Abstract
Background
Fulminant eosinophilic myocarditis (EM) is a rare and often fatal condition that may present atypically and be complicated by ventricular arrhythmias. Treatment involves high-dose corticosteroids to suppress eosinophilia, as well as increasing use of mepolizumab, an anti-interleukin-5 antibody with evidence for long-term efficacy and safety.
Case summary
A 38-year-old woman presented to the emergency department with neck pain and fatigue, and after extensive investigation was diagnosed with EM secondary to idiopathic hypereosinophilic syndrome. The patient was treated with methylprednisolone for eosinophil suppression and warfarin due to the presence of biventricular apical thrombi. Despite previously stable haemodynamics, the patient had a cardiac arrest due to ventricular fibrillation on Day 11 of hospitalization, requiring 30 min of cardiopulmonary resuscitation and commencement of veno-arterial extracorporeal membrane oxygenation support due to refractory ventricular arrhythmias. The patient was urgently listed for heart transplant but a suitable match was not identified, and she was weaned to pharmacologic support on Day 34. The patient survived with minimal sequelae and has returned to full-time work. She remains on mepolizumab as a steroid-sparing agent, therapeutic anti-coagulation, and heart failure therapy.
Discussion
This report describes an atypical presentation of fulminant EM requiring weeks of mechanical circulatory support due to refractory arrhythmia rather than heart failure. The case also highlighted the benefits of non-invasive cardiac magnetic resonance imaging in both diagnosis and prognostication of EM, as well as the need to maintain a high index of suspicion for this rare disease.
Keywords: Eosinophilic myocarditis, Myocarditis, Cardiac magnetic resonance imaging, Ventricular fibrillation, Case report
Learning points.
Eosinophilic myocarditis may present atypically, and there should be a high index of suspicion for cardiac involvement in the case of undifferentiated eosinophilia.
Eosinophilic myocarditis can cause rapid deterioration with arrhythmia, which may fail pharmacologic management and require mechanical support.
Cardiac magnetic resonance imaging may guide diagnosis and management strategy, for example in identifying extent of thrombus and scarring.
Introduction
