Abstract
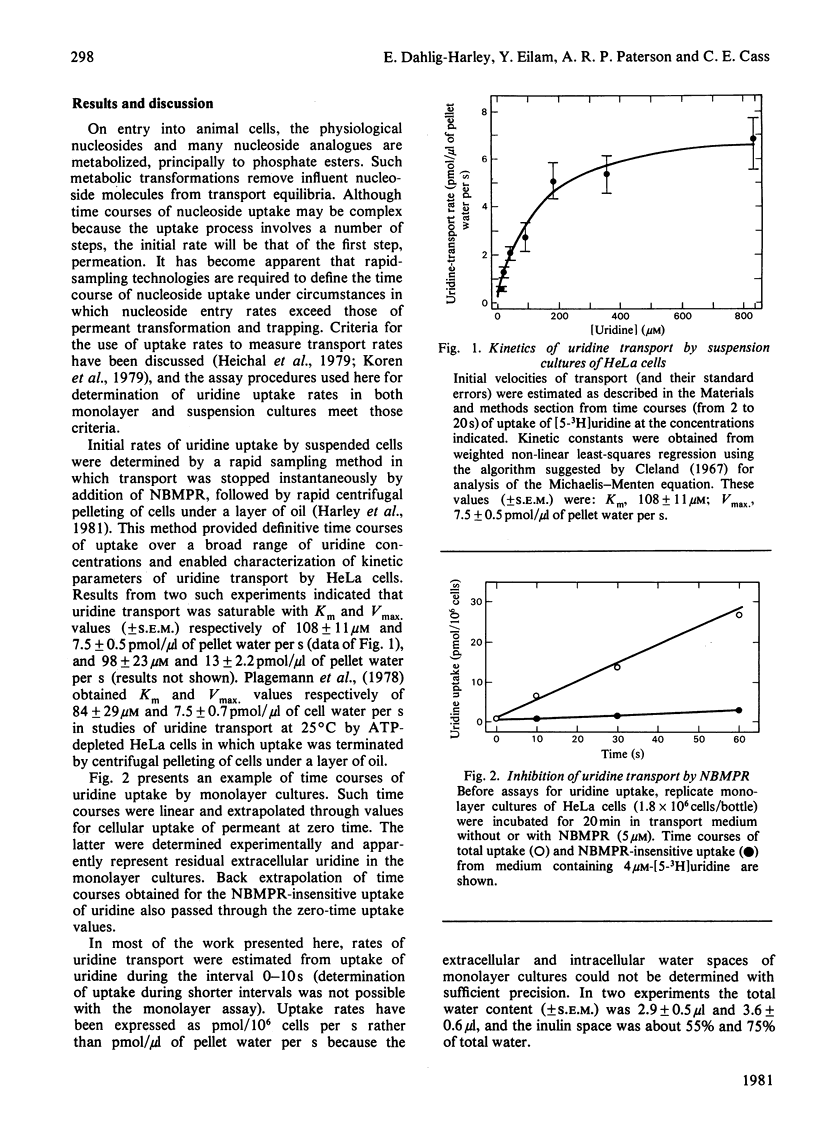
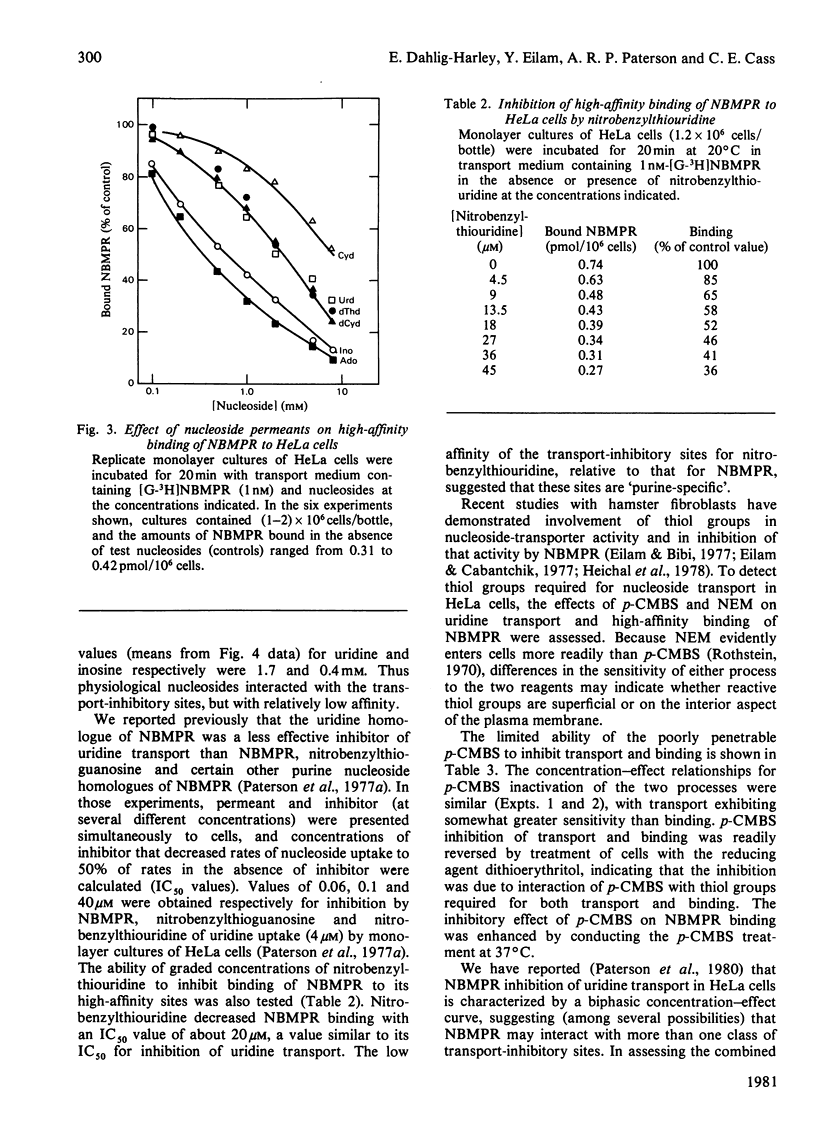
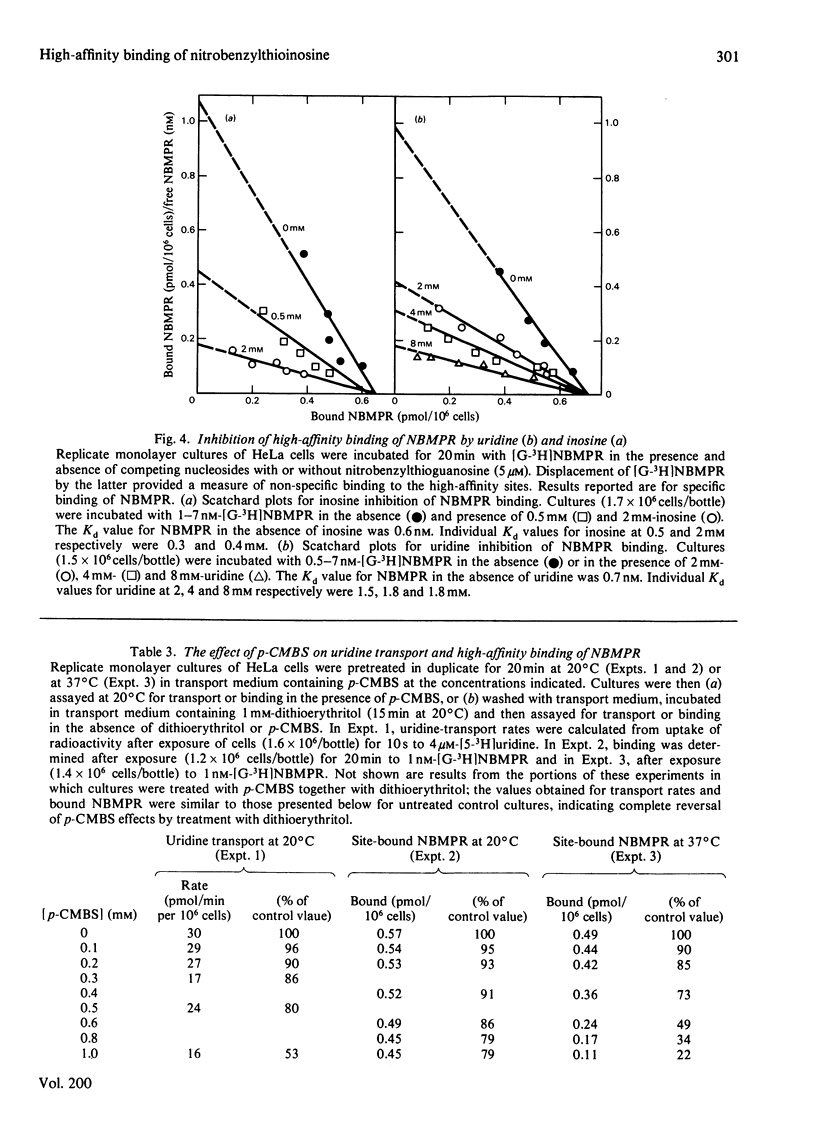
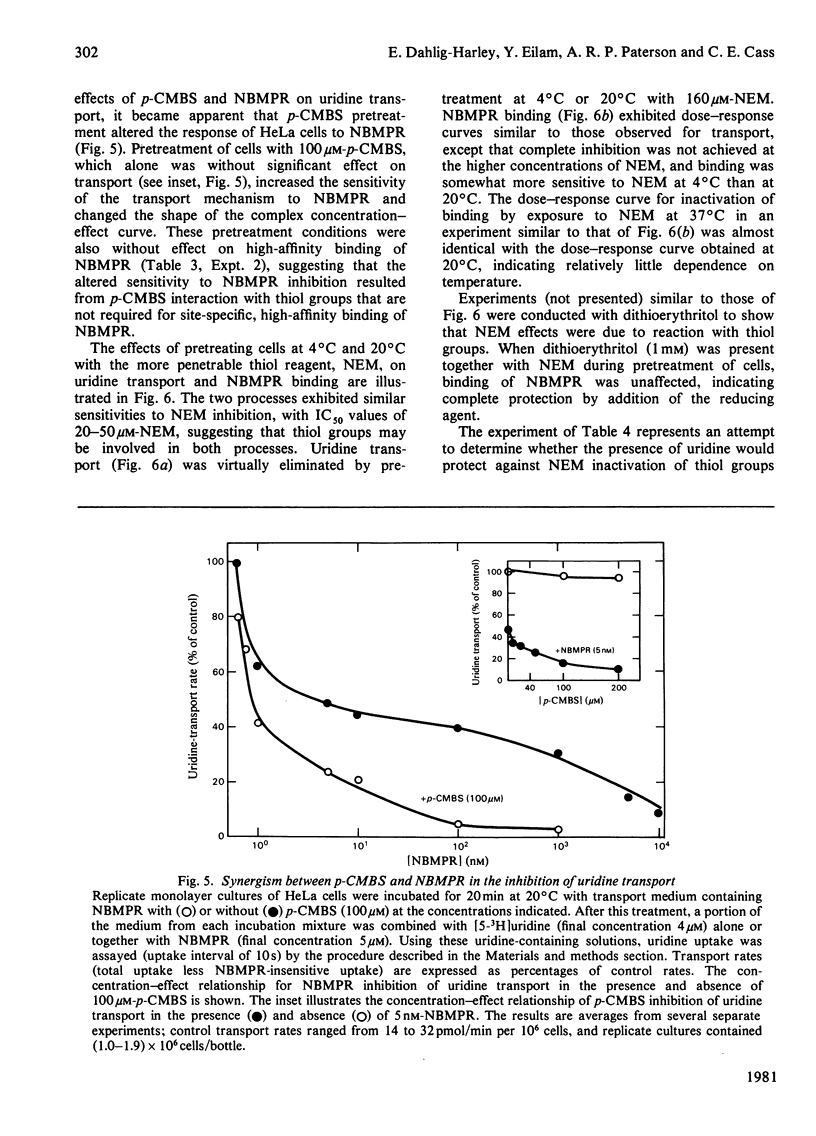
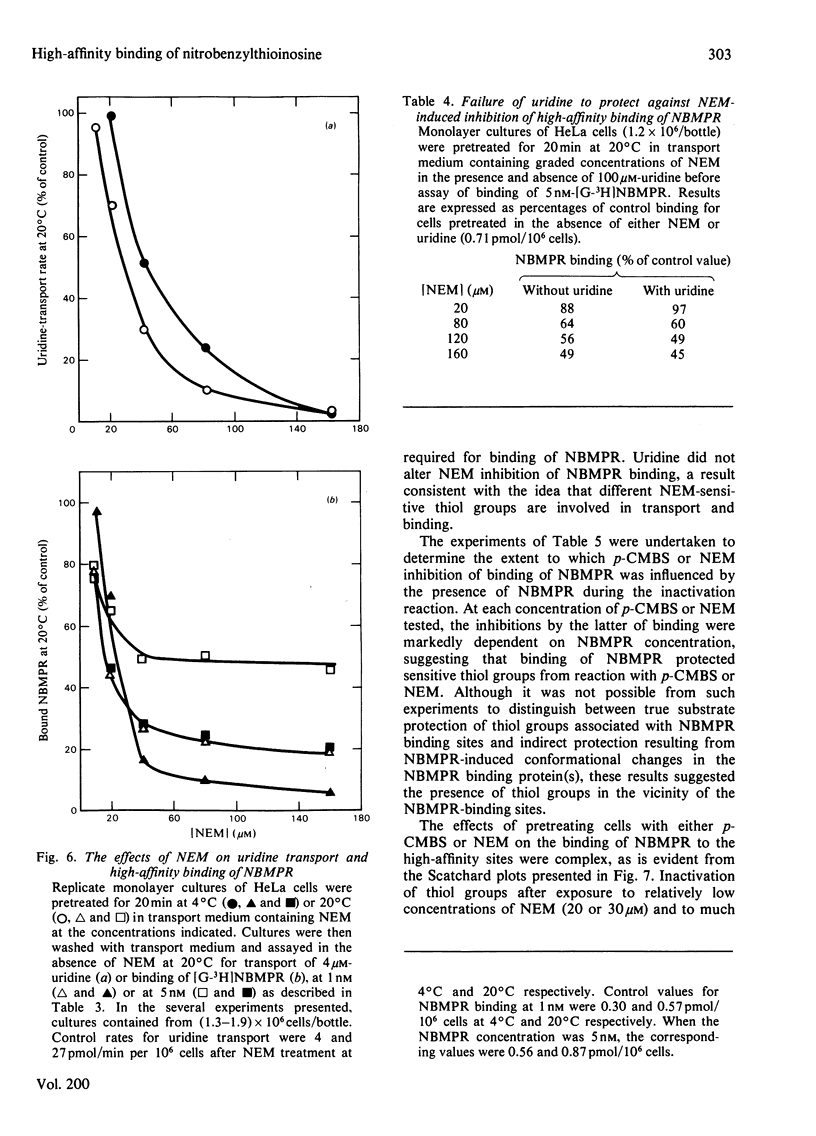
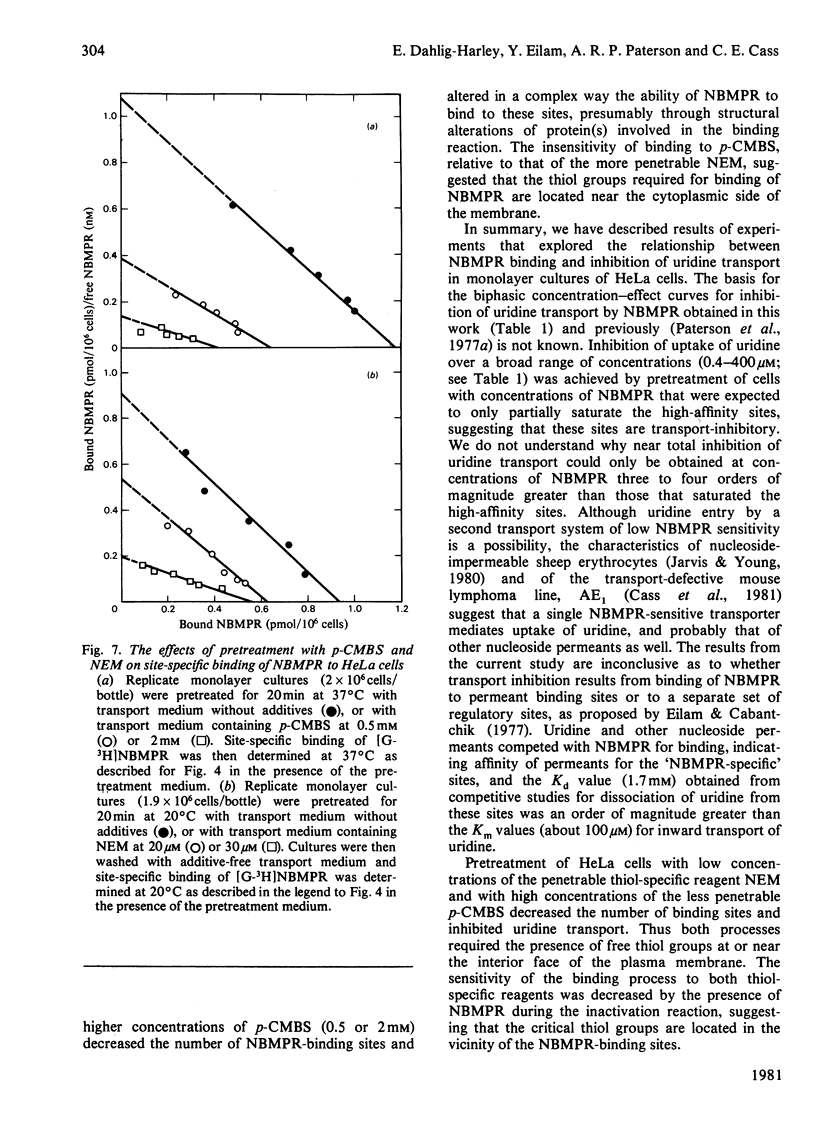
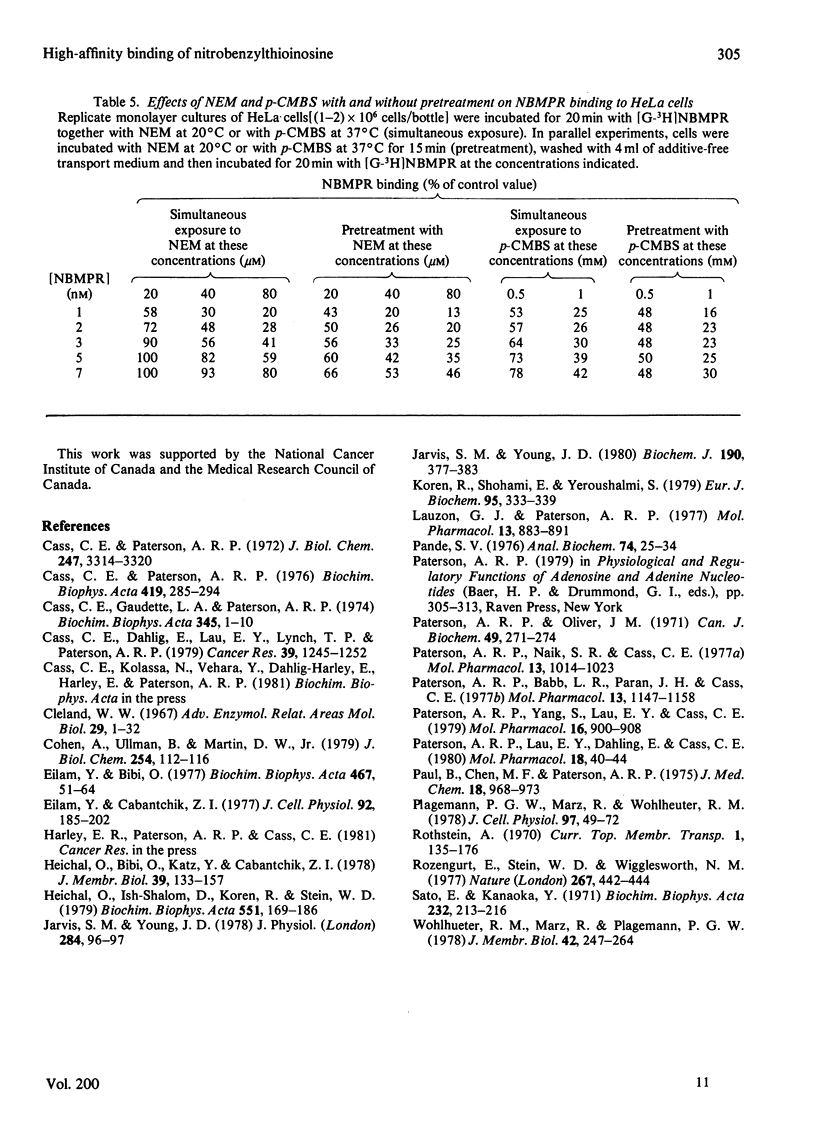
Nitrobenzylthioinosine (NBMPR) binds reversibly, but with high affinity (Kd 0.1--1.2 nM), to inhibitory sites on nucleoside-transport elements of the plasma membrane in a variety of animal cells. The present study explored relationships in HeLa cells between NBMPR binding and inhibition of uridine transport. The Km value for inward transport of uridine by HeLa cells in both suspension and monolayer culture was about 0.1 mM. The affinity of the transport-inhibitory sites for uridine (Kd 1.7 mM), inosine (Kd 0.4 mM) and other nucleoside permeants was low relative to that for NBMPR. The pyrimidine homologue of NBMPR, nitrobenzylthiouridine, also exhibited low affinity for the NBMPR-binding sites. Pretreatment of HeLa cells with p-chloromercuribenzene sulphonate (p-CMBS) or N-ethylmaleimide (NEM) decreased binding of NBMPR to its high-affinity sites and inhibited uridine transport, indicating the presence of thiol groups essential to both processes. NEM, a more penetrable reagent than p-CMBS, inhibited binding and transport at much lower concentrations than the latter compound. Pretreatment of cells with concentrations of p-CMBS that alone had no effect on either NBMPR binding or uridine transport increased the sensitivity of transport to NBMPR inhibition and changed the shape of the NBMPR concentration-effect curve, suggesting synergistic inhibiton of uridine-transport activity by these two agents.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cass C. E., Dahlig E., Lau E. Y., Lynch T. P., Paterson A. R. Fluctuations in nucleoside uptake and binding of the inhibitor of nucleoside transport, nitrobenzylthioinosine, during the replication cycle of HeLa cells. Cancer Res. 1979 Apr;39(4):1245–1252. [PubMed] [Google Scholar]
- Cass C. E., Gaudette L. A., Paterson A. R. Mediated transport of nucleosides in human erythrocytes. Specific binding of the inhibitor nitrobenzylthioinosine to nucleoside transport sites in the erythrocyte membrane. Biochim Biophys Acta. 1974 Apr 12;345(1):1–10. doi: 10.1016/0005-2736(74)90239-9. [DOI] [PubMed] [Google Scholar]
- Cass C. E., Paterson A. R. Mediated transport of nucleosides in human erythrocytes. Accelerative exchange diffusion of uridine and thymidine and specificity toward pyrimidine nucleosides as permeants. J Biol Chem. 1972 May 25;247(10):3314–3320. [PubMed] [Google Scholar]
- Cass C. E., Paterson A. R. Nitrobenzylthionionosine binding sites in the erythrocyte membrane. Biochim Biophys Acta. 1976 Jan 21;419(2):285–294. doi: 10.1016/0005-2736(76)90354-0. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- Cohen A., Ullman B., Martin D. W., Jr Characterization of a mutant mouse lymphoma cell with deficient transport of purine and pyrimidine nucleosides. J Biol Chem. 1979 Jan 10;254(1):112–116. [PubMed] [Google Scholar]
