IL-1 from monocytes, which makes it probable that it is responsible for RSV-CM-induced IL-8 secretion. This contrasts with the role for epithelial cell-derived IL-1α in the autocrine regulation of IL-8 secretion (54). It is likely that the autocrine and paracrine effects of IL-1 jointly act to drive epithelial cell IL-8 secretion in response to RSV infection. Our findings are consistent with studies in which coculture of A549 and peripheral blood mononuclear cells resulted in increased IL-8 secretion in the presence of RSV. The source of the IL-8 was not determined, and IL-1, TNF-α, and TNF-β were all implicated in IL-8 secretion (4). Our study represents the first report of the effects of mediators from a pure population of RSV-infected monocytes on epithelial cells and defines the molecular mechanisms leading to IL-8 secretion. We cannot exclude the possibility that other monocyte-derived mediators in RSV-CM may also be important, but our findings on the role of IL-1 as a critical mediator in this network mean that it will now be necessary to reconsider the role of this cytokine in vivo. There are few data on IL-1 concentrations during clinical RSV infection although increased IL-1β mRNA levels were detected in nasal epithelial cells of children with RSV (51).
The facts that IL-1 is identified as a key mediator in RSV-CM and that RSV-CM interacts synergistically with direct RSV infection are consistent with the many other synergistic interactions known to involve IL-1. For example, IL-1β and TNF-α act synergistically on IL-8 production in both synovial and lung fibroblasts as well as in human airway smooth muscle cells (26, 39, 57). More recently, IL-1 has been shown to interact with the virulence factor pyocyanin from Pseudomonas aeruginosa to cause synergistic IL-8 release from epithelial cells (22). With particular reference to the study of RSV infection, the only other synergistic interaction that has been identified as far as the authors are aware is of gamma interferon synergizing with direct RSV infection to induce RANTES secretion from respiratory epithelial cells (52).
In summary, we demonstrate a novel mechanism by which IL-8 secretion is upregulated in human RSV infection. CM from a pure population of RSV-infected monocytes synergize with direct RSV infection of the respiratory epithelium to augment IL-8 secretion. RSV-CM causes IL-8 secretion and gene expression in an NF-κB- and NF-IL6-dependent fashion. Rapid NF-κB activation by RSV-CM follows the rapid degradation of IκBα but not IκBβ, whereas NF-IL6 nuclear translocation is much more delayed. Since IL-1 is a crucial component of RSV-CM and has been described to be involved in autocrine IL-8 secretion (54), it is possible that this may provide a target for therapeutic intervention. Blocking the secondary, network-dependent IL-8 secretion could provide a means by which to prevent these amplification pathways and limit excessive inflammation without blocking the leukocyte recruitment initiated by direct cellular infection, which may be necessary for effective clearance of virus.
ACKNOWLEDGMENTS
We thank William Reed (University of North Carolina, Chapel Hill, N.C.) for providing the IL-8 promoter reporter constructs.
L. Thomas, M. Sharland, and J. S. Friedland were supported by Action Research, UK, grant no. S/P/3307. M. Wickremasinghe is an MRC (UK) Training Fellow.