Fulminant eosinophilic myocarditis (EM) is a rare and often fatal condition due to heart failure and arrhythmia, with cardiac arrest occurring in up to 27% of histologically proven cases.1,2 Eosinophilic myocarditis may present non-specifically with or without peripheral eosinophilia, and a high index of suspicion for cardiac involvement is required. We present a case of EM in a young woman complicated by a cardiac arrest due to ventricular fibrillation (VF), for which the most likely underlying cause was idiopathic hypereosinophilic syndrome (HES). Endomyocardial biopsy is required for formal diagnosis, but EM also has a characteristic appearance on cardiac magnetic resonance (CMR) imaging and serial imaging was critical in guiding management.3
Summary figure
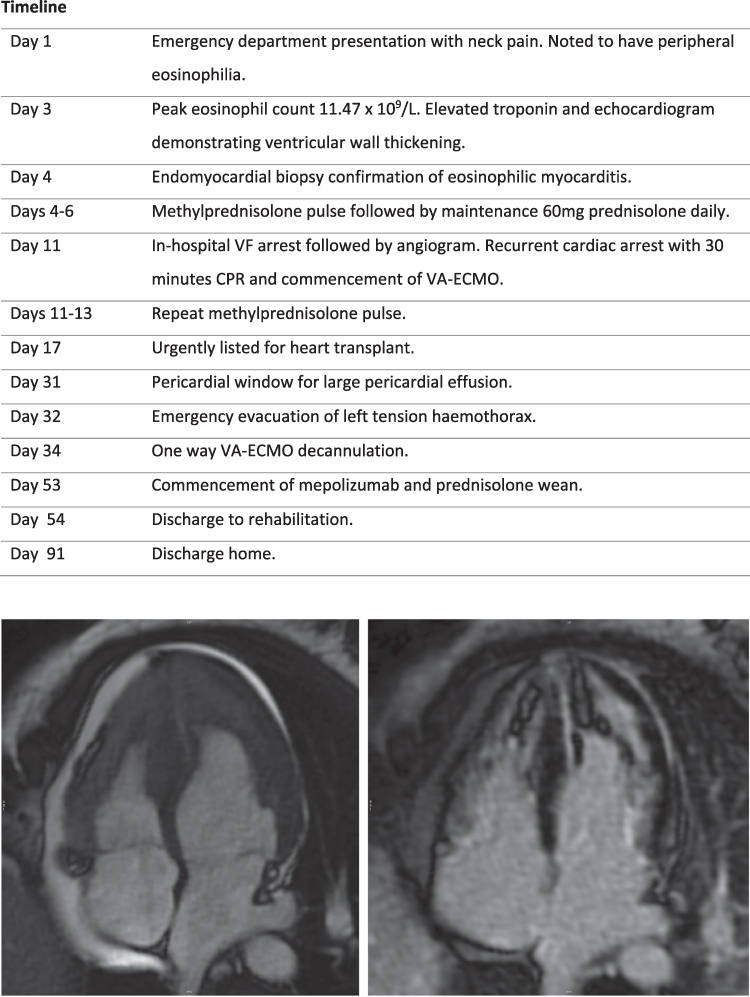
CMR imaging demonstrating biventricular apical thrombus (left panel) and transmural late gadolinium enhancement (right panel).
Case presentation
A 38-year-old woman presented with 3 days of severe right sided neck pain, fatigue, and splinter haemorrhages across multiple fingernails. She denied chest pain or dyspnoea. Blood tests demonstrated a peripheral eosinophilia of 9.7 × 109/L, C-reactive protein 75 mg/L, troponin 3261 ng/L, and N-terminal pro-brain natriuretic peptide 9120 ng/L. Electrocardiograms (ECGs) demonstrated sinus tachycardia without evidence of ischaemia. The patient had no significant past medical history and was a non-smoker with no drug or alcohol use.
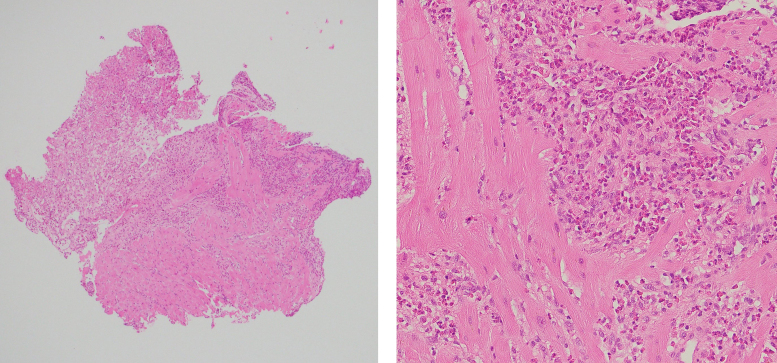
Initial transthoracic echocardiogram (TTE) demonstrated small biventricular cavities with thickened mid-apical segments (Videos 1 and 2). Endomyocardial biopsy on Day 4 confirmed the diagnosis of EM with extensive necrosis of the endomyocardium and eosinophilic infiltrate (Figure 1).
Figure 1.
Endomyocardial biopsy. Left: focally necrotic myocardium (top left) with viable myocardial fibres (bottom). Right: heavy infiltrate of eosinophils with associated necrosis. Haematoxylin and eosin staining.
Subsequent CMR confirmed biventricular thrombotic occlusion of the apices, patchy subendocardial late gadolinium enhancement of the apical segments and pericardium, and a reduced left ventricular ejection fraction (LVEF) of 41%.
Significant negative tests included parasitic serology, other infectious causes, autoimmune, and vasculitic serology. There were no features of hypersensitivity reaction. Bone marrow biopsy demonstrated normal karyotype without cytogenetic abnormalities, and fluorescent in situ hybridization analysis was negative for FIP1L1-PDGFRA, PDGFRB, or FGFR1 rearrangements seen in myeloid/lymphoid neoplasms with eosinophilia. A computed tomography scan did not demonstrate underlying malignancy.