- Eilam Y., Bibi O. Distinct properties of uridine transport systems in growing, quiescent and serum-stimulated hamster embryo cells. Biochim Biophys Acta. 1977 May 16;467(1):51–64. doi: 10.1016/0005-2736(77)90241-3. [DOI] [PubMed] [Google Scholar]
- Eilam Y., Cabantchik Z. I. Nucleoside transport in mammalian cell membranes: a specific inhibitory mechanism of high affinity probes. J Cell Physiol. 1977 Aug;92(2):185–201. doi: 10.1002/jcp.1040920207. [DOI] [PubMed] [Google Scholar]
- Heichal O., Bibi O., Katz J., Cabantchik Z. I. Nucleoside transport in mammalian cell membranes. III. Kinetic and chemical modification studies of cytosine-arabinoside and uridine transport in hamster cells in culture. J Membr Biol. 1978 Mar 10;39(2-3):133–137. doi: 10.1007/BF01870329. [DOI] [PubMed] [Google Scholar]
- Heichal O., Ish-Shalom D., Koren R., Stein W. D. The kinetic dissection of transport from metabolic trapping during substrate uptake by intact cells. Uridine uptake by quiescent and serum-activated Nil 8 hamster cells and their murine sarcoma virus-transformed counterparts. Biochim Biophys Acta. 1979 Feb 20;551(1):169–186. doi: 