REFERENCES
- 1.Aherne W, Bird T, Court S D M, Gardner P S, McQuillin J. Pathological changes in virus infections of the lower respiratory tract in children. J Clin Pathol. 1970;23:7–18. doi: 10.1136/jcp.23.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allport T D, Davies E G, Wells C, Sharland M. Ribavirin and bronchiolitis: variation in use in the UK. Arch Dis Child. 1997;76:385. doi: 10.1136/adc.76.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold R, Humbert B, Werchau H, Gallati H, König W. Interleukin-8, interleukin-6, and soluble tumour necrosis factor receptor type I release from a human pulmonary epithelial cell line (A549) exposed to respiratory syncytial virus. Immunology. 1994;82:126–133. [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold R, König B, Gallati H, Werchau H, König W. Cytokine (IL-8, IL-6, TNF-α) and soluble TNF receptor-I release from human peripheral blood mononuclear cells after respiratory syncytial virus infection. Immunology. 1995;85:364–372. [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold R, Werner F, Humbert B, Werchau H, Konig W. Effect of respiratory syncytial virus-antibody complexes on cytokine (IL-8, IL-6, TNF-α) release and respiratory burst activity in human granulocytes. Immunology. 1994;82:184–191. [PMC free article] [PubMed] [Google Scholar]
- 6.Baeuerle P A, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 7.Baggiolini M, Walz A, Kunkel S L. Neutrophil-activating peptide-1/interleukin-8, a novel cytokine that activates neutrophils. J Clin Investig. 1989;84:1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bazzoni F, Cassatella M A, Rossi F, Ceska M, Dewald B, Baggiolini M. Phagocytosing neutrophils produce and release high amounts of the neutrophil-activating peptide 1/interleukin 8. J Exp Med. 1991;173:771–774. doi: 10.1084/jem.173.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker S, Koren H S, Henke D C. Interleukin-8 expression in normal nasal epithelium and its modulation by infection with respiratory syncytial virus and cytokines tumor necrosis factor, interleukin-1 and interleukin-6. Am J Respir Cell Mol Biol. 1993;8:20–27. doi: 10.1165/ajrcmb/8.1.20. [DOI] [PubMed] [Google Scholar]
- 10.Becker S, Quay J, Soukup J. Cytokine (tumour necrosis factor, IL-6 and IL-8) production by respiratory syncytial virus-infected human alveolar macrophages. J Immunol. 1991;147:4307–4312. [PubMed] [Google Scholar]
- 11.Becker S, Reed W, Henderson F W, Noah T L. RSV infection of human airway epithelial cells causes production of the β-chemokine RANTES. Am J Physiol. 1997;272:L512–L520. doi: 10.1152/ajplung.1997.272.3.L512. [DOI] [PubMed] [Google Scholar]
- 12.Becker S, Soukup J M. Airway epithelial cell-induced activation of monocytes and eosinophils in respiratory syncytial viral infection. Immunobiology. 1999;201:88–106. doi: 10.1016/S0171-2985(99)80049-7. [DOI] [PubMed] [Google Scholar]
- 13.Bischoff S C, Krieger M, Brunner T, Rot A, von Tscharner V, Baggiolini M, Dahinden C A. RANTES and related chemokines activate human basophil granulocytes through different G protein coupled receptors. Eur J Immunol. 1993;23:761–767. doi: 10.1002/eji.1830230329. [DOI] [PubMed] [Google Scholar]
- 14.Biswas S, Remick D G, Davies E G, Sharland M. Elevated plasma interleukin-8 in respiratory syncytial virus bronchiolitis. Pediatr Infect Dis J. 1995;14:919. [PubMed] [Google Scholar]
- 15.Bont L, Heijnen C J, Kavelaars A, van Aalderen W M, Brus F, Draaisma J M T, Geelen S M, van Vught H J, Kimpen J L. Peripheral blood cytokine responses and disease severity in respiratory syncytial virus bronchiolitis. Eur Respir J. 1999;14:144–149. doi: 10.1034/j.1399-3003.1999.14a24.x. [DOI] [PubMed] [Google Scholar]