A 3-day course of intravenous methylprednisolone rapidly suppressed the peripheral eosinophil count followed by maintenance 60 mg prednisolone daily. Warfarin was commenced given the biventricular thrombus, and she tolerated low dose bisoprolol and ramipril. Continuous cardiac monitoring demonstrated sinus rhythm throughout.
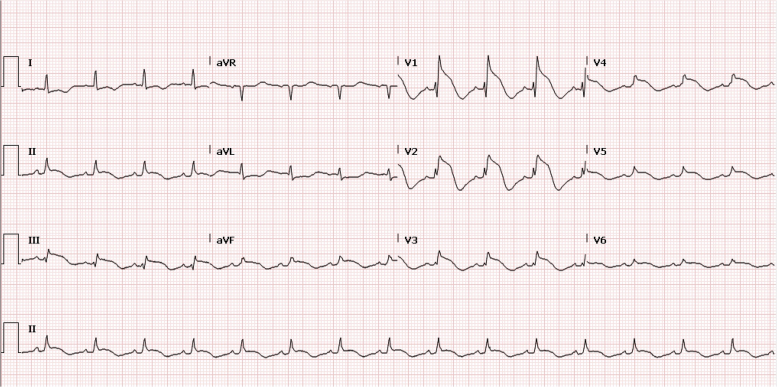
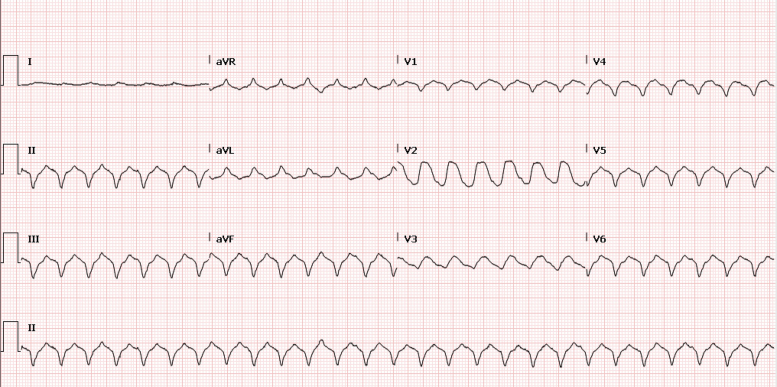
On Day 11, the patient had a cardiac arrest due to VF requiring a single defibrillator shock. Electrocardiograms post-arrest (Figure 2) demonstrated ST-segment elevation anteriorly, and coronary angiography was immediately performed. Although her coronary arteries were normal, increasing non-sustained ventricular tachycardia intra-procedurally culminated in a prolonged cardiac arrest with 13 defibrillations over 30 min. Multiple anti-arrhythmic agents were instigated, including amiodarone, lidocaine, isoprenaline, and procainamide. She was cannulated onto femoral veno-arterial extracorporeal membrane oxygenation (VA-ECMO) during cardiopulmonary resuscitation (CPR) and transferred to the intensive care unit with refractory arrhythmia (Figure 3).
Figure 2.
ECG demonstrating anterior ST elevation following cardiac arrest.
Figure 3.
ECG demonstrating ventricular arrhythmias during VA-ECMO period.
A second course of i.v. methylprednisolone was administered to suppress possible residual cardiac eosinophilia. Ongoing malignant arrhythmia requiring defibrillation despite trials of lidocaine, mexiletine, flecainide, and esmolol prompted urgent heart transplantation listing on Day 17. Long-term support with a ventricular assist device was not deemed an option due to extensive biventricular thrombus. No appropriate donor heart was available during the first two weeks of listing, and the likelihood of a match was low with 99% panel reactive antibodies, likely due to blood transfusions and prior pregnancies. Given the presence of an improvement in ventricular performance and the low likelihood of suitable organ match, it was decided to wean the patient to pharmacologic support, acknowledging the risk of recurrent arrhythmia.