10.1016/0005-2736(79)90363-8. [DOI] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D. Nucleoside transport in human and sheep erythrocytes. Evidence that nitrobenzylthioinosine binds specifically to functional nucleoside-transport sites. Biochem J. 1980 Aug 15;190(2):377–383. doi: 10.1042/bj1900377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren R., Shohami E., Yeroushalmi S. A kinetic analysis of the uptake of cytosine-beta-D-arabinoside by rat-B77 cells. Differentiation between transport and phosphorylation. Eur J Biochem. 1979 Apr 2;95(2):333–339. doi: 10.1111/j.1432-1033.1979.tb12970.x. [DOI] [PubMed] [Google Scholar]
- Lauzon G. J., Paterson A. R. Binding of the nucleoside transport inhibitor nitrobenzylthioinosine to HeLa cells. Mol Pharmacol. 1977 Sep;13(5):883–891. [PubMed] [Google Scholar]
- Pande S. V. Liquid scintillation counting of aqueous samples using triton-containing scintillants. Anal Biochem. 1976 Jul;74(1):25–34. doi: 10.1016/0003-2697(76)90306-7. [DOI] [PubMed] [Google Scholar]
- Paterson A. R., Babb L. R., Paran J. H., Cass C. E. Inhibition by nitrobenzylthioinosine of adenosine uptake by asynchronous HeLa cells. Mol Pharmacol. 1977 Nov;13(6):1147–1158. [PubMed] [Google Scholar]
- Paterson A. R., Lau E. Y., Dahlig E., Cass C. E. A common basis for inhibition of nucleoside transport by dipyridamole and nitrobenzylthioinosine? Mol Pharmacol. 1980 Jul;18(1):40–44. [PubMed] [Google Scholar]
- Paterson A. R., Naik S. R., Cass C. E. Inhibition of uridine uptake in HeLa cells by nitrobenzylthioinosine and related compounds. Mol Pharmacol. 1977 Nov;13(6):1014–1023. [PubMed] [Google Scholar]
- Paterson A. R., Oliver J. M. Nucleoside transport. II. Inhibition by p-nitrobenzylthioguanosine and related compounds. Can J Biochem. 1971 Feb;49(2):271–274. doi: 10.1139/o71-039. [DOI] [PubMed] [Google Scholar]
- Paterson A. R., Yang S. E., Lau E. Y., Cass C. E. Low specificity of the nucleoside transport mechanism of RPMI 6410 cells. Mol Pharmacol. 1979 Nov;16(3):900–908. [PubMed] [Google Scholar]