- 16.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 17.Brasier A R, Jamaluddin M, Casola A, Duan W, Shen Q, Garofalo R P. A promoter recruitment mechanism for tumor necrosis factor-a-induced interleukin-8 transcription in type II pulmonary epithelial cells. J Biol Chem. 1998;273:3551–3561. doi: 10.1074/jbc.273.6.3551. [DOI] [PubMed] [Google Scholar]
- 18.Breese-Hall C. Prospects for a respiratory syncytial virus vaccine. Science. 1994;265:1393–1394. doi: 10.1126/science.7915433. [DOI] [PubMed] [Google Scholar]
- 19.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of IκB-α proteolysis by site-specific, signal induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 20.Cannon M J. Microplaque immunoperoxidase detection of infectious respiratory syncytial virus in the lungs of infected mice. J Virol Methods. 1987;16:293–301. doi: 10.1016/0166-0934(87)90014-0. [DOI] [PubMed] [Google Scholar]
- 21.Chomczynski P, Sacchi N. Single-step method for RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–163. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 22.Denning G M, Wollenweber L A, Railsback M A, Cox C D, Stoll L L, Britigan B E. Pseudomonas pyocyanin increases interleukin-8 expression by human airway epithelial cells. Infect Immun. 1998;66:5777–5784. doi: 10.1128/iai.66.12.5777-5784.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Domurat F, Roberts N J, Walsh E E, Dagan R. Respiratory syncytial virus infection of human mononuclear leukocytes in vitro and in vivo. J Infect Dis. 1985;152:895–902. doi: 10.1093/infdis/152.5.895. [DOI] [PubMed] [Google Scholar]
- 24.Dowell S F, Anderson L J, Gary H E, Jr, et al. Respiratory syncytial virus is an important cause of community-acquired lower respiratory infections among hospitalised adults. J Infect Dis. 1996;174:456–462. doi: 10.1093/infdis/174.3.456. [DOI] [PubMed] [Google Scholar]
- 25.Durand D B, Shaw J P, Bush M R, Replogle R E, Belagaje R, Neilson E G. Characterization of antigen receptor response elements within the interleukin-2 enhancer. Mol Cell Biol. 1988;8:1715–1724. doi: 10.1128/mcb.8.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elias J A, Reynolds M M, Kotloff R M, Kern J A. Fibroblast interleukin 1 beta: synergistic stimulation by recombinant interleukin 1 and tumor necrosis factor and posttranscriptional regulation. Proc Natl Acad Sci USA. 1989;86:6171–6175. doi: 10.1073/pnas.86.16.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Everard M L, Swarbrick A, Wrightham M, McIntyre J, Dunkley C, James P D, Sewell H F. Analysis of cells obtained by bronchial lavage of infants with respiratory syncitial virus infection. Arch Dis Child. 1994;71:428–432. doi: 10.1136/adc.71.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falsey A R, Cunningham C K, Barker B W H, Kouides R W, Yuen J B, Menegus M, Weiner L B, Bonville C A, Betts R F. Respiratory syncytial virus and influenza A infections in the hospitalized elderly. J Infect Dis. 1995;172:389–394. doi: 10.1093/infdis/172.2.389. [DOI] [PubMed] [Google Scholar]
- 29.Fiedler M A, Wernke-Dollries K, Stark J M. Inhibition of viral replication reverses respiratory syncitial virus-induced NF-κB activation and interleukin-8 gene expression in A549 cells. J Virol. 1996;70:9079–9082. doi: 10.1128/jvi.70.12.9079-9082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiedler M A, Wernke-Dollries K, Stark J M. Mechanism of RSV-induced IL-8 gene expression in A549 cells before viral replication. Am J Physiol. 1996;271:L963–L971. doi: 10.1152/ajplung.1996.271.6.L963. [DOI] [PubMed] [Google Scholar]
- 31.Franke-Ullmann G, Pfortner C, Walter P, Steinmuller C, Lohmann-Matthes M-L, Kobzik L, Freihorst J. Alteration of pulmonary macrophage function by respiratory syncytial virus infection in vitro. J Immunol. 1995;154:268–280. [PubMed] [Google Scholar]