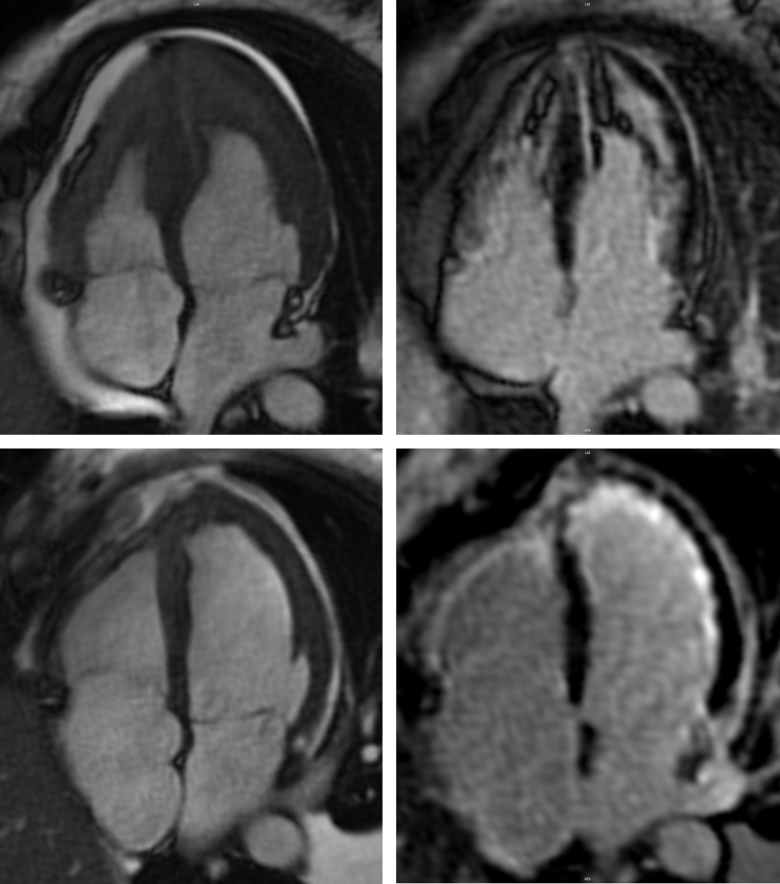
An enlarging pericardial effusion was recognized prior to decannulation and surgical pericardial window performed on Day 31, complicated by a tension haemothorax requiring emergency drainage. Despite this, her cardiac function improved and she was successfully decannulated on Day 34 with rhythm stabilization on oral amiodarone. A repeat CMR on Day 47 demonstrated significant improvement in stroke volume and resolution of the biventricular apical thrombi (Figure 4). Heparin was transitioned to warfarin, a secondary prevention implantable cardioverter defibrillator (ICD) inserted and bisoprolol and candesartan were continued. In consultation with colleagues from the immunology department, prednisolone was tapered and mepolizumab commenced as a steroid-sparing agent. The patient was discharged home from rehabilitation on Day 91 without residual renal, liver, or neurologic impairment and is now back working full-time.
Figure 4.
CMR. Comparison of initial four-chamber (top panels) and follow-up imaging on Day 47 (bottom panels) demonstrating interval improvement in stroke volume (left panels) and apical thrombus, with transmural late gadolinium enhancement of both ventricles (right panels).