- Paul B., Chen M. F., Paterson A. R. Inhibitors of nucleoside transport. A structure-activity study using human erythrocytes. J Med Chem. 1975 Oct;18(10):968–973. doi: 10.1021/jm00244a003. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Marz R., Wohlhueter R. M. Uridine transport in Novikoff rat hepatoma cells and other cell lines and its relationship to uridine phosphorylation and phosphorolysis. J Cell Physiol. 1978 Oct;97(1):49–72. doi: 10.1002/jcp.1040970107. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Stein W. D., Wigglesworth N. M. Uptake of nucleosides in density-inhibited cultures of 3T3 cells. Nature. 1977 Jun 2;267(5610):442–444. doi: 10.1038/267442a0. [DOI] [PubMed] [Google Scholar]
- Sato E., Kanaoka Y. Reaction of thiouracil and thiouridine with 2-hydroxy-5-nitrobenzyl bromide. Biochim Biophys Acta. 1971 Mar 11;232(2):213–216. doi: 10.1016/0005-2787(71)90572-7. [DOI] [PubMed] [Google Scholar]
- Wohlhueter R. M., Marz R., Plagemann P. G. Properties of the thymidine transport system of Chinese hamster ovary cells as probed by nitrobenzylthioinosine. J Membr Biol. 1978 Sep 19;42(3):247–264. doi: 10.1007/BF01870361. [DOI] [PubMed] [Google Scholar]