- 32.Garofalo R, Sabry M, Jamaluddin M, Yu R K, Casola A, Ogra P L, Brasier A R. Transcriptional activation of the interleukin-8 gene by respiratory syncytial virus infection in alveolar epithelial cells: nuclear translocation of the RelA transcription factor as a mechanism producing airway mucosal inflammation. J Virol. 1996;70:8773–8781. doi: 10.1128/jvi.70.12.8773-8781.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerszten R E, Garcia-Zepeda E A, Lim Y-C, Yoshida M, Ding H A, Gimbrone M A J, Luster A D, Luscinskas F W, Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 34.Ghosh S, May M J, Kopp E B. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 35.Henkel T, Machleidt I, Alkalay I, Kronke M, Ben-Neirah Y, Baeuerle P A. Rapid proteolysis of IκB-α is necessary for activation of transcription factor NF-κB. Nature. 1993;365:182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- 36.Jamaluddin M, Casola A, Garofalo R P, Han Y, Elliott T, Ogra P L, Brasier A R. The major component of IκBα proteolysis occurs independently of the proteosome pathway in respiratory syncytial virus-infected pulmonary epithelial cells. J Virol. 1998;72:4849–4857. doi: 10.1128/jvi.72.6.4849-4857.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jamaluddin M, Garofalo R, Ogra P L, Brasier A R. Inducible translational regulation of the NF-IL6 transcription factor by respiratory syncytial virus infection in pulmonary epithelial cells. J Virol. 1996;70:1554–1563. doi: 10.1128/jvi.70.3.1554-1563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaovisidha P, Peeples M E, Brees A A, Carpenter L R, Moy J N. Respiratory syncytial virus stimulates neutrophil degranulation and chemokine release. J Immunol. 1999;163:2816–2820. [PubMed] [Google Scholar]
- 39.John M, Au B T, Jose P J, Lim S, Saunders M, Barnes P J, Mitchell J A, Belvisi M G, Chung K F. Expression and release of interleukin-8 by human airway smooth muscle cells: inhibition by Th-2 cytokines and corticosteroids. Am J Respir Cell Mol Biol. 1998;18:84–90. doi: 10.1165/ajrcmb.18.1.2813. [DOI] [PubMed] [Google Scholar]
- 40.Kim H W, Canchola J G, Brandt C D, Pyles G, Chanock R M, Jensen K, Parrott R H. Respiratory syncytial virus disease in infants despite prior administration of an antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–433. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 41.Konig B, Krusat T, Streckert H-J, Konig W. IL-8 release from human neutrophils by the respiratory syncytial virus is independent of viral replication. J Leukoc Biol. 1996;60:253–260. doi: 10.1002/jlb.60.2.253. [DOI] [PubMed] [Google Scholar]
- 42.Kunsch C, Lang R K, Rosen C A, Shannon M F. Synergistic transcriptional activation of the IL-8 gene by NF-κB p65 (RelA) and NF-IL-6. J Immunol. 1994;153:153–164. [PubMed] [Google Scholar]
- 43.Lakshminarayanan V, Beno D W, Costa R H, Roebuck K A. Differential regulation of interleukin-8 and intercellular adhesion molecule-1 by H2O2 and tumor necrosis factor-alpha in endothelial and epithelial cells. J Biol Chem. 1997;272:32910–32918. doi: 10.1074/jbc.272.52.32910. [DOI] [PubMed] [Google Scholar]
- 44.Larsen C J, Anderson A O, Appella E, Oppenheim J J, Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989;243:1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- 45.Lieber M, Smith B, Szakal A, Nelsa-Rees W, Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer. 1976;17:62–70. doi: 10.1002/ijc.2910170110. [DOI] [PubMed] [Google Scholar]
- 46.Mastronarde J G, He B, Monick M M, Mukaida N, Matsushima K, Hunninghake G W. Induction of interleukin (IL)-8 gene expression by respiratory syncytial virus involves activation of nuclear factor (NF)-κB and NF-IL6. J Infect Dis. 1996;174:262–267. doi: 10.1093/infdis/174.2.262. [DOI] [PubMed] [Google Scholar]