Discussion
Fulminant EM (also known as acute necrotizing EM) is a rapidly progressive form with haemodynamic compromise requiring inotropes and/or mechanical circulatory support, and may be fatal despite early recognition: a recent registry identified a mortality rate of 37%.1 Eosinophil infiltration of cardiac tissue and degranulation of cytotoxic contents causes inflammation, necrosis, intracardiac thrombus, and later fibrosis. The characteristic hypercoagulable state is secondary to increased circulating thrombin and eosinophil release of tissue factor, the main initiator of blood coagulation.4
The underlying cause frequently remains unknown, although drug hypersensitivity, infection, malignancy, immune-mediated disorders such as eosinophilic granulomatosis with polyangiitis, and myeloproliferative disorders need to be excluded. Hypereosinophilic syndrome, the most likely diagnosis in this case, requires >1.5 × 105/L blood eosinophils in the absence of an alternative cause with attributable organ dysfunction.5
A high index of suspicion is needed: EM often goes undiagnosed, in part due to atypical presentations and the absence of peripheral eosinophilia in up to 25%, with 20% of cases diagnosed at autopsy.2 It is interesting in this case that cardiac arrest occurred on Day 11 despite steroid treatment, and it is possible that other patients with similar early symptoms may not have presented to medical attention prior to sudden cardiac death.
Definitive diagnosis of EM currently relies upon biopsy evidence of eosinophilic infiltrate.6 Cardiac magnetic resonance is the gold standard for non-invasive diagnosis of myocarditis, with the updated 2018 Lake Louise Criteria demonstrating a sensitivity of 87.5% and specificity of 96.2% for diagnosis of acute myocarditis.7 Although CMR cannot identify the inflammatory cell infiltrate and therefore histologic subtype of myocarditis, specific imaging features of EM are highly characteristic compared to other subtypes. This includes apical intra-cavity thrombus, a relatively preserved LVEF and extensive myocardial hyperintensity on T2 weighted imaging.8 Diffuse subendocardial late gadolinium enhancement not restricted to a vascular territory can be seen in all forms.9
There are no evidence-based criteria for classifying severity or treatment strategy for EM. However, unlike other non-invasive imaging, CMR accurately identifies extent of mural thrombus and the stage of myocardial disease, from early oedema and inflammation to late fibrosis and restriction. This is crucial for effective treatment, prognostication, and evaluation of response. It also visualizes post-inflammatory scarring that may predict risk of future arrhythmia and therefore assists risk stratification for ICD implantation.3 In this case, follow-up CMR demonstrated complete radiographic resolution of thrombus and improvement in LVEF. It had been unknown whether suppression of peripheral eosinophilia would correlate with cardiac involvement, and so this confirmation of response to therapy was crucial in guiding ongoing immunosuppression and the nuanced discussion of appropriate long-term anti-coagulation strategy in a young woman.
Eosinophil suppression in EM primarily involves early high-dose steroids, though there is a growing evidence base for the use of mepolizumab, an interleukin-5 antagonist, as a steroid-sparing agent: it has been demonstrated to be highly effective in HES and eosinophilic granulomatosis with polyangiitis, with case report data of its successful use in EM.10–13
In this unique case, survival would not have been possible without a prolonged period of mechanical circulatory support, with malignant arrhythmia lasting 21 days despite aggressive pharmacotherapy. Extracorporeal membrane oxygenation allowed the necessary time for resolution of inflammation and resultant electrical instability, although it was uncertain at the time whether it would be sustainable in the face of accumulating complications such as infection and bleeding. More durable options such as ventricular assist device as a bridge to transplant were contraindicated in the setting of LV thrombus and small biventricular cavity sizes.
Conclusions
We describe a rare case of EM due to idiopathic HES where the patient survived 30 min of CPR and 23 days of ECMO support. It highlights the use of CMR as an important non-invasive diagnostic and prognostic tool, and the utility of mechanical circulatory support in surviving refractory arrhythmias. The atypical presentation demonstrates the need to maintain a high index of suspicion for this rare disease.
Supplementary Material
Acknowledgements
We acknowledge the contribution from all healthcare personnel involved in this case at the Alfred Hospital, particularly Dr James Theuerle for his role in patient care and imaging.
Consent: The authors confirm that written consent for submission and publication of this case report has been obtained from the patient as per COPE guidelines.
Funding: There is no relevant funding to declare.
Contributor Information
Claudia Brick, Department of Cardiology, Alfred Hospital, 55 Commercial Road, Melbourne, Victoria 3004, Australia.