- 47.Mastronarde J G, Monick M M, Mukaida N, Matsushima K, Hunninghake G W. Activator protein-1 is the preferred transcription factor for cooperative interaction with nuclear factor κB in respiratory syncytial virus-induced interleukin-8 gene expression in airway epithelium. J Infect Dis. 1998;177:1275–1281. doi: 10.1086/515279. [DOI] [PubMed] [Google Scholar]
- 48.Matsusaka T, Fujikawa K, Nishio Y, Mukaida N, Matsushima K, Kishimoto T, Akira S. Transcription factors NF-IL6 and NF-κB synergistically activate transcription of the inflammatory cytokines, interleukin-6 and interleukin-8. Proc Natl Acad Sci USA. 1993;90:10193–10197. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelson K A, Yunis E J. Demonstration of respiratory syncytial virus in an autopsy series. Pediatr Pathol. 1990;10:491–502. doi: 10.3109/15513819009067138. [DOI] [PubMed] [Google Scholar]
- 50.Noah T L, Becker S. Respiratory syncytial virus-induced cytokine production by a human bronchial epithelial cell line. Am J Physiol. 1993;265:L472–L478. doi: 10.1152/ajplung.1993.265.5.L472. [DOI] [PubMed] [Google Scholar]
- 51.Noah T L, Henderson F W, Wortman I A, Devlin R B, Handy J, Koren H S, Becker S. Nasal cytokine production in viral acute upper respiratory infection of childhood. J Infect Dis. 1995;171:584–592. doi: 10.1093/infdis/171.3.584. [DOI] [PubMed] [Google Scholar]
- 52.Olszewska-Pazdrak B, Casola A, Saito T, Alam R, Crowe S E, Mei F, Ogra P L, Garafalo R P. Cell-specific expression of RANTES, MCP-1, and MIP-1α by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. J Virol. 1998;72:4756–4764. doi: 10.1128/jvi.72.6.4756-4764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Panuska J R, Cirino N M, Midulla F, Despot J E, McFadden E R, Jr, Young J T. Productive infection of isolated human macrophages by respiratory syncytial virus. J Clin Investig. 1990;86:113. doi: 10.1172/JCI114672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel J A, Jiang Z, Nakajima N, Kunimoto M. Autocrine regulation of interleukin-8 by interleukin-1 alpha in respiratory syncytial virus-infected pulmonary epithelial cells in vitro. Immunology. 1998;95:501–506. doi: 10.1046/j.1365-2567.1998.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pullan C R, Hey E N. Wheezing, asthma, and pulmonary dysfunction 10 years after infection with respiratory syncytial virus. Br Med J. 1982;284:1665–1669. doi: 10.1136/bmj.284.6330.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rathanaswami P, Hachicha M, Sadick M, Schall T J, McColl S R. Expression of the cytokine RANTES in human rheumatoid synovial fibroblasts. Differential regulation of RANTES and interleukin-8 genes by inflammatory cytokines. J Biol Chem. 1993;268:5834–5839. [PubMed] [Google Scholar]
- 57.Rathanaswami P, Hachicha M, Wong W L, Schall T J, McColl S R. Synergistic effect of interleukin-1 beta and tumor necrosis factor alpha on interleukin-8 gene expression in synovial fibroblasts. Evidence that interleukin-8 is the major neutrophil-activating chemokine released in response to monokine activation. Arthritis Rheum. 1993;36:1295–1304. doi: 10.1002/art.1780360914. [DOI] [PubMed] [Google Scholar]
- 58.Ray P, Yang L, Zhang D H, Ghosh S K, Ray A. Selective up-regulation of cytokine-induced RANTES gene expression in lung epithelial cells by overexpression of IkappaBR. J Biol Chem. 1997;272:20191–20197. doi: 10.1074/jbc.272.32.20191. [DOI] [PubMed] [Google Scholar]
- 59.Roebuck C A, Carpenter L R, Lakshminarayanan V, Page S M, Moy J N, Thomas L L. Stimulus-specific regulation of chemokine secretion involves differential activation of the redox-responsive transcription factors AP-1 and NF-κB. J Leukoc Biol. 1999;65:291–298. doi: 10.1002/jlb.65.3.291. [DOI] [PubMed] [Google Scholar]
- 60.Rot A, Krieger M, Brunner T, Bischoff S C, Schall T J, Dahinden C A. RANTES and macrophage inflammatory protein 1α induce the migration and activation of normal human eosinophil granulocytes. J Exp Med. 1992;176:1489–1495. doi: 10.1084/jem.176.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saito T, Deskin R W, Casola A, Häeberle H, Olszewska B, Ernst P B, Alam R, Ogra P L, Garofalo R. Respiratory syncytial virus induces selective production of the chemokine RANTES by upper airway epithelial cells. J Infect Dis. 1997;175:497–504. doi: 10.1093/infdis/175.3.497. [DOI] [PubMed] [Google Scholar]
- 62.Schall T J, Bacon K, Toy K J, Goedded G V. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 63.Sheeran P, Jafri H, Carubelli C, Saavedra J, Johnson C, Krisher K, Sanchez P J, Ramilo O. Elevated cytokine concentrations in the nasopharyngeal and tracheal secretions of children with respiratory syncytial virus disease. Pediatr Infect Dis J. 1999;18:115–122. doi: 10.1097/00006454-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 64.Standiford T J, Kunkel S L, Basha M A, Chensue S W, Lynch J P I, Toews G B, Westwick J, Strieter R M. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J Clin Investig. 1990;86:1945–1953. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stein B, Baldwin A S J. Distinct mechanisms for regulation of the interleukin-8 gene involve synergism and cooperativity between C/EBP and NF-κB. Mol Cell Biol. 1993;13:7191–7198. doi: 10.1128/mcb.13.11.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stein R T, Sherill D, Morgan W J, Holberg C J, Halonen M, Taussig L M, Wright A L, Martinez F D. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 67.Strieter R L, Wiggins S, Phan S H, Wharram B L, Showell H J, Remick D G, Chensue S W, Kunkel S L. Monocyte chemotactic protein gene expression by cytokine treated human fibroblasts and endothelial cells. Biochem Biophys Res Commun. 1989;162:694–700. doi: 10.1016/0006-291x(89)92366-8. [DOI] [PubMed] [Google Scholar]
- 68.Striz I, Mio T, Adachi Y, Robbins R A, Romberger D J, Rennard S I. IL-4 and IL-13 stimulate human bronchial epithelial cells to release IL-8. Inflammation. 1999;23:545–555. doi: 10.1023/a:1020242523697. [DOI] [PubMed] [Google Scholar]
- 69.Sun S, Ganchi P, Ballard D, Greene W. NF-κB controls expression of inhibitor IκBα: evidence for an inducible autoregulatory pathway. Science. 1993;259:1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- 70.Takeuchi R, Tsutsumi H, Osaki M, Sone S, Imai S, Chiba S. Respiratory syncytial virus infection of neonatal monocytes stimulates synthesis of interferon regulatory factor 1 and interleukin-1β (IL-1β)-converting enzyme and secretion of IL-1β. J Virol. 1998;72:837–840. doi: 10.1128/jvi.72.1.837-840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thomas L H, Friedland J S, Sharland M, Becker S. Respiratory syncytial virus-induced RANTES production from human bronchial epithelial cells is dependent on NF-κB nuclear binding and is inhibited by adenovirus-mediated expression of IκBα. J Immunol. 1998;161:1007–1016. [PubMed] [Google Scholar]
- 72.Thompson J E, Phillips R J, Erdjument-Bromage H, Tempst P, Ghosh S. IκB-β regulates the presistent response in a biphasic activation of NF-κB. Cell. 1995;80:573–582. doi: 10.1016/0092-8674(95)90511-1. [DOI] [PubMed] [Google Scholar]
- 73.Warhurst A C, Hopkins S J, Warhurst G. Interferon gamma induces differential upregulation of alpha and beta chemokine secretion in colonic epithelial cell lines. Gut. 1998;42:208–213. doi: 10.1136/gut.42.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wickremasinghe M I Y, Thomas L H, Friedland J S. Pulmonary epithelial cells are a source of IL-8 in the response to Mycobacterium tuberculosis: essential role of IL-1 from infected monocytes in a NF-kappa β-dependent network. J Immunol. 1999;163:3936–3947. [PubMed] [Google Scholar]