Angeline Leet, Department of Cardiology, Alfred Hospital, 55 Commercial Road, Melbourne, Victoria 3004, Australia.
Hui Tay, Department of Pathology, Alfred Hospital, Melbourne, Victoria, Australia.
David M Kaye, Department of Cardiology, Alfred Hospital, 55 Commercial Road, Melbourne, Victoria 3004, Australia; Baker Heart and Diabetes Institute, Melbourne, Victoria 3004, Australia.
Andrew J Taylor, Baker Heart and Diabetes Institute, Melbourne, Victoria 3004, Australia; Department of Cardiology, Royal Melbourne Hospital, Grattan Street, Parkville, Melbourne, Victoria 3050, Australia.
Lead author biography

Dr Claudia Brick is a first year cardiology advanced trainee at Austin Health in Melbourne, Australia. She completed her Royal Australasian College of Physician examinations in 2023 at Alfred Health, and completed medical school training through Monash Medical School in 2019.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Data availability
The data underlying this article are available in the main article and in its online Supplementary material.
References
- 1.Ammirati E, Veronese G, Brambatti M, Merlo M, Cipriani M, Potena L, et al. Fulminant versus acute nonfulminant myocarditis in patients with left ventricular systolic dysfunction. J Am Coll Cardiol 2019;74:299–311. [DOI] [PubMed] [Google Scholar]
- 2.Brambatti M, Matassini MV, Adler ED, Klingel K, Camici PG, Ammirati E. Eosinophilic myocarditis: characteristics, treatment, and outcomes. J Am Coll Cardiol 2017;70:2363–2375. [DOI] [PubMed] [Google Scholar]
- 3.Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 2018;72:3158–3176. [DOI] [PubMed] [Google Scholar]
- 4.Kuchynka P, Palecek T, Masek M, Cerny V, Lambert L, Vitkova I, et al. Current diagnostic and therapeutic aspects of eosinophilic myocarditis. Biomed Res Int 2016;2016:2829583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valent P, Klion AD, Roufosse F, Simon D, Metzgeroth G, Leiferman KM, et al. Proposed refined diagnostic criteria and classification of eosinophil disorders and related syndromes. Allergy 2023;78:47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caforio ALP, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636–2648. [DOI] [PubMed] [Google Scholar]
- 7.Luetkens JA, Faron A, Isaak A, Dabir D, Kuetting D, Feisst A, et al. Comparison of original and 2018 Lake Louise criteria for diagnosis of acute myocarditis: results of a validation cohort. Radiol Cardiothorac Imaging 2019;1:e190010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ammirati E, Veronese G, Bottiroli M, Wang DW, Cipriani M, Garascia A, et al. Update on acute myocarditis. Trends Cardiovasc Med 2021;31:370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Séguéla P-E, Iriart X, Acar P, Montaudon M, Roudaut R, Thambo J-B. Eosinophilic cardiac disease: molecular, clinical and imaging aspects. Arch Cardiovasc Dis 2015;108:258–268. [DOI] [PubMed] [Google Scholar]
- 10.Rothenberg ME, Klion AD, Roufosse FE, Kahn JE, Weller PF, Simon H-U, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med 2008;358:1215–1228. [DOI] [PubMed] [Google Scholar]
- 11.Roufosse F, Kahn J-E, Rothenberg ME, Wardlaw AJ, Klion AD, Kirby SY, et al. Efficacy and safety of mepolizumab in hypereosinophilic syndrome: a phase III, randomized, placebo-controlled trial. J Allergy Clin Immunol 2020;146:1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wechsler ME, Akuthota P, Khoury JD, Klion P, Langford A, A C, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med 2017;376:1921–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trovato V, Asada A, Fussner L, Curtis C, Kahwash R. Interleukin-5 antagonist monoclonal antibody therapy improves symptoms and reduces steroid dependence in eosinophilic myocarditis patients. JACC Case Rep 2024;29:102267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the main article and in its online Supplementary material.